Abstract
The antioxidant properties of various commonly consumed commercial teas were screened and compared to check what the consumers get in commercial tea bags in the range of consume preparation conditions. It includes flavored black teas and green teas, as well as some fruit teas. Electron transfer-based assays, such as phenolic content by Folin-Ciocalteu method and cupric ion reducing antioxidant capacity (CUPRAC), were applied. Higher antioxidant activity values were obtained for green and black tea infusions in comparison to fruit teas. The correlation between FC total phenolic and CUPRAC assay for all studied teas was significant (R2 = 0.871). The antioxidant power of tea infusions were also measured using cyclic voltammetry. The observed anodic waves were broadened in comparison with voltammograms of single flavonoids due to the response of several antioxidants with different oxidation potentials. The values of Trolox equivalents obtained by electrochemical approach were lower than in the CUPRAC assay; however, the increased order of the antioxidant capacity of tea infusions was the same.
INTRODUCTION
Natural antioxidants, particularly in fruits and vegetables, have gained increasing interest because of their ability to reduce free radical-mediated degradation of cells and tissues in an organism. The experimental studies have recognized that tea plant (Camellia sinensis) exhibits a significant health protecting activity due to its high flavonoid content.[Citation1,Citation2] Flavanoids are the most abundant compounds in fresh tea leaves and extracts.[Citation3] They have been considered to be responsible for the anticarcinogenic and antimutagenic properties of tea, as well as its protective action against cardiovascular diseases.[Citation4–6]
Tea is grown in about 30 countries but is consumed worldwide, although at greatly varying levels. Tea is manufactured in three basic forms. Green tea is prepared in such a way as to preclude the oxidation of green leaf polyphenols. During black tea production, oxidation is promoted so that most of these substances are oxidized. Oolong tea is a partially oxidized product. Fresh tea leaf is unusually rich in the flavanol group of polyphenols known as catechins, which may constitute up to 30% of the dry leaf weight.[Citation1] Other polyphenols include flavonols and their glycosides, as well as some phenolic acids, such as chlorogenic acid; coumarylquinic acid; and one unique to tea, theogallin (3-galloylquinic acid). Black and green teas are also used for the production of flavored tea where additional aromas and dry fruits or herbs are mixed with tea leaves in the last stage of processing before packing. Aromatized teas are popular because of their fragrance, antioxidant properties, and therapeutic applications.[Citation7] Fruit and herbal teas are milder to the human nervous system than black or green teas containing caffeine.[Citation8]
The antioxidant capacity of natural products in vitro has been assessed by various methods that have been recently reviewed.[Citation9–11 There is no single, widely acceptable assay method applicable to a reasonable variety of compounds in food matrices. Frankel and Meyer[Citation12] criticized one-dimensional methods to evaluate the antioxidant status of food. Electron transfer (ET)-based assays involving a redox reaction, where the change of color of the probe (oxidant) is proportional to the total antioxidant capacity, may results that are compatible with polyphenolic content.[Citation11–13
The aim of this work was to measure the antioxidant properties of various commonly consumed teas and to check what the consumers get in commercial tea bags in the range of consume preparation conditions. ET-based assays, such as phenolic content by Folin-Ciocalteu (FC) method and cupric ion reducing antioxidant capacity (CUPRAC) assay, were applied. The antioxidant power of tea infusion was also measured using cyclic voltammetry. It was demonstrated that electrochemical behavior of flavonoids and phenolic acids was related to their antioxidant activity.[Citation14]
REAGENTS AND APPARATUS
The standards of trolox, gallic acid, Folin-Ciocalteu's reagent, and DPPH (2,2-diphenyl-1-picryhydrazyl radicals) as well as the other chemicals were purchased from Sigma (Steinheim, Germany). Ultrapure water from Milli-Q system (Millipore, Bedford, MA, USA) with a conductivity of 18 MQ was used in all experiments. All solutions were filtered through 0.45 μm membranes (Millipore) and degassed prior to use.
Spectrophotometric determination was performed on a Perkin Elmer (Rodgau, Germany) model Lambda 20 UV-VIS spectrophotometer with cuvettes of 1 cm length. Data were processed with WinLab software (Perkin Elmer). Cyclic voltammetric experiments were performed using a one-compartment electrochemical cell equipped with a conventional three electrode system: glassy carbon working electrode, platinum wire auxiliary electrode, and Ag/AgCl reference electrode. The cyclic voltammograms were recorded in methanol with 0.1 M LiClO4 as supporting electrolyte from −100 to +1300 mV at a scanning rate of 100 mV/s. The working electrode was carefully polished with 0.05 μm alumina paste and rinsed in deionized water at the end of each cycle.
Teas and Preparation of Infusions
All bagged teas were purchased from a local market. Their compositions, as given by the producer, are presented in . The tea bags were dipped into 100 mL of freshly boiled water for 5 min. After the infusion time, the bags were removed and the partly turbid solutions were filtered after cooling to room temperature and then analyzed.
Table 1 Composition of studied teas
Table 2 The antioxidant capacities of tea infusions prepared from different kinds of tea measured by Folin and CUPRAC assays
Procedures for Antioxidant Capacity
Total phenolic content was determined using the Folin-Ciocalteu assay. One mL of tea infusion was introduced into test tubes followed by 0.1 mL of Folin-Ciocalteu's reagent and 0.9 mL of water. The tubes were allowed to stand for 5 min. At the end of this period, 1 mL of sodium carbonate (7%, w/v) and 0.4 mL of water were added and 10 more min were allowed for stabilization of the blue color formed. The absorbance against a reagent blank was measured at 765 nm. Total phenolic content was expressed as gallic acid (GA) and trolox (TR) equivalents as mg per g of dry matter.
For assessing cupric reducing ability (CUPRAC), the assay described by Apak et al. was adapted.[Citation15] In a test tube, 1 mL CuCl2 solution (1.0 × 10−2 M) was mixed with 1 mL of neocuproine alcoholic solution (7.5 × 10−3 M) and 1 mL of 1 M NH4AC buffer (pH 7), followed by mixing 0.5 mL of tea infusion and 0.6 mL of water. The tube containing sample and reagents was incubated in a water bath at a temperature of 50°C for 20 min. After cooling under running water, the absorbance against a reagent blank was measured at 450 nm. The infusions of teas were appropriately diluted with water to keep the absorbance between 0.2 and 0.4 absorbance units. The calibration curve was built up with TR and the antioxidant activity of the tea infusions was expressed as trolox equivalent (mmol TR/g of dry matter).
Cyclic voltammetric (CV) experiments were performed with a solution of infusions mixed with 0.1 M LiClO4 at a ratio of 1:1 (v/v).[Citation16] The CV method was actually based on the correlation between the total charge below the anodic wave of cyclic voltammograms and the antioxidant capacity of the sample and reference substance. Trolox was used as the reference compound and the results were expressed as mmol TR/g of dry tea. The total charge under the anodic wave of the background signal (solvent + supporting electrode) was subtracted from total charge under the anodic wave obtained for each standard and the samples.
Statistical Analysis
The data presented here are from single individual samples of the teas purchased. All analyses were run in triplicate and mean values, together with the standard deviations, are recorded.
RESULTS AND DISCUSSION
Different types of tea infusion had widely different antioxidant properties () ranging from 513.4 mg of GA/g for one of green tea (sample G1) to 122.9 mg of GA/g for one of the fruit teas (F2). Generally, the highest antioxidant power determined by Folin-Ciocalteu assay was obtained for green teas, followed by premium black tea, then flavored black teas, and in the end fruit teas (). This different behavior is due to the phenolic components present in these tea extracts. Infusion of Char Ming or Caribbean as fruit teas did not contain catechins but contained several compounds, including naringin and hesperidin (mainly) and others (in lower concentrations), namely quercetin as well as hydroxycinamic acids (ferrulic, chlorogenic, and synaptic acids), all with lower antioxidant activity. The lower antioxidant capacity of black teas compared to green teas may be attributed to the oxidative polymerization of some green tea antioxidants, mainly catechins and their gallic esters under the catalytic action of polyphenol oxidase during manufacture involving fermentation. The polyphenols contribute to astringency, but they were not responsible for the flavor, although there was evidence that interactions of flavanols in green tea extracts during heat processing and storage cause changes in the tea aroma.[Citation17] The cup of very popular black tea, commercial brand (Yellow label), exhibited antioxidant capacity equal to 835.6 mg of gallic acid in 100 mL of infusion. Benzie and Szeto[Citation18] estimated that a typical cup of green tea of usual strength (1.5%) contains an amount of antioxidant power similar to that found in 100–200 mg of pure ascorbic acid (vitamin C). The order of reported FC antioxidant capacities for studied tea infusions, calculated as trolox equivalents, was the same. The Folin-Ciocalteu method is the one adopted in almost all the published works regarding screening of natural antioxidants, and the obtained results are usually expressed as gallic acid equivalent (mg GA/g).[Citation10,Citation11] However, to standardize the results from various studies, the trolox equivalent antioxidant capacity (TEAC) has been used.[Citation9,Citation10]
In the CUPRAC method using copper(II)-neocuproine complex as an oxidant, the antioxidant capacity is assumed to be equal to the reducing capacity of a studied compound or sample.[Citation19] The CUPRAC values of antioxidant capacities of studied infusions reported in show that green and black teas gave a trolox equivalent of 27.1–11.4 mM TR/g. Fruit teas exhibited much lower antioxidant activity (in the range of 4.0–5.0 mM TR/g), similar like in Folin-Ciocalteu assay. However, the obtained TEAC values for fruit tea infusion were higher than reported for herbal teabags.[Citation20] Thus, these teas, with a nice smell of fruits, would support the human diet with a satisfactory source of antioxidants. The slightly higher results reported in CUPRAC assay in comparison with FC method may be a result of the fact that the redox reaction associated with FC may not reach completion at least for some antioxidants within the specific period of time (15 min vs. 20 min for FC and CUPRAC, respectively). Moreover, the samples in the CUPRAC method were incubated in a water bath at 50°C after mixing of reagents. Slow reacting flavonoids and hydroxycinamic acids, such as naringenin, sinapic, and chlorogenic acids, the most abundant phenolic in the citrus and berry families as well as in some other fruits, needed elevated temperature incubation to complete their oxidation.
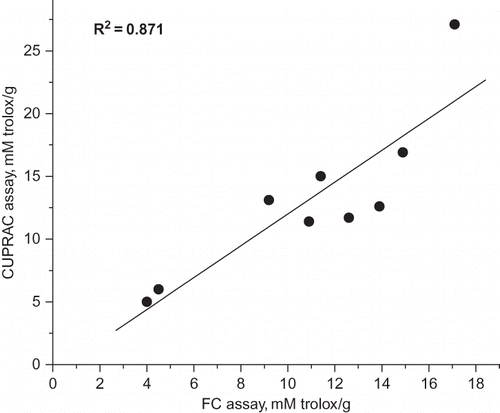
In order to correlate the used methods for evaluation of antioxidant capacity, a regression model was used. The correlation between FC total phenolic and CUPRAC assay for all studied teas, shown in , was significant (R 2 = 0.871). Both methods are based on the redox properties of phenolics, however, with different reduction potentials, different kinetics, and experimental conditions.
Figure 1 The correlation between the results of Folin-Ciocalteu method and CUPRAC assay for studied tea infusions.
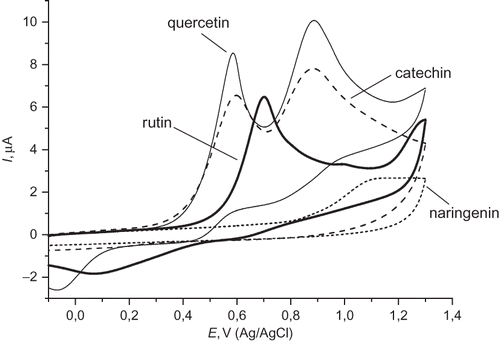
The cyclic voltammograms of some tea flavonoids (all at a concentration of 5 × 10−3 M) in 0.1 M LiClO4 solution are presented in . Catechin and quercetin present two oxidative peaks: the first at 0.59 V and 0.60 V, and a second at 0.88 V and 0.89 V, respectively. The first voltammetric peaks are attributed to the oxidation of the 3′,4′-dihydroxy substituent on the B-ring, while the second peaks correspond to the oxidation of the C-3 hydroxyl groups. Rutin presents only one peak at 0.70 V in the voltammograms as it has the C-3 hydroxyl group conjugated to a rutinose. Cyclic voltammograms of phenolic acids show single anodic peaks in more positive values; chlorogenic acid at 0.66 V and ferullic acid at 0.84 V (data not shown). There is a relationship between the antioxidant activity and the cyclic voltammetric oxidation peak potential for flavonoids; the lower the potential, the higher the antioxidant activity.[Citation21]
Figure 2 Cyclic voltammograms of some flavonoids recorded in 0.1 M LiClO4 solution at glassy carbon electrode, scan rate 100 mV/s (the background response due to the buffer solution has been subtracted from each curve).
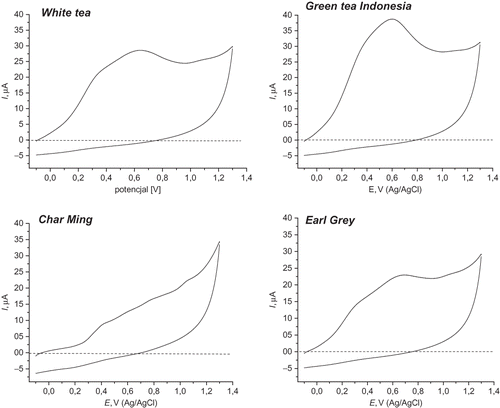
The cyclic voltammograms of analyzed tea infusions were recorded and some examples are shown in . The observed anodic wave was broadened due to the response of several antioxidants with different oxidation potentials, including mainly flavonoids. Antioxidants present in smaller amounts may appear only as a shoulder on some larger feature. As might be expected, the cyclic voltammograms for the green teas resembled that of epigallocatechin gallate, which is expected to be the major phenolic present.[Citation22] Total charge under anodic valves for all samples and calculated reducing capacities expressed in mM trolox per g of dry tea are presented in . Green tea infusions exhibit the highest antioxidant properties followed by black teas. The antioxidant capacity of flavored black teas determined by the CV method was much lower when compared to green teas. Comparison of cyclic voltammetry results with CUPRAC and FC assays has shown that these two methods yielded considerable different chemical information (). The TEAC values obtained by electrochemical approach are lower than in the CUPRAC assay; however, the increasing order of the antioxidant capacity of tea infusions was the same. Similar results were reported by Zielinska et al.[Citation16] for the evaluation of the antioxidant capacity of buckwheat products. The fact that the reducing activity did not show correlation with the content of total phenolics in the samples assayed does not signify that these do not contribute to it, but that this could be the result of the synergies (or antagonisms), still unknown.[Citation23]
Table 3 Reducing activity of tea infusions provided by cyclic voltammetry method
CONCLUSION
Higher antioxidant activity values were obtained for green and black tea infusions in comparison to fruit teas using the Folin-Ciocalteu method, cupric ion reducing antioxidant capacity, as well as cyclic voltammetry. The correlation between FC total phenolic and CUPRAC assay for all studied teas was significant (R 2 = 0.871). The values of trolox equivalents obtained by electrochemical approach were lower than in the CUPRAC assay, however, the increased order of the antioxidant capacity of tea infusions was the same. The antioxidant capacity of given samples are dependent on their chemical composition, particularly the content of phenolic compounds. The study regarding the correlation between the level of compounds exhibiting strong antioxidative properties and the antioxidative properties of different tea infusions will be investigated.
The higher level of antioxidants is expected to be beneficial for health; this can also lead to a more astringent beverage, which may not suit all tastes. For this reason, aromatized and fruit teas have become more and more popular in recent years. Despite an increasing importance of health-related and convenience-related dimensions on consumer acceptance of food, taste is reported to continue to be the prime consideration in food choice.[24]
REFERENCES
- Aoshima , H. , Hirata , S. and Ayabe , S. 2007 . Antioxidative and anti-hydrogen peroxide activities of various herbal teas . Food Chemistry , 1003 : 617 – 622 .
- Atoui , A.K. , Mansouri , A. , Boskou , G. and Kefalas , P. 2005 . Tea and herbal infusion . Their antioxidant activity and phenolic profile. Food Chemistry , 89 : 27 – 36 .
- Peterson , J. , Dwyer , J. , Bhagwat , S. , Haytowitz , D. , Holden , J. , Eldridge , A.L. , Beecher , G. and Aladesanmi , J. 2005 . Major flavonoids in dry tea . Journal of Food Composition and Analysis , 18 : 487 – 501 .
- Heo , H.J. , Kim , Y.J. , Chung , D. and Kim , D.O. 2007 . Antioxidant capacities of individual and combined phenolics in a model system . Food Chemistry , 104 : 87 – 92 .
- Sharangi , A.B. 2009 . Medicinal and therapeutic potentialities of tea (Camelia sinensis L.)—A review . Food Research International , 42 : 529 – 535 .
- Wang , H. , Provan , G.J. and Helliwell , K. 2000 . Tea flavonoids: Their functions, utilization and analysis . Trends in Food Science & Technology , 11 : 152 – 160 .
- Chen , H.Y. , Lin , Y.C. and Hsieh , C.L. 2007 . Evaluation of antioxidant activity of aqueous extract of some selected nutraceutical herbs . Food Chemistry , 104 : 1418 – 1424 .
- Aoshima , H. , Hirata , S. and Ayabe , S. 2007 . Antioxidative and anti-hydrogen peroxide activities of various herbal teas . Food Chemistry , 1003 : 617 – 622 .
- Magalhâes , L.M. , Segundo , M.A. , Reis , S. and Lima , J.L.F. 2008 . Methodological aspects about in vitro evaluation of antioxidant properties . Analytica Chimica Acta , 613 : 1 – 19 .
- Moon , J.K. and Shibamoto , T. 2009 . Antioxidant assays for plant and food components . Journal of Agricultural and Food Chemistry , 57 : 1655 – 1666 .
- Prior , R.L. , Wu , X. and Schaich , K. 2005 . Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements . Journal of Agricultural and Food Chemistry , 53 : 4290 – 4302 .
- Frankel , E.N. and Meyer , A.S. 2000 . The problems of using one-dimensional methods to evaluate multifunctional food and biological antioxidants . Journal of the Science of Food and Agriculture , 80 : 1925 – 1941 .
- Celik , S.E. , Ozyurek , M. , Altun , M. , Bektasoglu , K. , Guclu , K. , Berkel , K.I. , Ozyurek , M. and Apak , R. 2008 . Antioxidant capacities of herbal plants used in the manufacture of Van herby cheese “Otlu Peynir” . International Journal of Food Properties , 11 : 747 – 761 .
- Buratti , S. , Scampicchio , M. , Giovanelli , G. and Manino , S. 2008 . A low-cost and low-tech electrochemical system for the evaluation of total phenolic content and antioxidant power of tea infusions . Talanta , 75 : 312 – 316 .
- Apak , R. , Güçlü , K. , Özyürek , M. and Karademir , S.E. 2004 . Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducting capability in the presence of neocuproine: CUPRAC method . Journal of Agricultural and Food Chemistry , 52 : 7970 – 7981 .
- Zielinska , D. , Wiczkowski , W. and Piskula , M.K. 2008 . Determination of the relative contribution of quercetin and its glucosides to the antioxidant capacity of onion by cyclic voltammetry and spectrophotometric methods . Journal of Agricultural and Food Chemistry , 56 : 3524 – 3531 .
- Wang , L.F. , Kim , D.M. and Lee , C.Y. 2002 . Interaction of flavanols in green tea extract during heat processing and storage . Bioactive Compounds in Foods , 816 : 58 – 72 .
- Benzie , I.F.F. and Szeto , Y.T. 1999 . Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay . Journal of Agriculture and Food Chemistry , 47 : 633 – 636 .
- Huang , D. , Ou , B. and Prior , R.L. 2005 . The chemistry behind antioxidant capacity assays . Journal of Agricultural and Food Chemistry , 53 : 1841 – 1856 .
- Apak , R. , Güçlü , K. , Özyürek , M. , Karademir , S.E. and Erçağ , E. 2006 . The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas . International Journal of Food Sciences and Nutrition , 57 : 292 – 304 .
- Chevion , S. , Roberts , M.A. and Chevion , M. 2000 . The use of cyclic voltammetry for the evaluation of antioxidant capacity . Free Radical Biology & Medicine , 6 : 860 – 870 .
- Kilmartin , P.A. and Hsu , C.F. 2003 . Characterisation of polyphenols in green, oolong, and black teas, and in coffee, using cyclic voltammetry . Food Chemistry , 82 : 501 – 512 .
- Etsuo , N. 2002 . Antioxidant activity: Are we measuring it correctly? . Nutrition , 18 : 524 – 525 .
- Verbeke , W. 2005 . Consumer acceptance of functional foods: Socio-demographic, cognitive and attitudinal determinants . Food Quality and Preference , 16 : 45 – 57 .