Abstract
The electron paramagnetic resonance tests carried out indicated that both the technique and the conditions of sterilization of dried white mulberry leaves were chosen correctly, as no significant differences were found between the concentration and the properties of free radicals in the analyzed samples. The high temperature short time method steam sterilization process, the temperature, as well as the time of exposure to it during the preparation of water extracts resulted in no significant change in the content of polyphenol compounds in the samples analyzed. However, the total antioxidant activity increased, which suggested that despite technological processing mulberry leaves do not lose their antioxidative properties.
INTRODUCTION
White mulberry (Morus alba L.) leaves have found medical application in Far East medicine for centuries. They contain many nutritive substances, such as proteins, fats, carbohydrates, cellulose, mineral salts, and vitamins.Citation[1] Mulberry leaves and fruits are particularly rich in antioxidative polyphenol compounds, such as phenolic acids and flavonoids.Citation[2] Among them, the most important are chlorogenic acid, quercetin, quercetin 3-(6-malonylglucoside), rutin, isoquercitrin, kaempferol, astragalin, scopolin, skimmin, roseoside II, benzyl D-glucopyranoside,Citation[3,Citation4] kuwanons,Citation[5] moracins,Citation[6] and many other, newly discovered compounds.Citation[7] Among the biologically active constituents of mulberry leaves there are also compounds displaying counter-diabetic properties, such as 1-deoxynojirimycin.Citation[8]
Many scientific reports indicate that the chemical substances contained in mulberry leaves contribute to the prevention and treatment of civilization diseases, such as diseases of the circulatory system,Citation[9,Citation10] diabetes,Citation[11,Citation12] obesity,Citation[13,Citation14] diseases of the nervous system (Alzheimer's),Citation[15] and cancer.Citation[6] It has been proven that mulberry leaves have antibacterial propertiesCitation[16] and, by inhibiting the synthesis of melanin, they may be used for removing skin hyperpigmentation.Citation[17]
Although the health benefits of mulberry leaves are quite well known, it is important to study the influence processing—drying, sterilization, and the technique of extract preparation—has on the content of bioactive constituents. With mulberry leaves, just as with all herbal raw material, there is a threat of microbiological contamination presenting a serious danger to health. The contamination may be primary (contamination with soil, dust, bird excrement, fertilizers) or secondary (incorrect harvesting, drying, storing, packaging, and transportation of the plants).Citation[18,Citation19]
Over the years, various methods have been used to remove microorganisms from food products. The first method—sterilization with ethylene oxide gas—is nowadays forbidden in European countries.Citation[20] Radiation is still in use as a method of sterilization.Citation[21,Citation22] As the influence of radiation on sterilized products is not fully known, the method is becoming less and less popular in many countries, because of the pressure of consumer organizations. When using radiation in the sterilization process, information about it must be put on the product packaging. The most popular method used in the herb and spice processing industry, which allows for the sterilization of raw materials, both whole and in the ground form, involves steaming.Citation[23] Modern steam sterilization methods are, first of all, ecological and ensure microbiological purity. One such method is NMC® (Natural Microbiological Clear) steam sterilization. It is based on high temperature short time (HTST) principle, and involves the use of a special Nauta® mixer (Hosokawa Micron B.V., The Netherlands). Sterilization is performed using saturated steam, brought in under 10 bar pressure at the temperature of 110–140°C, within a relatively short time (30–180 s). The product is being constantly stirred while sterilized. Steam vapor, together with essential oils, pass through the condenser, where oils liquefy and are introduced back into the cooled product. Owing to this, the loss of essential oils is marginal. The humidity of the products obtained does not exceed 2–10%.
As the composition and the quality of raw material exposed to high temperatures may change, the aim of this work was to assess the influence of steam sterilization on the emergence of free radicals in dried mulberry leaves, as well as on the antioxidative activity and the content of polyphenols in water extracts of mulberry leaves.
MATERIALS AND METHODS
Materials
The objects of research were unsterilized dried white mulberry (Morus alba L.) leaves as well as mulberry leaves sterilized by steaming. The material for the tests was supplied by K.P.P.S. Interjarek Company (Gołuchów, Poland), which sterilizes herbs and spices using the NMC® steam sterilization method.
Sample Preparation and Conditions of Electron Paramagnetic Resonance (EPR) Spectra Measurements
Samples of white mulberry leaves in their initial state, as well as samples of the powdered sterilized leaves, were placed in thin-walled glass tubes, measuring 3 mm in the outer diameter. The weight of the samples contained in the test tubes was measured. Empty tubes gave no EPR signal while the maximum line amplification was used at the maximum microwave power (70 mW). EPR spectra of the samples tested were registered in room temperature, using electron spectrometer of paramagnetic resonance in X frequency band (9.3 GHz) with the magnetic field modulation of 100 kHz, by RADIOPAN Company (Poznań, Poland). The frequency of microwave radiation was registered by means of a MCM 101 meter produced by EPRAD Company (Poznań, Poland).
The samples in the test tubes were placed between electromagnetic poles and EPR spectra were registered by computer in the form of the first absorption derivative. The JAGMAR Company (Kraków, Poland) numerical data acquisition was used, together with the lab View program and spectroscopic analysis software. EPR spectra were measured using microwave power within the range of 2.2–70 mW. The power of microwave radiation used during the measurement of EPR spectra was calculated by means of the pattern determining the attenuation of the circuit in decibels:Citation[24]
where Mo
is total microwave power produced by klystron (70 mW) and M is microwave power used during the EPR spectrum measurement. The maximum suppression used was 15 dB.
EPR Parameters Analyzed
The following parameters of EPR spectra were measured: amplitude (A [a.u.]), line width (ΔBpp
[mT]), integral intensity (I [a.u.]) and g factor. The integral intensity of EPR line registered as the first absorption derivative was measured by double integration of the area under the resonance curve. The integral intensity of EPR line was used for calculating the concentration of free radicals in the sample. The value of the g factor provides information on the kind of atom on which the unpaired electron is located. The apparent g value was calculated directly from the resonance condition as follows.Citation[24] The apparent g value was measured directly based on the resonance condition as:Citation[24]
where h is Planck constant (J × s), ν is microwave frequency (GHz), μ
B
is Bohr magneton (J × T
−1), and Br
is resonance magnetic field (T). Microwave frequency ν was measured and magnetic induction Br
was determined from the experimental spectra. The g factor in the study was measured to ±0.0002 tolerance. The concentration of free radicals in the samples was measured according to the pattern:
where nu
is the number of paramagnetic center in ultramarine (the references) (nu
= 1.2 × 1019 spin), W and Wu
are the receiver gains for sample and ultramarine, A and Au
are the amplitudes of ruby signal for the sample and ultramarine, I and Iu
are the integral intensities for the sample and ultramarine, and m is the mass of the sample.
Ultramarine (sodium aluminosilicate containing sulphur) was used as the reference of concentration, because the paramagnetic centers present in the substance are quite stable. In order to increase the accuracy of the measurement, the so-called ‘internal reference’ was used—a ruby crystal (Al2O2: Cr3+), introduced into the resonator by means of a goniometer, below the tested sample. The amplitude of the EPR line for the ruby was measured.
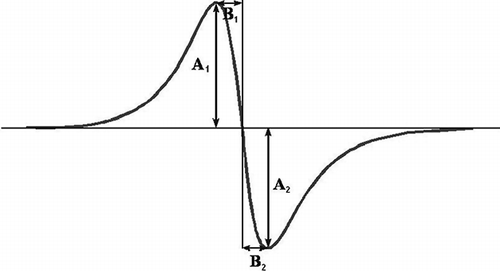
The following parameters of EPR spectrum shapes were analyzed: A 1 - A 2, |A 1 - A 2|, A 1/A 2, B 1 - B 2, |B 1 - B 2| as well as B 1/B 2. shows the values of A 1, A 2, B 1, and B 2. The shape parameters were measured for the EPR spectra in the power range between 2.2–70 mW. It was checked whether the shape of EPR lines for the samples tested changed together with the microwave power. The influence of microwave power on the amplitude (A) and the width (ΔBpp ) of EPR lines recorded for the samples was analyzed, in order to determine the character of spectra widening (homogenous and non-homogenous).
Preparation of Water Extracts
Home-made water extracts of plants are often used for medical purposes. Infusion, decoction, and cold and hot maceration are the most popular ways of preparing water extracts of medicinal herbs.
Infusion
A 0.5 g sample of the raw material was weighed and soaked in 5 cm3 of deionized boiling water. Then it was cooked, under a lid, for 15 min. The infusion was set aside for another 15 min and then filtered and filled up with water to achieve the stable capacity.
Decoction
A 0.5 g sample of the raw material was weighed and soaked in 5 cm3 of room temperature deionized water. It was cooked, under a lid, for 30 min. Then the decoction was filtered when still hot, and filled up with water to achieve the stable capacity.
Cold Macerate
A 0.5 g sample of the raw material was weighed and soaked in 5 cm3 of room temperature deionized water. It was stirred well and left for 30 min. Then the extract was filtered and filled up with water to achieve the stable capacity.
Hot Macerate
A 0.5 g sample of the raw material was weighed and soaked in 5 cm3of deionized boiling water, stirred well and left for 15 min. Then the extract was filtered and filled up with water to achieve the stable capacity.
Measuring Total Antioxidant Status and the Polyphenol Content
In the extracts obtained, using a diluted sample, the total antioxidant status (TAS) was measured, as well as the polyphenol content, using colorimetric techniques. UV/VIS Unicam spectrophotometer was used to perform the measurement. The measurement of the total antioxidant potential was performed using the TAS set (Randox Laboratories Ltd., UK). This method of TAS measurement consists of inhibiting the appearance of ABTS•+ cationic radical, proportionally to the concentration of oxidants contained in the sample. The total antioxidant activity was expressed as Trolox antioxidant activity— Trolox equivalent antioxidant capacity (TEAC). The technique of measuring polyphenolsCitation[25] is based on chromatic reaction involving Folina-Ciocalteau reagent. The total polyphenol concentration was expressed as gallic acid (GAE) equivalent.
RESULTS AND DISCUSSION
EPR Analysis
A sterilization process, removing microorganisms from stored herb products, is performed using steam, high temperature, and pressure. Herbs owe their medical properties mainly to the large content of antioxidant substances and essential oils. These latter ones are fatty acid esters, which in favorable conditions (humidity and high temperature) may easily undergo hydrolysis and oxidation processes involving the appearance of peroxide radicals. The EPR tests the authors have conducted showed, however, that HTST steam sterilization process does not produce a significant increase in the amount of free radicals. Free radicals in white mulberry had not been studied before. Free radical properties of Calami rhizoma were known from our previous study. In this study, the usefulness of EPR spectroscopy in the assessment of the herb sterilization process has been demonstrated.Citation[26]
Tests using elecron paramagnetic resonance (EPR) spectroscopy showed that both samples in the initial state and sterilized samples are paramagnetic. There were EPR spectra of white mulberry registered even using the small power of 2.2 mW. EPR spectra of white mulberry in the initial state (a) and of sterilized mulberry (b) are shown in . The parameters of EPR spectra and the concentrations of free radicals in the samples of white mulberry are shown in .
Table 1 Concentration (N) of free radicals and the parameters of EPR spectra recorded for white mulberry
Figure 2 EPR spectra of white mulberry registered using the microwave power of 2.2 mW (attenuation of 15 dB) on the sample of mulberry in the initial state (a) and sterilized (b).
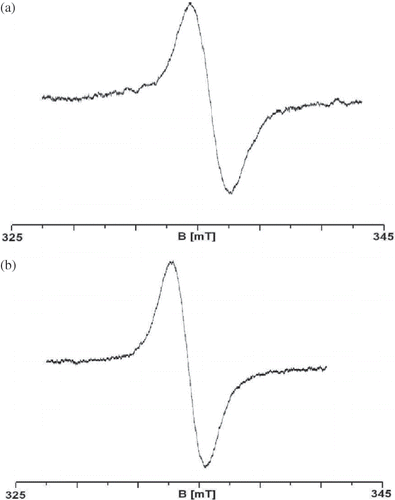
The amplitude (A) of the EPR line rises together with the increase in the amount of free radicals present in the sample and the width (ΔBpp ) of EPR lines depends on the magnetic interactions between the unpaired electrons in the sample. Dipole interactions widen the EPR lines and the exchange interactions may cause the lines to narrow down.Citation[24,Citation27] The amplitude (A) of EPR lines recorded for the sample of sterilized white mulberry is wider than the amplitude recorded for the sample of white mulberry in the initial state (). This means that weaker dipole interactions between free radicals take place in the samples of sterilized mulberry. EPR lines recorded for the samples of white mulberry in the initial state and the samples of sterilized white mulberry were characterized by large values of spectrum width. It may be said, therefore, that powerful dipole interactions of unpaired electrons in free radicals take place in the samples—consequently, the distances between the radicals must be short.
Free radicals were present both in the samples of white mulberry in the initial state and the samples of sterilized white mulberry and their concentration amounts to 1018 spin/g (). The concentrations of free radicals in the samples of white mulberry in the initial state and the samples of sterilized white mulberry were similar. The process of sterilization did not generate large amounts of free radicals in the sample.
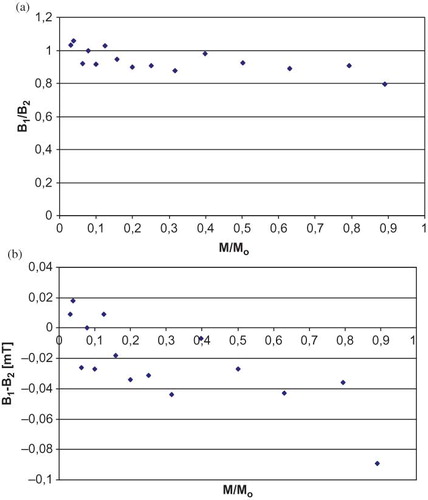
The shapes of EPR spectrum changed with the altering of microwave power, in the case of samples in which there were a few types of free radicals present.Citation[24,Citation27] All parameters of EPR spectrum shapes recorded for the white mulberry samples tested depended on the microwave power used. Similar correlations were observed both in the samples of white mulberry in the initial state and the samples of sterilized white mulberry ( and ). These correlations indicate that there are a few types of free radicals present in white mulberry.
Figure 3 Correlation of (a) A1/A2 and (b) A1-A2 parameters of sterilized white mulberry EPR spectra and the microwave power (M). M0 = 70 mW (maximum power). (Color figure available online.)
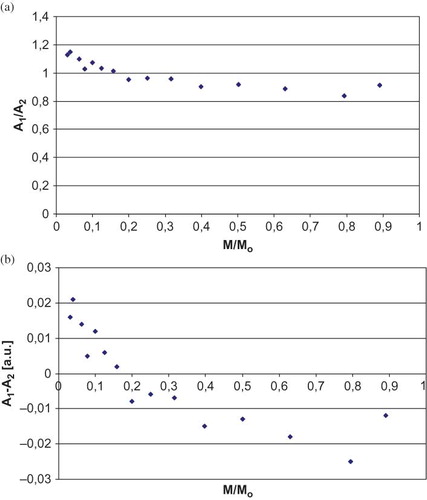
Figure 4 Correlation of (a) B1/B2and (b) B1-B2 parameters of sterilized white mulberry EPR spectra and the microwave power (M). M0 = 70 mW (maximum power). (Color figure available online.)
The values of g factor indicated that there were mainly free radicals with unpaired electrons located in the oxygen atoms (O) and carbon (C) present in white mulberry. The obtained low g values are characteristic for unpaired electrons in oxygen and carbon atoms with the low spin-orbit coupling.Citation[24] The authors propose that such type of paramagnetic centers exist in the examined herbs. EPR lines reflecting these radicals changed differently with the increase of microwave power, which resulted in the change of the shape of the resultant spectra.
The amplitude of EPR line homogenously broadened rose together with the increase of microwave power and, having reached the maximum value, it decreases. The width of the homogenously broadened line grew together with the increase of microwave power, whereas the amplitude and the width of unhomogenously broadened EPR lines, having reached the maximum value, did not change with further increase of microwave power.Citation[24] The influence of microwave power on the amplitude and the width of EPR lines recorded for the samples of sterilized white mulberry were presented, respectively, in and . Similar relationships were observed in the case of white mulberry in the initial state. The amplitude of EPR lines recorded for white mulberry rose with the increase of microwave power (). The relationships characterizing the amplitude and the width of EPR lines recorded for white mulberry were typical for homogenous distribution of free radicals in the sample. This result is particularly significant in the case of sterilized white mulberry, because it indicates that the sterilization process carried out had a uniform impact on the herb sample and spin islands did not appear in it. This means that the sterilization of white mulberry was carried out correctly.
Figure 5 The influence of the microwave power (M) on the amplitude (A) of sterilized white mulberry EPR lines. M0 = 70 mW (maximum power). (Color figure available online.)
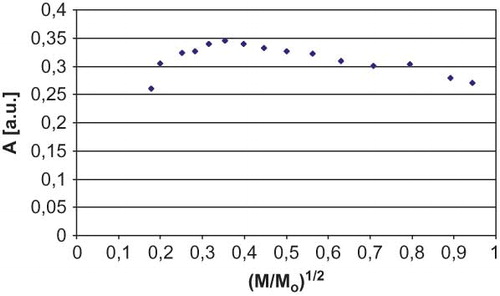
Figure 6 The influence of the microwave power (M) on the width (ΔBpp) of sterilized white mulberry EPR lines. M0 = 70 mW (maximum power). (Color figure available online.)
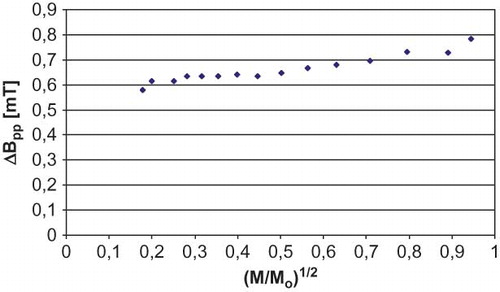
Constant microwave saturation of EPR lines, and the observed changes in the amplitude of EPR lines taking place together with the increase in microwave power, provide information on the speed of spin-lattice relaxation processes in the sample. In the case of slow spin-lattice relaxation processes, EPR lines are saturated at low microwave power, while in fast spin-lattice relaxation processes EPR lines are saturated at high microwave power.Citation[24] White mulberry EPR lines were saturated at low microwave power (), which indicates that slow spin-lattice relaxation processes took place in the samples. Saturation of EPR lines resulted from the inversion of the electron location in the energetic levels of the system.
Antioxidant Activity and Polyphenol Content
In recent years, the demand for herbal infusions and pharmacological preparations made of mulberry leaves has been increasingly widespread. Also, dried and powdered mulberry leaves are taken, among others, in the form of water extracts. The manner in which herbs are infused influences the content of antioxidant compounds present in them. This is connected with the length of the process and the temperature at which it takes place. There are many empirical studies concerning the antioxidant activity of white mulberry leaves, but the majority of them are concerned with the extraction of the active compounds using organic solvents.Citation[28]
In the research that was carried out, the authors found that antioxidant activity was higher in sterilized mulberry extracts than in extracts of unsterilized white mulberry. This relationship characterizes all water extracts prepared hot (infusions, decoctions, hot macerates; ). Changes in the activity of herbs that had undergone sterilization depended on the manner of extraction and diminished in the following order: infusion > decoction > hot macerate > macerate.
Table 2 Average antioxidant activity of white mulberry extracts expressed in TEAC*
The influence of temperature on the antioxidant activity of mulberry leaves is not unambiguous and depends, among others, on its value and the time of exposure to it.Citation[29] The increase in the antioxidant activity produced by the high temperature the authors observed may be caused by many factors. As a result of heating, increased pressure or enzymatic hydrolysis, severing of cellular walls takes place and with that comes greater bioavailability of antioxidant substances.Citation[30,Citation31] Moreover, in the course of thermal processing, new antioxidant substances may be created.Citation[32] The most significant is, however, that thermal processing of plant products, which is, on the one hand, detrimental as it causes scouring away of various constituents, on the other hand, inactivates enzymes responsible for enzymatic oxidation of natural antioxidants. Inactivation of these enzymes is, therefore, conducive for retaining antioxidant activity.Citation[33] The examples here are fruit and vegetables exposed to, for example, blanching, which retain their antioxidant activity to a much greater extent than raw materials which have not been blanched.Citation[34]
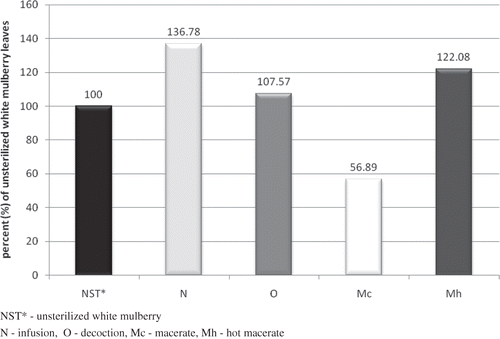
The results of the research ascertained also the opposite relationship: antioxidant activity was higher in the samples of unsterilized mulberry than in the extracts prepared cool (macerates). The antioxidant activity of unsterilized mulberry macerate (13.621 ± 0.162 mmol Trolox/l) was the highest in value of all the measured samples. At the same time, the macerate of sterilized mulberry displayed the lowest antioxidant activity (7.749 ± 0.078 mmol Trolox/l) of all the measured samples (). The antioxidant activity of unsterilized mulberry extracts diminished in the following order: macerate > decoction > infusion > hot macerate. The diminishing of the antioxidant activity of white mulberry as a result of steam sterilization was about 56.89% (). The remaining mulberry extracts showed an increase in the antioxidant activity in the following sequence: decoction (7.57%) < hot macerate (22.08%) < infusion (36.78%).
Figure 7 Changes in antioxidant activity of white mulberry extracts tested, as a result of steam sterilization.
Comparing the values of the antioxidant activity of sterilized and unsterilized mulberry leaf water extracts prepared cold (macerates), allows for drawing conclusions concerning the influence of steam sterilization process on antioxidant activity. Probably, at high temperatures, decomposition of antioxidant substances takes place, for example, of the ascorbic acid, which is present in large amounts (100–200 mg/100 g) in white mulberry leaves.Citation[1]
The ascorbic acid relatively easily undergoes oxidation when exposed to the activity of enzymes or oxygen. It also undergoes thermal decomposition during processes, such as blanching, cooking, pasteurization, sterilization, and drying.Citation[35] However, as the study by Munyaka et al. demonstrated, blanching using high temperature short time (HTST) technique was the best way of preserving vitamin C while processing broccoli.Citation[36] In all the water extracts tested, statistically significant differences (p < 0.05) were found between sterilized and unsterilized herbs.
The highest concentrations of polyphenols in the extracts of unsterilized mulberry were found in the water extracts prepared hot: the decoction (2.328 ± 0.0272 mg GAE/ml) and the infusion (2.121 ± 0.0129 mg GAE/ml) (). It was lower in the extracts prepared by means of room temperature water maceration (1.627 ± 0.0057 mg GAE/ml) and boiling water maceration (1.882 ± 0.0153 mg GAE/ml). A similar relationship was observed in sterilized mulberry extracts. The lowest concentration was measured for room temperature water maceration (1.609 ± 0.0044 mg GAE/ml); it was higher in the extracts prepared hot: infusion, decoction, and hot macerate. Apart from the macerate, in all cases the steam sterilization process performed caused a slight increase in the concentration of polyphenols. presents the changes in polyphenol concentrations caused by steam sterilization in percentages. Only in the case of the macerate, the concentration of polyphenols slightly decreased to the amount of 98.89%. In the remaining cases, the sterilization process caused an increase in the concentration of polyphenols up to the following amounts: 107.73% in the decoction, 114.88% in the hot macerate, and—the largest increase observed—127.96% in the macerate.
Figure 8 Changes in the content of polyphenols in white mulberry extracts tested as a result of steam sterilization.
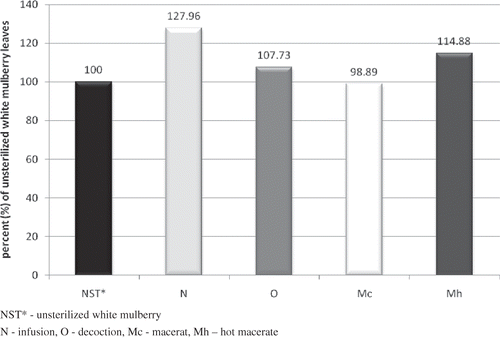
Table 3 Average total polyphenol concentration in mulberry leaf extracts, expressed in GAE/ml
Lowering in the total concentration of polyphenols in sterilized mulberry macerates prepared with the use of room temperature water may be caused by thermal decomposition of polyphenol compounds exposed to temperature or moisture. The polyphenols, which are predominantly present in white mulberry, are flavonols, and among them, glycosides of quercetine. According to some reports, high temperature processes, especially short ones, do not cause significant flavonol loss.Citation[37] The significant factor influencing the polyphenol content is not only temperature as such, but also the manner in which it influences the sample. Heating plant products in water causes fast penetration of warmth into the tissues, which prolongs the exposition of the whole volume of the processed product to this factor and results in the high loss of antioxidants. However, while being heated in the air the inside of the product retains lower temperature than the surface of it. This lowers the loss of antioxidants, including polyphenols.Citation[38,Citation39]
The increase in polyphenol concentration in the sterilized mulberry extracts prepared hot may be explained by the inactivation process of enzymes mentioned earlier, causing decomposition of polyphenol compounds and taking place during a short sterilization process. The increase in the concentration of polyphenols in the extracts of sterilized mulberry may be also related to the high pressure used during the sterilization process, as it was demonstrated in the study by Prasad et al.Citation[40] All the average polyphenol concentrations measured in each of the extracts of sterilized and unsterilized mulberry demonstrated significant statistic differences (p < 0.05). Also, the differences between the parameters measured for each particular extraction technique were statistically significant (p < 0.05).
Dependence of Antioxidant Activity on Polyphenol Concentration
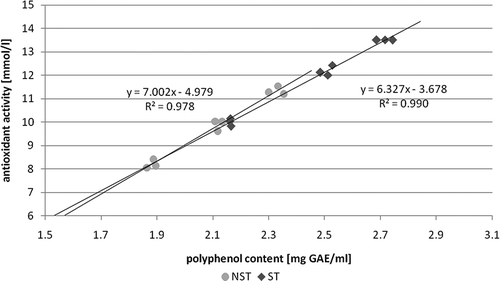
Trying to establish the correlation between antioxidant activity and the polyphenol concentration in the decoctions, infusions, macerates, and hot macerates of sterilized mulberry, the authors found a linear correlation of R 2 = 0.9905, while in the case of unsterilized mulberry the correlation was R 2 = 0.9785 (). The correlation between the content of phenol compounds and antioxidant activity in various extracts of mulberry leaves was observed by Arabshahi-Delouee and Urooj.Citation[28]Similar correlations were demonstrated in various plant extracts,Citation[41] fruits,Citation[42] and cereals.Citation[43]
CONCLUSION
Based on the EPR spectroscopic testing it may be stated that the methods and the conditions of white mulberry leaves sterilization were chosen correctly, as no significant changes were found in the concentration and properties of free radicals in the samples analyzed. The HTST steam sterilization process—the temperature and the time of exposure to the temperature—used during the preparation of water extracts only slightly influenced the content of polyphenol compounds in the samples analyzed. Moreover, the total antioxidant activity increases, which may indicate that despite high temperature technological processes performed, white mulberry leaves do not lose their antioxidant properties.
REFERENCES
- Butt, M.S. , Nazir, A. , Sultan, T. , and Schroën, K. , 2008. Morus alba L. nature's functional tonic , Trends in Food Science and Technology 19 (10) (2008), pp. 505–512.
- Gungor, N. , and Sengul, M. , 2008. Antioxidant activity, total phenolic content and selected physicochemical properties of white mulberry (Morus alba L.) fruits , International Journal of Food Properties 11 (1) (2008), pp. 44–52.
- Doi, K. , Kojima, T. , Makino, M. , Kimura, Y. , and Fujimoto, Y. , 2001. Studies on the constituents of the leaves of Morus alba L , Chemical and Pharmaceutical Bulletin 49 (2) (2001), pp. 151–153.
- Kim, S.Y. , Gao, J.J. , Lee, W.C. , Ryu, K.S. , Lee, K.R. , and Kim, Y.C. , 1999. Antioxidative flavonoids from the leaves of Morus alba , Archives of Pharmacal Research 22 (1) (1999), pp. 81–85.
- Nomura, T. , Hano, Y. , and Fukai, T. , 2009. Chemistry and biosynthesis of isoprenylated flavonoids from Japanese mulberry tree , Proceedings of the Japan Academy–Series B: Physical and Biological Sciences 85 (9) (2009), pp. 391–408.
- Yang, Y. , Gong, T. , Liu, C. , and Chen, R.Y. , 2010. Four new 2-arylbenzofuran derivatives from leaves of Morus alba L , Chemical and Pharmaceutical Bulletin 58 (2) (2010), pp. 257–260.
- Yang, Y. , Zhang, T. , Xiao, L. , and Chen, R.Y. , 2010. Two novel flavanes from the leaves of Morus alba L , Journal of Asian Natural Products Research 12 (3) (2010), pp. 194–198, .
- Kimura, T. , Nakagawa, K. , Kubota, H. , Kojima, Y. , Goto, Y. , Yamagishi, K. , Oita, S. , Oikawa, S. , and Miyazawa, T. , 2007. Food-grade mulberry powder enriched with 1-deoxynojirimycin suppresses the elevation of postprandial blood glucose in humans , Journal of Agricultural and Food Chemistry 55 (14) (2007), pp. 5869–5874.
- Enkhmaa, B. , Shiwaku, K. , Katsube, T. , Kitajima, K. , Anuurad, E. , Yamasaki, M. , and Yamane, Y. , 2005. Mulberry (Morus alba L.) leaves and their major flavonol quercetin 3-(6-malonylglucoside) attenuate atherosclerotic lesion development in LDL receptor-deficient mice , The Journal of Nutition 135 (4) (2005), pp. 729–734.
- Liu, L.K. , Chou, F.P. , Chen, Y.C. , Chyau, C.C. , Ho, H.H. , and Wang, C.J. , 2009. Effects of mulberry (Morus alba L.) extracts on lipid homeostasis in vitro and in vivo , Journal of Agricultural and Food Chemistry 57 (16) (2009), pp. 7605–7611.
- Nakamura, M. , Nakamura, S. , and Oku, T. , 2009. Suppressive response of confections containing the extractive from leaves of Morus alba on postprandial blood glucose and insulin in healthy human subjects , Nutrition and Metabolism 14 (6) (2009), p. 29.
- Mudra, M. , Ercan-Fang, N. , Zhong, L. , Furne, J. , and Levitt, M. , 2007. Influence of mulberry leaf extract on the blood glucose and breath hydrogen response to ingestion of 75 g sucrose by type 2 diabetic and control subjects , Diabetes Care 30 (5) (2007), pp. 1272–1274.
- Lee, J. , Chae, K. , Ha, J. , Park, B.Y. , Lee, H.S. , Jeong, S. , Kim, M.Y. , and Yoon, M. , 2008. Regulation of obesity and lipid disorders by herbal extracts from Morus alba, Melissa officinalis and Artemisia capillaris in high-fat diet-induced obese mice , Journal of Ethnopharmacology 115 (2) (2008), pp. 263–270.
- Oh, K.S. , Ryu, S.Y. , Lee, S. , Seo, H.W. , Oh, B.K. , Kim, Y.S. , and Lee, B.H. , 2009. Melanin-concentrating hormone-1 receptor antagonism and anti-obesity effects of ethanolic extract from Morus alba leaves in diet-induced obese mice , Journal of Ethnopharmacology 122 (2) (2009), pp. 216–220.
- Niidome, T. , Takahashi, K. , Goto, Y. , Goh, S. , Tanaka, N. , Kamei, K. , Ichida, M. , Hara, S. , Akaike, A. , Kihara, T. , and Sugimoto, H. , 2007. Mulberry leaf extract prevents amyloid beta-peptide fibril formation and neurotoxicity , NeuroReport 18 (8) (2007), pp. 813–816.
- Park, K. , You, J. , Lee, H. , Baek, N. , Hwang, J. , and Kuwanon, G , 2003. an antibacterial agent from the root bark of Morus alba against oral pathogens , Journal of Ethnopharmacology 84 (2–3) (2003), pp. 181–185.
- Zhang, X. , Hu, X. , Hou, A. , and Wang, H. , 2009. Inhibitory effect of 2,4,2’,4’-tetrahydroxy-3-(3-methyl-2-butenyl)-chalcone on tyrosinase activity and melanin biosynthesis , Biological and Pharmaceutical Bulletin 32 (1) (2009), pp. 86–90.
- Kneifel, W. , Czech, E. , and Kopp, B. , 2002. Microbial contamination of medicinal plants–A review , Planta Medica 68 (1) (2002), pp. 5–15.
- Schweiggert, U. , Reinhold, C. , and Schieber, A. , 2007. Conventional and alternative processes for spice production–A review , Trends in Food Science and Technology 18 (5) (2007), pp. 260–268.
- Tateo, F. , and Bononi, M. , 2006. Determination of ethylene chlorohydrin as marker of spice fumigation with ethylene oxide , Journal of Food Composition and Analysis 19 (1) (2006), pp. 83–87.
- Farkas, J. , 2006. Irradiation for better food , Trends in Food Science and Technology 17 (4) (2006), pp. 148–152.
- Polovka, M. , and Suhaj, M. , 2010. The effect of irradiation and heat treatment on composition and antioxidant properties of culinary herbsand spices–A Review , Food Reviews International 26 (2) (2010), pp. 138–161.
- Almela, L. , Nieto-Sandoval, J.M. , and Fernández-López, J.A. , 2002. Microbial inactivation of paprika by a high-temperature short-X time treatment. Influence on color properties , Journal of Agricultural and Food Chemistry 50 (6) (2002), pp. 1435–1440.
- Weil, J. , Bolton, J.R. , and Wertz, J.E. , 1994. Electron Paramagnetic Resonance: Elementary Theory and Practical Applications . New York: John Wiley and Sons, Inc; 1994.
- Singleton, V.L. , and Rossi, J.A. , 1965. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents , American Journal of Enology and Viticulture 16 (1965), pp. 144–158, .
- Pawłowska-Góral, K. , Kurzeja, E. , Pilawa, B. , and Ramos, P. , 2009. EPR studies of Rhizoma Calami , Engineering of Biomaterials 12 (89–91) (2009), pp. 159–161.
- 1998. Eaton, G.R. , Eaton, S.S. , and Salikhov, K.M. , eds. Foundations of Modern EPR . Singapore: World Scientific; 1998.
- Arabshahi-Delouee, S. , and Urooj, A. , 2007. Antioxidant properties of various solvent extracts of mulberry (Morus indica L.) , Food Chemistry 102 (4) (2007), pp. 1233–1240.
- Katsube, T. , Tsurunaga, Y. , Sugiyama, M. , Furuno, T. , and Yamasaki, Y. , 2009. Effect of air-drying temperature on antioxidant capacity and stability of polyphenolic compounds in mulberry (Morus alba L.) , Food Chemistry 113 (4) (2009), pp. 964–969.
- McInerney, J.K. , Seccafien, C.A. , Stewart, C.M. , and Bird, A.R. , 2007. Effects of high pressure processing on antioxidant activity, and total carotenoid content and availability, in vegetables , Innovative Food Science and Emerging Technologies 8 (4) (2007), pp. 543–548.
- Kurzeja, E. , Stec, M. , Pawłowska-Góral, K. , Dudek, M. , and Wardas, M. , 2009. Wpływ obróbki termicznej na właściwości antyoksydacyjne soków z wybranych odmian pomidora , Bromatologia i Chemia Toksykologiczna 42 (3) (2009), pp. 861–864.
- Jeong, S.M. , Kim, S.Y. , Kim, D.H. , Jo, S.C. , Nam, K.C. , Ahn, D.U. , and Lee, S.C. , 2004. Effect of heat treatment on the antioxidant activity of extracts from citrus peels , Journal of Agricultural and Food Chemistry 52 (11) (2004), pp. 3389–3393.
- Gonçves, E.M , Pinheiro, J. , Abreu, M. , Brandão, T.R.S. , and Silva, C.L.M. , 2010. Carrot (Daucus carota L.) peroxidase inactivation, phenolic content and physical changes kinetics due to blanching , Journal of Food Engineering 97 (4) (2010), pp. 574–581.
- Patras, A. , Tiwari, B.K. , and Brunton, N.P. , 2010. Influence of blanching and low temperature preservation strategies on antioxidant activity and phytochemical content of carrots, green beans and broccoli , Food Science and Technology 43 (9) (2010), pp. 1313–1319.
- Jiratanan, T. , and Liu, R.H. , 2004. Antioxidant activity of processed table beets (Beta vulgaris var. conditiva) and green beans (Phaseolus vulgaris L.) , Journal of Agricultural and Food Chemistry 52 (9) (2004), pp. 2659–2670.
- Munyaka, A.W. , Oey, I. , Van Loey, A. , and Hendrickx, M. , 2010. Application of thermal inactivation of enzymes during vitamin C analysis to study the influence of acidification, crushing and blanching on vitamin C stability in Broccoli (Brassica oleracea L var. italica) , Food Chemistry 1209 (2) (2010), pp. 591–598.
- Rodrigues, A.S. , Pérez-Gregorio, M.R. , García-Falcón, M. S , and Simal-Gándara, J. , 2009. Effect of curing and cooking on flavonols and anthocyanins in traditional varieties of onion bulbs , Food Research International 42 (9) (2009), pp. 1331–1336.
- Turkmen, N. , Sari, F.Y. , and Velioglu, S. , 2005. The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables , Food Chemistry 93 (4) (2005), pp. 713–718.
- Francisco, M. , Velasco, P. , Moreno, D.A. , García-Viguera, C. , and Cartea, M.E. , 2010. Cooking methods of Brassica rapa affect the preservation of glucosinolates, phenolics and vitamin C , Food Research International 43 (5) (2010), pp. 1455–1463.
- Prasad, N.K. , Yang, E. , Yi, C. , Zhao, M. , and Jiang, Y. , 2009. Effects of high pressure extraction on the extraction yield, total phenolic content and antioxidant activity of longan fruit pericarp , Innovative Food Science and Emerging Technologies 10 (2) (2009), pp. 155–159.
- Miliauskas, G. , Venskutonis, P.R. , and van Beek, T.A. , 2004. Screening of radical scavenging activity of some medicinal and aromatic plant extracts , Food Chemistry 85 (2) (2004), pp. 231–237.
- Yu, C. , Ranieri, M. , Lv, D. , Zhang, M. , Charles, M.T. , Tsao, R. , Rekika, D. , and Khanizadeh, S. , 2011. Phenolic composition and antioxidant capacity of newly developed strawberry lines from British Columbia and Quebec , International Journal of Food Properties 14 (1) (2011), pp. 59–67.
- Djordjevic, T.M. , Šiler-Marinkovic, S.S. , and Dimitrijevic-Brankovic, S.I. , 2011. Antioxidant activity and total phenolic content in some cereals and legumes. International , Journal of Food Properties 14 (1) (2011), pp. 175–184.