Abstract
Enzyme immobilization is an effective method to improve enzyme properties. There are varieties of immobilization techniques. In this study, lactoperoxidase was purified using different chromatography techniques. Polyaniline polymer was used due to its unique physical and chemical properties to immobilize lactoperoxidase. Polyaniline polymer was activated with glutaraldehyde first, and then lactoperoxidase was successfully immobilized on it. Then the effects of enzyme concentration, time, and pH on the immobilization efficiency were investigated. The bare polyaniline polymer and polyaniline polymer-enzyme surface topographies were investigated via atomic force microscope. Calculated binding efficiency showed that immobilized lactoperoxidase conserved 91% of its native activity. Also, stability studies showed that the stability of immobilized enzyme in comparison with free enzyme was increased; moreover, the immobilized enzyme can be reused for several times without loss of activity.
INTRODUCTION
Lactoperoxidase (LPO) (donor: hydrogen peroxide oxidoreductase, EC 1.11.1.7) is one of the most abundant enzymes in bovine milk. It is a glycoprotein with a single chain and heme prosthetic group. It has 595 amino acid residues that give the molecular mass of approximately 80 kDa.[Citation1,Citation2] It has a carbohydrate content of about 10% (four or five carbohydrate chains) and an isoelectric point of 9.6. It catalyzes oxidizing halide and pseudohalide ions, such as thiocyanate, using hydrogen peroxide to produce potent antimicrobial agents, which can inactivate a wide range of microorganisms, such as viruses, gram positive and gram negative bacteria, fungi, and parasites by killing or inhibiting their growth via blocking their metabolism leading to a change in cellular functions in a lactoperoxidase system (LP-s). Moreover LP-s has been identified as a natural antimicrobial system in human secretions.[Citation2–4CitationCitation−4 The antitumor activity that has been reported for this enzyme can be considered as an important biological effect due to exterminate tumor cells and mycoplasma as well, but it is harmless to mammalian cells.[Citation4] The field of factual and potential applications of these natural antimicrobial systems is widely spread. It also can be used as a natural antimicrobial tool (preservative agent) in different industries, such as food products, dairy products, cosmetics, drugs, and medicine.[Citation5–7CitationCitation−7 Therefore, we prompted to immobilize lactoperoxidase. It should be mentioned that enzyme immobilization provides increased resistance against environmental changes, such as pH or temperature.[Citation8] It also allows enzymes to be held in place through the reaction, following which they are easily separated from the products and may be used again—a far more efficient process and so is widely used in industry for enzyme catalyzed reactions.[Citation9] There are different organic and inorganic supports, such as polymers and nanoparticles, which can be chosen as suitable supports for enzyme immobilization to ensure the highest retention of enzyme activity and its stability.[Citation10–14CitationCitationCitationCitation−14
Polyaniline is one of the most important electrically conducting polymers with unique electrical, optical, and chemical properties, such as ease of synthesis, light weight, high yield, less expensive, high stability against greatest temperature and pH, and resistance against microorganisms attacks. Therefore, it has considerable application in recent years and is a suitable candidate for a variety of technological applications, such as batteries, separation membranes, sensors, etc.[Citation15–18CitationCitationCitation−18 It also behaves as suitable polymeric supports for enzyme immobilization.[Citation18–21CitationCitationCitation−21
In this study, we purified lactoperoxidase from skimmed bovine milk and immobilized it using polyaniline polymer (PANI) according to the manner described by Fernandes et al.[Citation18,Citation22] Glutaraldehyde was used to immobilize the enzyme covalently, which is one of the most popular used technologies for enzyme immobilization and is quite simple and efficient.[Citation23] Then the immobilized enzyme was characterized.
MATERIALS AND METHODS
Reagents
2,2-Azinobis [3-ethyl benzothiazo line-6-sulphonic acid] diammonium salts (ABTS; Sigma, USA), hydrogen peroxidase (30% solution; Merck, Germany) were obtained from Merck. Polyaniline was purchased from Sigma Chemical Co. (USA). CG-50 resin and Sephadex G-100 were purchased from Sigma, USA. CM-Sephadex C-50 was obtained from Sigma, USA. All other reagents that were used in this work were of analytical grade and were used without further purification.
Purification of Lactoperoxidase
Lactoperoxidase was purified according to the method described by Ozdemir et al.[Citation24,Citation25] First, bovine milk was centrifuged to separate fat. Then amberlite CG 50 NH4+ resin equilibrated in 5 mM sodium acetate solution (pH 6.8) was added and stirred slowly for 1 h. The resin was washed with distilled water and 20 mM of sodium acetate solution (pH 6.8). In order to elute the bound proteins, 0.5 M of acetate solution (pH 6.8), was used. Solid ammonium sulfate was slowly added to the obtained green colored mixture from the previous step. After dialyzing against 5 mM sodium phosphate buffer (pH 6.8), the obtained supernatant was loaded on a CM Sephadex C-50 column (3 × 10 cm), which was equilibrated with 10 mM sodium phosphate buffer (pH 6.8). The elution of LPO was performed with a linear gradient of 100–200 mM NaCl in 10 mM sodium phosphate buffer (pH 6.8), followed by ammonium sulfate addition and dialysis. The dialyzed sample was passed through a Sephadex G-100 column (2.5 × 100 cm). A Sephadex-bound enzyme was eluted using 0.1 M of phosphate buffer (pH 6.8), followed by an ammonium sulfate addition and dialysis against 0.5 M of sodium phosphate buffer (pH 6.0). Then it was lyophilized and controlled for purity. Purification degree of the purified lactoperoxidase was confirmed by SDS-PAGE and Rz(A412/A280) value. Finally the secondary structure of purified enzyme was analyzed using a circular dichroism spectroscopy.
Activation of Supports
The activation process of the polymer was carried out according to Olsson and Gren methods.[Citation26] A 2.5% (v/v) glutaraldehyde solution as a bifunctional agent was provided in 0.1 M sodium phosphate buffer (pH 6.0). The mixture was permitted to react under reflux for 2 h. The activated dried polymer was called PANIG.
Enzyme Immobilization
LPO immobilization was performed via adding 1.0 ml of an enzyme solution containing 12 μg LPO in 0.1 M sodium phosphate buffer, pH 6.0, to 5.0 mg PANIG. The mixture was slowly stirred for 1 h at 4°C; the solids were separated and washed with the same buffer to extract the unreacted enzyme. The obtained solids were tested for enzymatic activity.
Enzyme Activity Assay
Activity assay of lactoperoxidase was performed following the procedure of Shindler et al.[Citation27] with some modifications. The assay was performed at room temperature, using 2,2-azino-bis(3-ethylbenzthiazoline-6 sulfonic acid) diammonium salt (ABTS) and H2O2 as substrate. An amount of 100 μL of H2O2 (1 mM) was added to 900 μL of phosphate buffer 0.1 M, pH 6.0, containing 100 μL of ABTS (10 mM) and 50 μL of free LPO. The absorbance was measured at 412 nm as a function of time for 2 min. The assay for immobilized enzyme was performed in the same condition except for reaction interruption before spectrophotometric measurements to separate PANIG-LPO from the reaction mixture. Each test was performed three times for statistical validity.
Enzyme Binding Efficiency
After performing immobilization under the best conditions, the solids were separated. The supernatant was collected, dialyzed against water, and lyophilized. The protein content was determined via the Bradford method by dissolving the lyophilized sample in sodium phosphate buffer. The binding efficiency (amount of retained protein that remains active) was calculated as the ratio between the percentage of immobilized active enzyme and the percentage of retained protein. This experiment was performed three times for statistical validity.
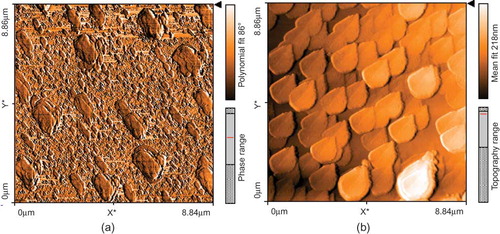
Atomic Force Microscopy
Atomic force microscope (AFM) is one of the most powerful techniques to determine surface topography at subnanometer resolution. It can be used to obtain highly accurate images and manipulate atoms and structures on a variety of surfaces, including polymers, ceramics, composites, glass, and biological samples. We used it as an effective analytical tool to study bare polyaniline polymer surface topography and polymer modified with enzyme surface topography.[Citation28,Citation29]
Storage Stability
To determine the storage stability of the immobilized enzyme, the PANIG-LPO and native enzyme were stored at 4°C and at room temperature (approximately 30°C). Then at regular intervals of time, aliquots were tested to assay the enzyme activity according to the method described previously.
Thermal Stability of PANIG-LPO
Thermal stability studies were carried out by suspending both of the free and immobilized enzymes in 0.1 mol L−1 phosphate buffer, pH 6.0, and incubating the mixture in a water bath at 65°C for different time intervals. After incubation time, the mixture was immediately transferred to an ice bath and then tested for remaining activity.
RESULTS AND DISCUSSION
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis
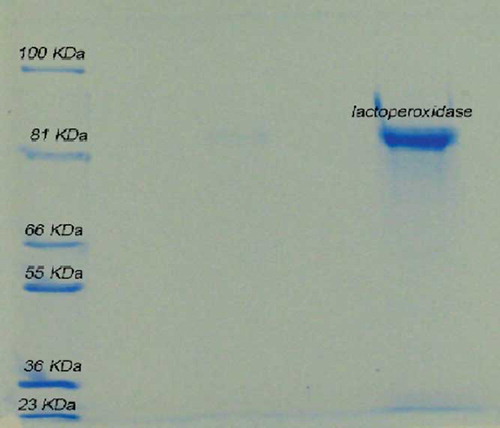
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Rz(A412/A280) were used to evaluate the purity of the enzyme quantitatively. The measured Rz(A412/A280) value after enzyme purification by a CM Sephadex C-50 column was 0.7, although it can be increased up to 0.8 by performing further purification using a Sephadex G-100 column. The protein concentration was detected via the Bradford method. SDS-PAGE of purified bovine LPO and the purification steps are shown in and , respectively.
Table 1 Purification steps of lactoperoxidase from bovine milk
Circular Dichroism Studies
The secondary structure of the enzyme was studied using circular dichroism (CD). Results showed that the contents of the secondary structure were as follows: 67.7% beta-structure, 19% alpha-helix, and 13.3% unordered structure. Similar studies on lactoperoxidase structure using CD were performed by Sievers and Sharique et al.[Citation30,Citation31] It should be noted that purification processes caused no changes in the secondary structure content.
Coupling of LPO and Binding Efficiency of PANIG-LPO
Several parameters were investigated to find the best condition for enzyme immobilization on PANIG.
Influence of pH
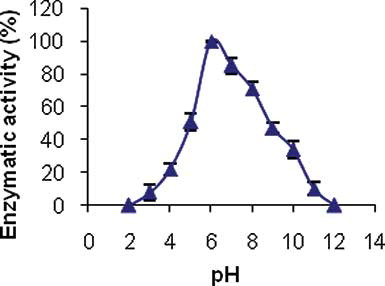
To investigate pH effect on enzyme immobilization, the enzymatic activity measurement was performed at different solution pHs. We used 0.1 M sodium acetate solution (pH 2.0 to 5.0), 0.1 M sodium phosphate buffer (pH 6.0 to 8.0), and 0.1 M Tris buffer (pH 9.0 to 12.0). The result is shown in . The maximum activity was obtained at pH 6.0, so it was chosen as the best pH. Similar results were obtained by Mecitoglu and Yemenicioglu[Citation32] using alginate film for immobilization of lactoperoxidase. This result was also reported by other researchers in the processes of horseradish and soybean peroxidases immobilization on polyaniline polymer.[Citation18,Citation22]
Influence of Enzyme Amount
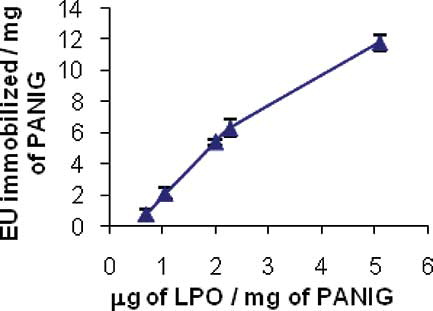
The relationship between quantities of LPO and relative amounts of PANIG was tested and shown in . The best enzyme amount for immobilization was around 2.5 μg of LPO per mg of PANIG. At this ratio, we found a linear relationship between the immobilized enzyme and the amount of enzyme available per mg of PANIG. At higher quantities there is a nonlinear relationship, which is indicative of polymer saturation.
Influence of Time
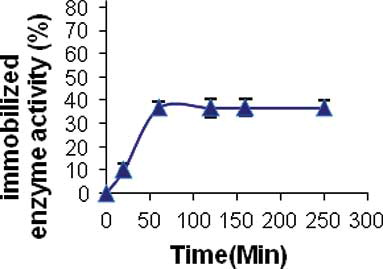
The required time to complete LPO immobilization process was detected through measurement of immobilized enzyme activity and the activity of enzyme in supernatant. The result is shown in . As it is obvious with the rising of immobilization time, the activity of the immobilized enzyme increased up to 60 min after that; there were no observed significant changes in the enzyme activity. Therefore, considering operational reasons, 60 min was chosen as the best time period for enzyme immobilization.
Efficiency of Enzyme Immobilization
The amounts of enzyme and protein linked to PANIG under the best condition for immobilization is given in . The results show that 23.2% of the available enzyme was immobilized and 25.31% of the protein was retained. Determination of the binding efficiency indicates that 91% of the immobilized protein has maintained its native activity. Similar investigations were carried out by Fernandes et al. and Magri et al.[Citation18,Citation22] They used polyaniline polymer to immobilize horseradish peroxidase and soybean seed coat peroxidase, respectively. The binding efficiency for horseradish peroxidase was 100% and for soybean seed coat peroxidase was 82%. Results indicate that polyaniline polymer is a suitable polymer for immobilization.
Table 2 Efficiency of PANIG on active enzyme retention
It should be noted that, for a long lasting enzyme immobilization, glutaraldehyde is an efficient tool.
AFM Characterization of the Polyaniline Surfaces
The surface topography of polyaniline polymer containing immobilized enzyme and that of bare polyaniline polymer was investigated using AFM. The AFM images are shown in . The images demonstrate significant differences between bare and immobilized enzyme polyaniline polymer surfaces topography, which indicate that the process of enzyme immobilization was successfully performed. Similar studies were done by Zhang and Tan[Citation28] to characterize immobilized enzyme molecules on biosensor surfaces and Masato et al.[Citation33] to study immobilized ferritin onto gold electrode.
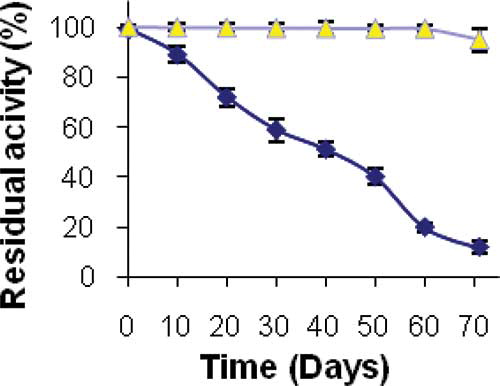
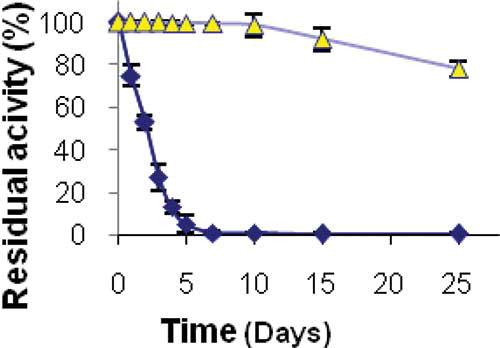
Stability of Immobilized Enzyme During Storage
The activity measurement assays of the PANIG-LPO and free enzyme during storage at 4 and 30°C indicated that the immobilization processes enhanced stability of the enzyme. The result of storage stability at 4°C is shown in . It should be added that when free and immobilized enzymes were stored at 4°C, the immobilized enzyme maintained 100% of its activity during 60 days of storage and then began to lose its activity slowly, whereas the native enzyme lost 80% of its initial activity within 60 days. The result of storage stability at 30°C is shown in . As seen when stored at room temperature, the free enzyme was inactive absolutely after 5 days while the immobilized enzyme kept its full activity during 10 days. Also, reuse assessment gave excellent results, the immobilized enzyme retained 100% of its activity after seven reuses during the storage period.
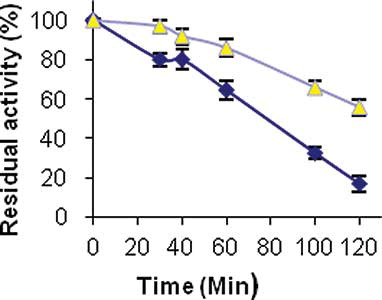
Thermal Stability of PANIG-LPO
The thermal stability curves of immobilized and free enzymes at 60°C are shown in . As seen, the free and immobilized enzymes presented quite different behaviors against heat treatment. The immobilized enzyme in comparison with the free enzyme has a higher resistance against thermal denaturation. The immobilized enzyme maintained 60% of its initial activity after 120 min lined at 60°C, whereas the native enzyme kept only 20% of its activity during this period of time. This improvement in denaturation resistance indicates that the immobilized enzyme makes a strong binding with polyaniline polymer during the immobilization process.
CONCLUSION
LPO is known as an antibacterial enzyme that has attracted increasing interest during the past few years, and can be used in a variety of industrial operations as a biopreservative. Thus, LPO immobilization in order to improve its stability is economical.
Polyaniline polymer, because of its special properties, has been studied extensively in recent years. Its high effectiveness makes it a suitable polymeric support to immobilize enzymes. In this study, polytaniline was applied to immobilize LPO. Results indicated that immobilization processes caused no significant changes on the LPO structure. This polymer also showed a good capability for enzyme retention (23.2%) according to 600 μg of LPO per gram of PANIG and an acceptable capability for activity maintenance. Also, stability studies showed that the stability of the immobilized enzyme is more than that of the free enzyme. Therefore, polyaniline immobilization can be considered as an effective method to improve LPO stability and a good option for its commercial applications in a large-scale food industry.
REFERENCES
- Amit, K.S., Nagendra, S., Sujata, S., Baskar, S., Punit, K., Bhushan, A., and Tej, P.S., 2008. Crystal structure of lactoperoxidase at 2, 4 A resolution. Journal of Molecular Biology 376 (2008), pp. 1060–1071.
- Klaas, D.K., and van Hooijdonk, A.C.M., 2000. Lactoperoxidase: Physico-chemical properties, occurrence, mechanism of action and application, British Journal of Nutrition 84 (2000), pp. 19–25.
- Dewit, J.N., and Van Hooydonk, A.C.M., 1996. Structure, function and application of lactoperoxidase in natural antimicrobial system, Nederlands Melk 50 (1996), pp. 227–244.
- Florence, A.F., Alistair, S.G., and Michael, J.L., 2005. Factors affecting lactoperoxidase activity, International Journal of Dairy Technology 58 (2005), pp. 233–236.
- Uguz, M.T., and Ozdemir, H., 2005. Purification of bovin milk lactoperoxidase and investigation of antibacterial properties at different thiocyanate mediated, Applied Biochemistry and Microbiology 41 (2005), pp. 349–353.
- Keri, M., 2004. Therapeutic application of whey protein, Alternative Medicine Review 9 (2004), pp. 136–156.
- Boots, J.W., and Floris, R., 2009. Lactoperoxidase: From catalytic mechanism to practical applications, International Journal of Dairy Technology 16 (2009), pp. 1272–1276.
- Yadav, D.N., Patki, P.E., Srihari, S.P., Sharma, G.K., and Bawa, A.S., 2010. Studies on polyphenol oxidase activity of heat stabilized whole wheat flour and its chapatti making quality, International Journal of Food Properties 13 (2010), pp. 142–154, .
- Cesar, M., Jose, M., Palomo, G.F.L., Jose, M., and Guisan, R.F.L., 2007. Improvement of enzyme activity, stability and selectivity via immobilization techniques, Enzyme and Microbiol Technology 40 (2007), pp. 1451–1463.
- Kuhlmeyer, C., and Klein, J., 2003. Improvement of enzyme activity, stability and selectivity via immobilization techniques, Enzyme and Microbiol Technology 32 (2003), pp. 99–106.
- Gomez, J.L., Bodalo, A., Gomez, E., Bastida, J., Hidalgo, A.M., and Gomez, M., 2006. Immobilization of peroxidase on glass beads: An improved alternative for phenol removal, Enzyme and Microbiol Technology 36 (2006), pp. 1016–1022.
- Temocin, Z., and Yigitoglu, M., 2008. Studies on the activity and stability of immobilized horseradish peroxidase on poly(ethylene terephthalate) grafted acrylamide fiber, Bioprocess and Biosystems Engineering 32 (2008), pp. 467–474.
- Gogoi, B.K., Alavi, S.H., and Rizvi, S.S.H., 2000. Mechanical properties of protein-stabilized starch-based supercritical fluid extrudates, International Journal of Food Properties 3 (2000), pp. 37–58.
- Alben Ercelebi, E., and Ibanoglu, E., 2010. Stability and rheological properties of egg yolk granule stabilized emulsions with pectin and guar gum, International Journal of Food Properties 13 (2010), pp. 618–630.
- Gerard, M., Chaubey, A., and Malhotra, B.D., 2002. Application of conducting polymers to biosensors, Biosensors and Bioelectronics 173 (2002), pp. 45–59.
- Raghavendra, S.C., Revansiddappa, M., Narsimha, P., and Ambika, M.V.N., 2006. Synthesis, transport and dielectric properties of polyaniline/CO3O4, Bulletin of Material Science 78 (2006), pp. 15–27.
- Srivastava, A., Singh, V., Dhand, C., Kaur, M., Singh, T., Witte, K., and Scherer, U., 2006. Study of swift heavy ion modified conducting polymer composites for application as gas sensor, Sensors 6 (2006), pp. 262–269.
- Fernandes, K.F., Lima, C.S., Pinho, H., and Collins, C.H., 2003. Immobilization of horseradish peroxidase onto polyaniline polymer, Process Biochemistry 38 (2003), pp. 1379–1384.
- Samantha, S.C., and Katia, F.F., 2004. Covalent immobilisation of horseradish peroxidase onto poly(ethylene terephthalate)-poly(aniline) composite, Process Biochemistry 39 (2004), pp. 883–888.
- Parente, A.H., Marques, E.T.A., Azeyedo, W.M., Diniz, F.B., Melo, E.H., and Lima Filho, J.L., 1969. Glucose biosensor using glucose oxidase immobilized in polyaniline, Applied Biochemistry and Microbiology 37 (1969), pp. 267–273.
- Nadruz, W.J., Marques, E.T.A., Azevedo, W.M., Lima, J.L., and Caryalho, L.B., 1969. Immobilization of xentine on a polyaniline silicone, Brazilian Journal of Medical and Biological Research 29 (1969), pp. 347–350.
- Magri, M.L., Miranda, M.V., and Cascone, O., 2005. Immobilization of soybean seed coat peroxidase on polyanilin: Synthesis optimization and catalytic properties, Biocatalysis and Biotransformation 23 (2005), pp. 339–346.
- Betancor, L., Lopez-Gallego, F., Hidalgo, A., Alonso-Morales, N., Dellamora-Ortiz, C., Mateo, G., Fernandez-Lafuente, R., and Guisan, J., 2006. Different mechanisms of protein immobilization on glutaraldehyde activated supports: Effect of support activation and immobilization conditions, Enzyme and Microbiol Technology 39 (2006), pp. 877–882.
- Ozdemir, H., Hacibeyoglu, H., and Uslu, H., 2002. Purification of lactoperoxidase from creek buffalo milk and investigation of kinetic and antibacterial properties, Preparative Biochemistry and Biotechnology 32 (2002), pp. 143–155.
- Ozdemir, H., Aygul, I., and Kufrevioglu, O.I., 2001. Purification of lactoperoxidase from bovin milk and investigation of the kinetic properties, Preparative Biochemistry and Biotechnology 31 (2001), pp. 125–134.
- Olsson, B., and Gren, L., 1983. Optimization of peroxidase immobilization and of design of packed–bed enzyme reactors for flow injection analysis, Analytica Chimica Acta 45 (1983), pp. 87–99.
- Shindler, J.S., Chlds, R.D., and Bardsley, W.G., 1976. Peroxidase from human cervical mucus, European Journal of Biochemistry 65 (1976), pp. 325–331.
- Zhang, P., and Tan, W., 2001. Atomic force microscopy for the characterization of immobilized enzyme molecules on biosensor surfaces, Journal of Analytical Chemistry 369 (2001), pp. 302–307, .
- Johnson Travis, W., 2008. Imaging DNA in Solution with the AFM. Agilent Technologies, USA; 2008, 5989-9932EN.
- Sievers, G., 1980. Structure of lactoperoxidase: A study using circular dichroism and difference absorption spectroscopy, Journal of Analytical Chemistry 369 (1980), pp. 302–307.
- Sharique Zahida, M., Deva, W., and Digambar, V.B., 1999. Fluorescence and circular dichroism spectroscopic studies on bovine lactoperoxidase, Biometals 12 (1999), pp. 219–225.
- Mecitoglu, C., and Yemenicioglu, A., 2007. Partial purification and preparation of bovine lactoperoxidase and characterization of kinetic properties of its immobilized form incorporated into cross-linked alginate films, Food Chemistry 104 (2007), pp. 726–733.
- Masato, T., Akihiro, O., Yoshihisa, Y., and Masashi, K., 2003. Electrochemical, AFM and QCM studies on ferritin immobilized onto a self-assembled monolayer-modified gold electrode, Journal of Electroanalytical Chemistry 566 (2003), pp. 323–329.