Abstract
The phenolic composition, antioxidant activity, and antimicrobial activity of extracts from vine leaves of six grape varieties collected in May, August, and September was studied. The phenolic potential of the extracts was dependent on variety and picking-time. Extracts of leaves collected in September were the richest in total phenols, flavonoids, flavonols, and stilbenes. The antioxidant properties determined by ferric reducing antioxidant power and 2,2-diphenyl-1-picrylhydrazyl assays, and antimicrobial activity against Stapylococcus aureus, Bacillus cereus, Campylobacter jejuni, Escherichia coli, and Salmonella Infantis were good and in correlation with the chemical composition changes of the leaf extracts. The results indicated that leaves remaining on the vine in September after the grape harvest could be especially promising as an inexpensive source of effective antioxidant/antimicrobial agents.
INTRODUCTION
In order to avoid or retard oxidative deterioration and undesirable microbial growth, a wide range of food grade chemicals are added during food preparation, storage, and distribution. The growing interest in the substitution of synthetic food antioxidants and antimicrobial agents by natural ones has fostered research on plant sources and the screening of raw materials for identifying new natural food additives with a broad spectrum of antioxidant and antimicrobial activity. Special attention has been focused on their extraction from inexpensive or residual sources from the agricultural and food-processing industries.[Citation1–5]
The utilization of vine leaves in the food-making process is not unfamiliar. Traditional medicine indicates therapeutic aspects of preparations made of vine leaves,[Citation6–9] while old cooking recipes mention the use of fresh and brined or fermented leaves as food throughout the year, especially in the winter months.[Citation10] In spite this, contemporary use of vine leaves in negligible and knowledge of its chemical composition and properties is insufficient. On the other hand, grapes and grape products have been subjected to very intense research,[Citation11–13] especially after the epidemiological studies proving an inverse correlation between consumption of red wine and the incidence of cardiovascular disease.[Citation14] It has been suggested that polyphenol compounds (flavonoids and stilbenes) are responsible for wine health benefits.[Citation15,Citation16] Recent research suggests potential use of phenolics in food processing for improving quality, safety, and stability of food products.[Citation3,Citation4,Citation12] Scarcity of information on polyphenolic composition of leaves brings out the need for research on this plant material as a potential rich source of phenolic compounds.[Citation12]
The aim of the present study was to investigate the chemical composition and the in vitro antioxidant and antimicrobial activity of phenolic extracts obtained from Vitis vinifera L. leaves. Knowledge of the polyphenolic profile of vine leaf extracts is relevant to their future use. The compounds of interest in the present study were the flavonoids (+)-catechin, (−)-epicatechin, apigenin, kaempherol, quercetin, myricetin, quercetin-4′-glucoside, and rutin, and the stilbenes cis- and trans-resveratrol monomers, piceid and astringin. These polyphenols were selected because of their suggested health-beneficial properties.[Citation16,Citation17] Separation and quantitative determination of individual polyphenolic compounds was performed using high-performance liquid chromatography (HPLC). The antioxidant properties of extracts were determined as the free radical scavenging ability and the ferric reducing power. Additionally, all extracts were screened by the broth microdilution test for antimicrobial activity against gram-positive (Staphylococcus aureus, Bacillus cereus) and gram-negative (Escherichia coli O157:H7, Salmonella Infantis, Campylobacter jejuni) food-borne pathogenic bacteria. This study was conducted with the aim of evaluating the potential of vine leaves as a natural source of biologically active polyphenolic extracts for further use in the food industry to improve the quality and safety and prolong the shelf-life of food products.
MATERIALS AND METHODS
Reagents, Solvents, and Standards for HPLC Method
HPLC standards were purchased from Extrasynthese (Genay, France), Sigma (Milwaukee, WI, USA), and Polyphenols Laboratories (Sandnes, Norway). Acetic acid and acetonitril (HPLC grade) were purchased from Merck (Darmstadt, Germany). The ultrapure water was prepared with a Millli-Q-water purification system (Millipore, Bedford, MA, USA). All of the other reagents and solvents used in the experiments were of adequate analytical grade and were obtained from Fluka (Buch, Switzerland), Kemika (Zagreb, Croatia), Merck (Darmstadt, Germany), and Sigma (Sigma–Aldrich GmbH, Steinheim, Germany).
Instruments
All spectrophotometric analyses were performed using a UV-VIS double beam spectrophotometer (Specord 200, Analytik Jena Inc., Jena, Germany), equipped with a six-cell holder and a thermostatically controlled bath. The content of individual phenolics was determined by a Varian UV-VIS poly diode-array (PDA) 330 detector, a ternary gradient liquid Pro Star 230 pump, heater model 500, and Star chromatography workstation version 6.0 (Varian Inc., Walnut Creek, USA). Microplate Reader Safire II (Tecan, Mannedorf/Zurich, Switzerland) was used for bioluminescence measurements in microbiological microtiter plate assays.
Plant Material
Plant material used in the present research includes Vitis vinifera L. leaves of six grape varieties (white: Maraština, Pošip; red: Lasin, Merlot, Syrah, Vranac). Fully expanded, green, healthy leaves with petioles were collected from Teskera vineyards, Kijevo, Dalmatia, Croatia, during the months of May, August (after verasion), and September (at the end of grape ripening). The sample size was 350 g. The plant material was air dried in the shade at room temperature. The leaf petioles were carefully manually separated and dry leaves were pulverized (3 × 1 min in a high speed grinder) into powder. Three groups of samples—May leaves, August leaves, and September leaves—were prepared.
Extractions of Polyphenols
The polyphenolic compounds were extracted from the homogenized dry plant material (20 g) using alcoholic solvent (ethanol/water 80/20, v/v; 100 mL) at 60°C, contact time was 60 min.[Citation13] The extract was filtered with Whatman No. 1 filter paper and the residual tissue was washed with 2 × 25 mL of alcoholic solvent. The filtrates were combined in total volume, which was dried under a vacuum using a rotary evaporator, at 50°C. The dry residues were redissolved with methanol-water mixture (50:50, v/v) reaching a volume of 10 mL. Extractions were done in three repetitions and extracts (3 × 10 mL) were combined in total leaf extract (LE). Thus, obtained LE was centrifuged at 5000 rpm for 10 min and used for further analysis. Three extracts were obtained for each grape variety: May LE, August LE, and September LE.
Determination of Total Phenols, Flavonoids, Non-Flavonoids, and Flavanols
Total phenols were determined with Folin-Ciocalteu reagent according to the procedure described by Singleton and Rossi.[Citation18] Gallic acid was used as the standard for calibration curve and results are expressed as gallic acid equivalents (GAE)/L of LE. Measurement of total non-flavonoid concentration in LE was based on the method described by Kramling and Singleton and concentration of flavonoid compounds in LE was calculated as difference between total phenols and non-flavonoids.[Citation19] The results for total phenols, non-flavonoids, and flavonoids are reported in g GAE/L of extracts. The total flavanol content was estimated using the p-dimethylaminocinnamaldehyde (DMACA), which has great advantage over the widely used vanillin method, since there is no interference with anthocyanins.[Citation20] The concentration of total flavanols was estimated from a calibration curve, using (−)-epicatechin as a standard. Results are reported in mg of epicatechin equivalents (ECE)/L of LE.
HPLC Analysis
Separation of polyphenols was carried out on an octadecyl column (Zorbax Eclipse XDB-C18; 4.6 × 250, 5μ, Agilent, Palo Alto, USA), using the gradient eluting method, with eluent (A) being water/acetic acid (98:2, v/v) and eluent (B) acetonitrile/acetic acid (98:2, v/v). The elution program used was as follows: 0 min 92% A and 8% B; 18 min 80% A and 20% B; 25 min 60% A and 40% B; 30 min 55% A and 45% B; 40 min 35% A and 65% B; 50 min 20% A and 80% B; 54 min 20% A and 80% B; 57 min 90% A and 10% B; 60 min 90% A and 10% B. Column temperature was 25°C and flow rate was 1.0 mL min−1. Monitoring of the eluate was at 280 nm. Extracts were filtered through 0.45-μm syringe filters and directly injected through a 20-μL fixed loop into a guard C18 column. Each sample was injected twice in order to check reproducibility. Polyphenolic compounds were identified on the basis of their retention times and quantified using external standard calibration curves. The results are expressed as mg/L of LE.
Antioxidant Properties of LEs
Free radical scavenging activity
Radical-scavenging activity was determined by use of stable 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical according to the procedure reported by Katalinić et al.[Citation13] The DPPH assay, which has wide spread use in free radical-scavenging assessment, is based on reaction between the free DPPH radical and molecules that can donate hydrogen atoms (such as most antioxidants).[Citation21] As a result, a stable non-radical form of the DPPH is obtained, with simultaneous change of the violet color to pale yellow due to the picryl group present in solution.[Citation5] The disappearance of the DPPH radical absorption by the action of antioxidants is taken as a measure of antioxidant activity. The decrease in absorbance (A) was measured at 517 nm. The percentage inhibition of the DPPH radical (% Inh DPPH) after adding individual samples was calculated according to the following equation:
Ferric Reducing Antioxidant Power (FRAP)
FRAP of LEs was estimated following the procedure originally described by Benzie and Strain.[Citation22] At low pH, when a ferric-tripyridyltriazine (FeIII-TPTZ) complex is reduced to the ferrous (FeII) form, an intense blue color with an absorption maximum at 593 nm develops. The change in absorbance, therefore, is directly related to the combined or “total” reducing power of the electron-donating antioxidants present in the reaction mixture. Increased absorbance of the reaction mixture indicates greater reduction capability.[Citation5,Citation22] Standard curve was prepared using different concentrations (100–2000 μmol/L) of Trolox and the antioxidant efficiency of LE was calculated with reference to the reaction signal given by the Trolox solution of known concentration.[Citation13] Results are expressed as millimolar Trolox equivalents (TE)/L of extract.
Antimicrobial Activity
Bacterial strains, culture media, and growth conditions
Antimicrobial effects were individually tested against Bacillus cereus WSBC 10530 (clinical isolate), Staphylococcus aureus ATCC 25923 (clinical isolate), Campylobacter jejuni ATCC 33560 (bovine faeces isolate), Escherichia coli O157:H7 ZMJ 129 (clinical isolate), and Salmonella Infantis ZM9 (poultry meat isolate). Bacterial cultures of B. cereus, S. aureus, and S. Infantis for antimicrobial were grown in 5 mL of Müeller Hinton broth (MHB, Oxoid, Hampshire, UK) aerobically for 20 h, continuously shaken at 100 rpm at 37°C. E. coli was incubated in Triptone soya broth (TSB) or Triptone soya agar (TSA) (Oxoid, Hampshire, UK), while C. jejuni was incubated microaerobically at 42°C in MHB with defibrinated horse blood (Oxoid, Hampshire, UK) added. Bacterial cultures for antimicrobial testing were incubated for 20 h in MHB and for antibacterial activity assays 1 mL of each culture was diluted with MHB medium to ca. 106 CFU/mL.
Determination of the minimum inhibitory concentration (MIC)
The extracts prepared as described above were diluted to 10 and 15% (v/v) stock solutions in MHB (or TSB when E. coli was tested). For the broth microdilution test, 50 μL of bacterial suspension was added to the wells of a sterile 96-well microtiter plate containing 50 μL of two-fold serially diluted plant extracts in MHB. The concentrations ranged from 4.0–0.06 mg GAE/mL growth medium. The final volume in each well was 100 μL. Control wells were prepared with culture medium, bacterial suspension only, plant extracts only, and ethanol in amounts corresponding to the highest quantity present. The contents of each well were mixed on a microplate shaker (Eppendorf, Hamburg, Germany) at 900 rpm for 1 min prior to incubation for 24 h at 37°C. The MIC was defined as the lowest concentration where no viability was observed after 24 h on the basis of metabolic activity. To indicate respiratory activity, the presence of the purple color was determined visually after adding 10 μL/well of INT (2-p-iodophenyl-3-p-nitrophenyl-5-phenyl tetrazolium chloride, Sigma-Aldrich GmbH, Steinheim, Germany) dissolved in water (2 mg/mL) and incubated at 37°C for 30 min in the dark.[Citation23] To analyze the adenosine triphosphate activity, the absence of a bioluminescence signal was checked by a Microplate Reader after adding 100 μL/well of BacTiter‐GloTM reagent (Promega, Madison, WI, USA) and 5 min incubation in the dark.[Citation24] MICs were expressed in mg GAE/mL of growth medium.[Citation13] All measurements of MIC values were repeated in triplicate and the most representative values were used.
Statistical Analysis
In all cases, analyses were performed in triplicate, unless elsewhere specified. Data are presented as mean values ± standard deviation. Statistical analysis was performed with the GraphPad InStat3 package (Version 4.03 for Windows, GraphPad Software, San Diego, USA). The t-test was used to determine whether the mean of a variable differed between the samples. Relationships between variables were assessed by Pearson's product-moment correlation coefficient “r.” A value of P < 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Understanding of the chemical composition and potential biological properties of plant extracts is of essential importance for their further use in the food industry. A range of spectrophotometric and chromatographic assays was applied to create a data base that could be used to evaluate the potential of grape vine leaves as an inexpensive and accessible natural resource for the production of polyphenol-rich extracts with strong antimicrobial and antioxidant activity.
Polyphenolic Composition
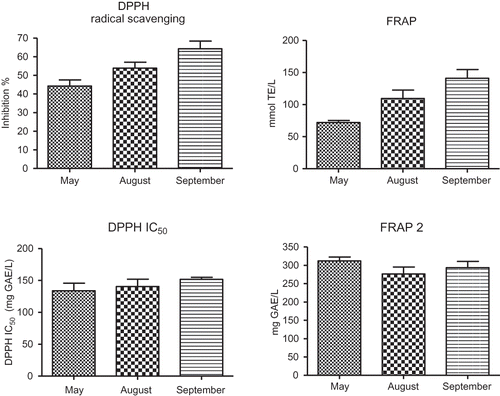
Results of the investigation of the phenolic composition of vine LEs are presented in and and The total phenolic content of the LEs, as estimated by the Folin-Ciocalteu method, was cultivar-dependent and ranged from 18.8 to 28.0 g GAE/L in May LEs, from 25.2 to 35.0 g GAE/L in August LEs, and from 32.5 to 46.7 g GAE/L in September LEs (). The results indicate that both the variety and leaf-picking time strongly influenced the Les' phenolic content and composition. The differences between the contents of total phenolics, or phenolic subgroup, in the extracts of grape vine leaves collected during different vegetation phases () were statistically significant (P < 0.05). The average amount of total phenols in September LEs (40.5 ± 6.3 g GAE/L) was almost 2-fold higher compared to May LEs (22.5 ± 3.4 g GAE/L). A dramatic increase in the mean total flavonoid content from May to September (3.9 ± 2.1 g GAE/L of May LEs; 7.3 ± 3.6 g GAE/L of August LEs; 15.5 ± 6.9 g GAE/L of September LEs) was followed by an equally rapid increase of total flavanols. The average content of total flavonoids, as well as total flavanols (estimated by the DMACA method), was about 4-fold higher in September LEs compared to May LEs ( and ). The total phenol content calculated per mass of plant material ranged from 9.4 to 23.4 g GAE/kg of dry leaves (N = 18), which confirms high phenolic potential of vine leaves.
Table 1 Phenolic content and related antioxidant properties of Vitis vinifera L. leaf extracts
Table 2 The content of individual flavonoids and stilbenes in the extracts from Vitis vinifera L. leaves
Figure 1 The phenolic profile of grapevine leaf extracts (LEs). The content of flavonols (*) was calculated as the sum of apigenin, kaempherol, quercetin, myricetin, quercetin-4′-glucoside and rutin, and the content of stilbenes (**) calculated as the sum of trans-resveratrol and astringin. The results are mean ±SD for LEs of six grape varieties.
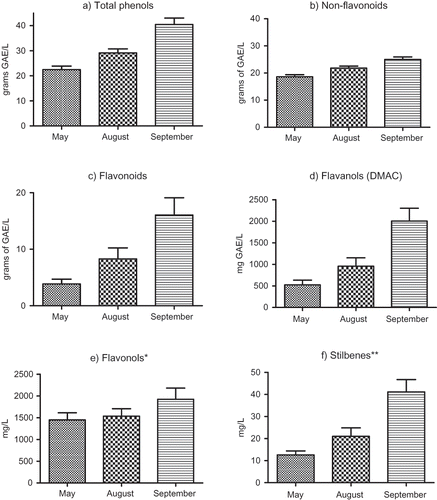
By applying the HPLC-RP-PDA method, a successful separation, identification, and quantification of (+)-catechin and (−)-epicatechin (flavan-3-ols); apigenin, kaempherol, quercetin, myricetin, quercetin-4′-glucoside, and rutin (flavonols); cis- and trans-resveratrol monomers; and astringin (stilbenes) was performed. According to the results presented in , the contents of the principal polyphenolic compounds in Vitis vinifera LEs were variety dependent and strongly influenced by the sample collection period. The dominant flavan-3-ol monomer in all LEs was (+)-catechin. The average concentrations of this flavonoid compound with good antioxidant properties were 328 ± 249 mg/L, 328 ± 302 mg/L, and 225 ± 349 mg/L, given for May, August, and September LEs, respectively. The best results were obtained for the red varieties, Vranac (up to 164 mg/kg of dry leaves) and particularly Merlot (up to 467 mg/kg of dry leaves). Compared to (+)-catechin, its epimer (−)-epicatechin was found in significantly lower concentrations, especially in September LEs in which the ratio of catechin/epicatechin was 80:1. The significantly smaller amounts of (−)-epicatechin monomer compared to (+)-catechin, confirmed in all LEs analyzed, could be the consequences of a more intensive embedding of (−)-epicatechin into complex polymeric forms. This is in line with results reported by Bogs et al.,[Citation25] who stated that the condensed leaf tannins or proanthocyanidin polymers comprise smaller polymers than those in berry skin, with epicatechin the major subunit.
Contrary to the decrease in the monomer content of catechins, a significant increase in flavonol content could be noticed in September LEs compared to May LEs (, ). The dominant flavonol monomer was quercetin. The concentration of this powerful, well-known antioxidant ranged from 10 to 35 mg/kg of dry leaves, depending on the variety and the time of sampling. While the average concentration of kaempherol remained almost the same, larger amounts of apigenin (10.6 ± 2.9 mg/L) and myricetin (23.0 ± 9.9 mg/L) were found in the extracts of leaves picked in September. It can be noticed that the decrease in free quercetin content in September LEs compared to August LEs was followed by a significant increase in the content of quercetin derivatives (1475 mg/L in August LEs; 1857 mg/L in September LEs), calculated as the sum of rutin and quercetin-4′-glucoside. Due to the extremely high concentrations of quercetin derivatives (1–3 g/kg of dry leaves), the total relative content of flavonols amounted to 90%, or more of the total quantity of identified phenolic compounds. The results for flavonols are in agreement with literature data.[Citation9,Citation26] These authors also identified quercetin and quercetin glycosides as the main compounds responsible for the antioxidant activity of grape vine leaves.
Because of the great interest that has recently been devoted to resveratrol and its derivatives, special attention was given to the stilbene compounds.[Citation16] The presence of trans-resveratrol and astringin, confirmed in all LEs, was the highest in September LEs (, ). The presence of cis-resveratrol was found only in September LEs of the Merlot and Vranac varieties. The trans-resveratrol content calculated per mass of plant material ranged from 0.2 to 9.4 mg/kg of dry leaves, which is consistent with data published by Balik et al.,[Citation12] who confirmed the presence of this phytoalexin in a concentrations from 2.5 to 10.3 mg/kg of dry healthy leaves. Quantification of the resveratrol derivatives piceid and isorhapontin was not possible because the HPLC-RP method applied could not separate these two stilbenes. Regarding the trans-resveratrol content, concentrations above 10 mg/L were found in September LEs of the Syrah (red) and Pošip (white) varieties. Regarding the astringin contents, above 20 mg/L were found in the three August LEs and five September LEs. It may be generally concluded that the content of certain stilbenes, as well as flavonoids, in LEs analyzed depended on the variety and the phenophase in which the leaves had been picked, but based on the basis of the available data it cannot be concluded that there are differences between the white and the red varieties.
Antioxidant Activity
The antioxidant properties of LEs were determined as DPPH radical scavenging ability and ferric reducing/antioxidant power (FRAP method). Appreciable antioxidant potential of all LEs () was observed and related to the presence of a mixture of polyphenolic compounds with good antioxidant properties. An extremely significant correlation between the free radical scavenging ability (% Inh DPPH) and the reducing power of extracts (FRAP) was confirmed (P < 0.0001; N = 18).
Free Radical-Scavenging Ability
The DPPH assay was based on the measurement of the loss of the deep violet color of the DPPH radical at 517 nm after reaction with an antioxidant compound. It is a simple and rapid test widely used in antioxidant screening to provide information on antioxidant or antioxidant mixture capability in preventing reactive radical species from reaching biomolecules, such as lipoproteins or polyunsaturated fatty acids, in biological and food systems. All LEs, especially September LEs, were potently active and exhibited strong and concentration-dependent DPPH radical-scavenging ability. Significant correlations were observed between total phenol content, total flavonoids, or total flavanol content and free radical scavenging activity of LEs (Pearson's correlation coefficients r = 0.882, 0.868, 0.907; N = 18). The results imply that vine leaves' phenolic extracts may be useful for preventing radical-related food deterioration, especially at higher concentrations.
The concentration of total phenols that causes a decrease in the initial DPPH concentration by 50% is defined as IC50. A lower DPPH IC50 value indicates a better free radical scavenging capacity of the extracted polyphenolic mixture. The ethanolic LEs of selected grape varieties, regardless of the month in which the leaves had been picked, were able to interact with the stable free DPPH radicals efficiently and quickly, with an average IC50 of 142 ± 24 (range 104 to 180) mg GAE/L, which is very similar to the IC50 values of grape skin extracts (148 ± 70 mg GAE/L) reported by Katalinić et al.[Citation13] Although a slight increase of IC50 can be noticed during the monitoring period (May: 134 mg GAE/L; August: 141 mg GAE/L; September: 152 mg GAE/L), the differences between the mean values for May, August, and September LEs () were not statistically significant. This indicates that the changes in concentration of single phenolic constituents () were not crucial and that the total antioxidant capacity of the extracts is a result of the joint, additive, or even synergistic, activity of all, not just a single phenolic compound.
Reducing Power (FRAP)
FRAP detects compounds with redox potentials of <0.7 V, the redox potential of the Fe3+-TPTZ product. Unlike the DPPH method that includes more reaction mechanisms, the FRAP assay is an entirely electron transfer reaction and cannot detect compounds that act by hydrogen atom transfer.[Citation27,Citation28] The reducing power of all LEs, according to FRAP, was good and ranged from 63.6 to 183.5 mmol TE/L (average 108 ± 39 mmol TE/L; N = 18), depending on the variety and the period in which the leaves had been picked. Comparison of the reducing power of LEs picked during different months is displayed in A significant increase in FRAP was noticed in August (110 ± 32 mmol TE/L), and particularly in September (141 ± 33 mmol TE/L), compared to May LEs (72 ± 7.8 mmol TE/L), and can be related to changes in phenol content and composition. The extremely significant correlation between FRAP and total phenol, or flavonoid, content (P < 0.0001; N = 18) was expected because the FC method is based on the reaction of reducing groups, and, in fact, measures the reducing capacity of any phenolic compound relative to the reducing capacity of gallic acid as standard.[Citation11] The high Pearson's correlation coefficient (r = 0.907; P < 0.0001; N = 18) between FRAP and flavanols (DMACA) indicates the importance of this group of compounds in the total reducing power of the LEs. Interestingly, although the proportion of trans-resveratrol and astringin in LEs is small compared to the total phenol content, a significant positive correlation was found between FRAP and the detected stilbenes (P < 0.05; N = 18). FRAP2, the quantity of phenolic compounds with a reducing power of 1 mM of Trolox, was calculated for each extract. The means of FRAP2 for May, August, and September LEs did not differ significantly (), regardless of the fact that HPLC analyses showed differences in the phenolic content of the extracts.
Antimicrobial Effect of Vine Leaf Extracts
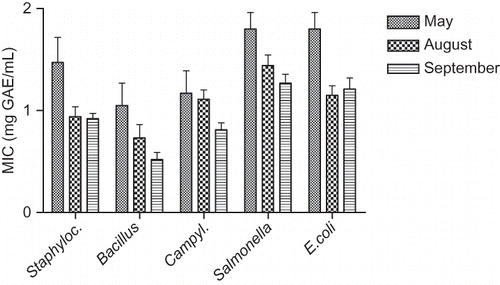
Although grape leaves have been confirmed as rich sources of phenolic compounds, both flavonoids and non-flavonoids, few reports on the antimicrobial activity of grape LEs or a comparison with the efficiency of extracts from other by-products of grape processing are available.[Citation29,Citation30] The antimicrobial activity of all 18 leaf phenolic extracts was screened by the broth microdilution test using gram-positive (Stapylococcus aureus, Bacillus cereus) and gram-negative bacteria (Campylobacter jejuni, Escherichia coli O157:H7, and Salmonella Infantis). Activity was confirmed against all the tested organisms. MICs were expressed in mg of GAE/mL of phenolic compounds per mL of microbiological growth medium and ranged between 0.38–2.24 mg GAE/mL for different organisms and extracts (), which is somewhat higher in comparison with the MIC values of grape skin extracts.[Citation13] Differences in the efficiency of phenolic LEs from six grapevine varieties were observed against different testing organisms, but these differences were not significant in all phenophases. It was not possible to select a specific grape vine variety as a preferable source of antimicrobial compounds. In addition, there was no great difference in activity against gram-positive and gram-negative bacteria, which is usually reported for plant phenolic extracts,[Citation23] but we found a similar situation in the case of grape skin extracts.[Citation13] The sequence of microorganisms according to their sensitivity to LEs, expressed as average MICs (in mg GAE/mL, N = 18) was: Bacillus (0.77 ± 0.34) > Campylobacter (1.03 ± 0.29) > Staphylococcus (1.11 ± 0.36) > Escherichia (1.39 ± 0.36) > Salmonella (1.50 ± 0.30) (). On average, lower MICs were confirmed in phenolic mixtures from August and September LEs, compared to May LEs, for all tested species (, ). This is correlated with the differences found in chemical composition of LEs prepared from leaves collected in August and September that were richer not only in flavonols but also in stilbenes, which are known as good phytoalexins. The very good antioxidant properties and antimicrobial activity of vine leaves, especially September LEs, is very important from the economic point of view regarding the availability of plant material for production of added-value antimicrobial and antioxidant phenolic extracts after grape harvest.
Table 3 Antimicrobial activity of phenolic extracts from Vitis vinifera L. leaves collected during different phenophases. Antimicrobial activity is expressed as minimal inhibitory concentrations (MICs) of total phenols, i.e., GAE* per mL of growth medium in broth microdilution test
Figure 3 Comparison of the in vitro antimicrobial activity of phenolic extracts from Vitis vinifera L. leaves collected during three phenophases, against gram-positive (Staphylococcus aureus, Bacillus cereus) and gram-negative (Campylobacter jejuni, Escherichia coli O157:H7, Salmonella) bacteria. The results are mean ±SD for LEs of six grape varieties.
CONCLUSIONS
The results of this study indicate an interesting polyphenolic composition of Vitis vinifera L. LEs. The high contents of flavan-3-ols and flavonols, especially of quercetin and its derivatives, as well as the presence of compounds from the resveratrol family, make grape-vine leaves a promising, inexpensive source of biologically active polyphenolic mixtures. The high polyphenolic potential and significant antioxidant and antimicrobial activities of vine LEs, especially of leaves collected after verasion (August LEs) and at the end of grape ripening (September LEs), offer possibilities for their application in preventing oxidative deterioration and/or microbial spoilage of food products. The leaves that remain on the vine after the grape harvest (September LEs) could be especially interesting for further exploitation because leaf picking does not interfere with grape ripening or the grape harvest.
ACKNOWLEDGMENTS
The authors would like to thank the Ministry of Science, Education and Sports of the Republic of Croatia and the Ministry of Education, Science and Sport of the Republic of Slovenia for financing the national (011-2160547-2226) and bilateral (BI-CRO-SLO-044-09/10) projects. This work was partly supported by the European Union as part of the Integrated Project BIOTRACER (contract 036272) in the sixth RTD framework. The authors are most grateful to Ana Teskera for her assistance in collecting the plant material.
REFERENCES
- Moure , A. , Cruz , J.M. , Franco , D. , Dominguez , J.M. , Sineiro , J. , Dominguez , H. , Nunez , M.J. and Parajo , J.C. 2001 . Natural antioxidants from residual sources . Food Chemistry , 72 : 145 – 171 .
- Holley , R.A. and Patel , D. 2005 . Improvement in shelf-life and safety of perishable foods by essential oils and smoke antimicrobials . Food Microbiology , 22 : 273 – 292 .
- Balasundram , N. , Sundram , K. and Samman , S. 2006 . Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses . Food Chemistry , 99 : 191 – 203 .
- Albayrak , S. , Aksoy , A. , Sagdic , O. and Hamzaoglu , E. 2010 . Compositions, antioxidant and antimicrobial activities of Helychrysum (Asteraceae) species collected from Turkey . Food Chemistry , 119 : 114 – 122 .
- Tohma , H.S. and Gulç , I. 13 . Antioxidant and radical scavenging activity of aerial parts and roots of Turkish liquorice (Glycyrrhiza Glabra L.) . International Journal of Food Properties , 2010 : 657 – 671 .
- Marušić , R. 1990 . Ljekovitim Biljem do Zdravlja (Through Herbal Medicine to Health , 358 Zagreb , , Croatia : Mladost .
- Lardos , A. and Kreuter , M.H. 2000 . “ Red Vine Leaf ” . In Intercity Pharmaceuticals and Extracts Edited by: Kreuter , M.H. and Flachsmann , A.G. 1 – 7 . Zurich , , Switzerland
- Orhan , D.D. , Orhan , N. , Ozcelik , B. and Ergun , F. 2009 . Biological activities of Vitis vinifera L. leaves . Turkish Journal of Biology , 33 : 341 – 348 .
- Dani , C. , Oliboni , L.S. , Agostini , F. , Funchal , C. , Serafini , L. , Henriques , J.A. and Salvador , M. 2009 . Phenolic content of grapevine leaves (Vitis labrusca var. Bordo) and its neuroprotective effect against peroxide damage . Toxicology In Vitro , 24 : 148 – 153 .
- Kosar , M. , Küpeli , E. , Malyer , H. , Uylaser , V. , Türkben , C. and Can Baser , K.H. 2007 . Effect of brining on biological activity of leaves of Vitis vinifera L. (Cv Sultani cekirdeksiz) from Turkey . Journal of Agricultural and Food Chemistry , 55 : 4596 – 4603 .
- Frankel , E.N. , Waterhouse , A.L. and Teissedre , P.L. 1995 . Principal phenolic phytochemicals in selected California wines and their antioxidant activity in inhibiting oxidation of human low-density lipoproteins . Journal of Agricultural and Food Chemistry , 43 : 890 – 894 .
- Balik , J. , Kyselakova , M. , Vrchotova , N. , Triska , J. , Kumšta , M. , Veverka , J. , Hic , P. , Totušek , J. and Lefnerova , D. 2008 . Relations between polyphenols content and antioxidant activity in vine grapes and leaves . Czech Journal of Food Sciences , 26 : S24 – S32 .
- Katalinić , V. , Smole Možina , S. , Skroza , D. , Generalić , I. , Abramovič , H. , Miloš , M. , Ljubenkov , I. , Piskernik , S. , Pezo , I. , Terpinc , P. and Boban , M. 2010 . Polyphenolic profile, antioxidant properties and antimicrobial activity of grape skin extracts of 14 Vitis vinifera varieties grown in Dalmatia (Croatia) . Food Chemistry , 119 : 715 – 723 .
- Renaud , S. and Lorgeril , M. 1992 . Wine, alcohol, platelets and French paradox for coronary hearth . Lancet , 339 : 1523 – 1526 .
- Monagas , M. , Garrido , I. , Bartolome , B. and Gomez-Cordoves , C. 2006 . Chemical characterization of commercial dietary ingredients from Vitis vinifera L . Analitica Chimica Acta , 563 : 401 – 410 .
- Burjonroppa , S. and Fujise , K. 2006 . “ Resveratrol as cardioprotective agent: Evidence from bench and bedside ” . In Resveratrol in Health and Disease , Edited by: Aggarwal , B.B. and Shishodia , S. 539 – 556 . London , , England : CRC Press .
- Hakkinen , S. 2000 . Flavonols and phenolic acids in berries and berry products. Doctoral dissertation , 17 – 21 . Finland : Kuopio University Publications D. Medical Sciences 221 .
- Singleton , V.L and Rossi , J. 1965 . Colorimetry of total phenolics with phospho-molybdic-phosphotungstic acid reagents . American Journal of Enology and Viticulture , 16 : 144 – 158 .
- Kramling , T.A. and Singleton , V.L. 1969 . An estimate of the nonflavonoid phenols in wines . American Journal of Enology and Viticulture , 20 : 86 – 92 .
- Arnous , A. , Makris , D. and Kefalas , D. 2001 . Effect of principal polyphenolic components in relation to antioxidant characteristics of aged red wines . Journal of Agricultural and Food Chemistry , 4 : 5736 – 5742 .
- Szabo , M.R. , Radu , D. , Gavrilas , S. , Chambre , D. and Iditoiu , C. 2010 . Antioxidant and antimicrobial properties of selected spice extracts . International Journal of Food Properties , 13 : 535 – 545 .
- Benzie , I.F.F. and Strain , J.J. 1996 . The ferric reducing ability of plasma (FRAP) as measurement of “antioxidant power”: The FRAP assay . Analytical Biochemistry , 239 : 70 – 76 .
- Klančnik , A. , Piskernik , S. , Jeršek , B. and Smole Možina , S. 2009 . Evaluation of diffusion and dilution methods to determine the antimicrobial activity of plant extracts . Journal of Microbiological Methods , 81 : 121 – 186 .
- Klančnik , A. , Guzej , B. , Hadolin-Kolar , M. , Abramovič , H. and Smole Možina , S. 2009 . In vitro antimicrobial and antioxidant activity of commercial rosemary extract formulations . Journal of Food Protection , 72 : 1744 – 1752 .
- Bogs , J. , Downey , M.O. , Harvey , J.S. , Ashton , A.R. and Tanner , G.J. 2005 . Proanthocyanidin synthesis and expression of genes encoding leucoanthocyanidin reductase and anthocyanidin reductase in developing grape berries and grapevine leaves . Plant Physiology , 139 : 652 – 663 .
- Park , H.J. and Cha , H.C. 2003 . Flavonoids from leaves and exocarps of Grape Kyoho . Korean Journal of Biological Sciences , 7 : 327 – 330 .
- Prior , L.R. , Wu , X. and Schaich , K. 2005 . Standardized methods for determination of antioxidant capacity and phenolics in foods and dietary supplements . Journal of Agricultural and Food Chemistry , 53 : 4290 – 4302 .
- Çelik , S.E. , Özyürek , M. , Altun , M. , Bektasoglu , B. , Güçlü , K. , Berker , K.I. , Özgökçe , F. and R , Apak' . 2008 . Antioxidant capacities of herbal plants used in the manufacture of Van Herby cheese: ‘Otlu Peynir’ . International Journal of Food Properties , 11 : 747 – 761 .
- Baydar , N.G. , Özkan , G. and Sagdiç , O. 2004 . Total phenolic contents and antibacterial activities of grape (Vitis vinifera L.) extracts . Food Control , 15 : 335 – 339 .
- Luther , M. , Parry , J. , Moore , J. , Meng , J. , Zhang , Y. , Cheng , Z. and Yu , L. 2007 . Inhibitory effect of Chardonnay and black raspberry seed extracts on lipid oxidation in fish oil and their radical scavenging and antimicrobial properties . Food Chemistry , 104 : 1065 – 1073 .