Abstract
Solid-state bioprocessing of foxtail millet by Lactobacillus paracasei Fn032 is a biotechnological strategy to produce fermented foxtail millet meal with more beneficial components. The objective of this study was to evaluate the antioxidant, antimicrobial properties and nutritional values of water extracts from fermented foxtail millet flour and its bran with and without protease. Fermented foxtail millet flour with added protease extract showed higher scavenging ability on 1,1-diphenyl-2-picrylhydrazyl radicals as well as reducing power than fermented foxtail millet flour and fermented foxtail millet bran extracts. Both extracts, fermented foxtail millet flour and fermented foxtail millet flour with protease, showed significant (P < 0.05) effectiveness inhibition abilities on microbial growth when compared with fermented foxtail millet bran extracts. Amino acid profile revealed that fermented foxtail millet flour with protease, with relatively strongest antioxidant, antimicrobial activity, also had the highest total hydrophobic amino acids content (51.39%) and hydrophobic index (8.47 Kj/mol amino acid residue). Moreover, fermented foxtail millet flour with protease revealed the highest protein content, predicted protein efficiency ratio, and protein digestibility. Molecular weight of the whole extracts varied from 180–5000 Da. Based on the results obtained, fermented foxtail millet flour extracts were relatively effective in the antioxidant, antimicrobial properties assayed and might be potential biological values for application in food products.
INTRODUCTION
Millet is a general term for a wide range of cereals. Foxtail millet (Setaria italica) is one of the most important food crops of the semi-arid tropics; it originated from China, and is now planted all over the world. It plays a very important role in the agriculture and food of many developing countries because of its ability to grow under adverse heat and limited rainfall conditions. It was reported that foxtail millet has many nutritious and medical functions.[Citation1 Citation2] The millet bran is used extensively as animal feed in China.[Citation3] Millet is an important cereal and nutritious food in traditional diets, especially for people in the continents of Asia and Africa. However, cereals such as millet are also deficient in some of the basic components (e.g., essential amino acids); fermentation may be the most simple and economical way of improving their nutritional value, sensory properties, and functional qualities.[Citation4 Citation5] Thus, fermented foods have become very important to human beings all over the world. About 20 to 40% of our food supply is from fermented foods.[Citation6]
Production of antimicrobial peptides is found as a widespread strategy used by plants, animals, and microorganisms to combat pathogenic microorganisms. Besides the variable structural characteristics, these peptides are mostly cationic, showing an amphipathic nature, containing about 30 to 100 amino acid residues in a linear or cyclic arrangement.[Citation7] Lactobacillus spp. are gram-positive, non-motile, non-sporulating, facultative anaerobes. Lactobacillus paracasei isolated from healthy humans showed antibacterial and anticandidal activities against oral pathogens, such as S. mutans, S. salivarius, Streptococcus sanguis, Staphylococcus aureus, Actinomyces viscosus, P. gingivalis, Candida albican, Candida tropicalis, and Candida grabata.[Citation8]
There is a wide range of oxygen-free radicals and other reactive oxygen species (ROS), which include free radicals, such as superoxide anion radicals (O2 −•); hydroxyl radicals (HO•); and non-free-radical species, such as hydrogen peroxide (H2O2) and singlet oxygen ( | O2), which may form in the human body and in the food system. These radicals induce not only lipid peroxidation that causes deterioration of foods, but also cause oxidative damage by oxidizing biomolecules leading to cell death and tissue damage, such as atherosclerosis, cancer, emphysema, cirrhosis, and arthritis.[Citation9] Antioxidant agents can act against free radicals either by retarding their formation (preventive antioxidants) or by inactivating radicals in reaction medium (chain-breaking antioxidants).[Citation10] Currently, the natural antioxidant α-tocopherol and some synthetic antioxidants, such as butylated hydroxytoluene, butylated hydroxyanisole, and propyl gallate, are commonly used to act against free radicals in food and biological systems. However, the use of synthetic antioxidants in food products is under strict regulation owing to their potential health hazards.[Citation11]
Antioxidative property of Lactobacilli would be useful in the food manufacturing industry. They could beneficially affect the consumer by providing another dietary source of antioxidants or by providing probiotic bacteria with the potential of producing antioxidants during their growth in the intestinal tract. It was found that superoxide dismutase “SOD” produced by the engineered Lactobacillus gasseri reduces the levels of superoxide radicals in the gut imposed by specific members of the gut microbiota and the immune system,[Citation12] demonstrating the potential use of the antioxidative Lactobacilli. They also could modulate a redox state in the colonic fermentation system, which is related to their free radical scavenging ability or antibacterial effect. Sun et al.[Citation13] have previously demonstrated that antioxidative Lactobacillus paracasei Fn032 is a probiotic with the ability to scavenge or prevent the production of hydroxyl radical in the system mimicking the colon environment with Fe2+ addition.
Nutritional, proximate analysis, and functionality of foxtail millet were widely studied. [Citation5 Citation14–17] However, information on the aqueous extract's antimicrobial effect, antioxidant activity of fermented foxtail millet by Lactobacillus paracasei Fn032 is yet to be studied. Therefore, the present study was aimed to test the antibacterial effect and antioxidative properties including reducing power of aqueous extracts of fermented foxtail millet by L. paracasei Fn032, and also to evaluate their nutritional values using amino acid profile.
MATERIALS AND METHODS
Materials
Foxtail millet bran and foxtail millet meal were purchased from a local producer (Hebei, China) and Da-Run-Fa Market (Wuxi, China), respectively. 1,1-Diphenyl-2-picrylhydrazyl (DPPH), was purchased from Sigma-Aldrich, Inc. (Shanghai, China). Acid protease (Acid protease–537 from Asp.) was purchased from Sunson Industry Group Co. Ltd. (Beijing, China). Gentamycin was purchased from Xuzhou RYEN PHARMA. Co. Ltd. (Xuzhou, China). All other reagents were of analytical grade.
Bacterial Strains
Lactobacillus paracasei Fn032 was obtained by the Key Laboratory of Industrial Biotechnology, Ministry of Education, Jiangnan University (Wuxi, China). Staphylococcus aureus ATCC 6538, Escherichia coli ATCC 8099, and Salmonella typhi CMCC 50013 were provided by the microbiology laboratory culture collection of Jiangnan University (Wuxi, China). Bacteria were maintained as glycerol stocks and stored at −70°C. All strains were serially transferred at least three times prior to use in this study. The strains were inoculated into the Mann Rogosa Sharpe (MRS) broth and incubated for 18 h at 37°C in an anaerobic jar. Cells were collected by centrifugation at 3000× g for 15 min and the bacterial pellets were washed twice and re-suspended in 0.1 mol/L phosphate buffered saline (PBS;pH 7.3).
Fermentation and Preparation of Foxtail Millet Extracts
An amount of 0.025 mL of L. paracasei Fn032 with and without protease was mixed aseptically with 25 g of defatted foxtail millet bran (FFMB and FFMBp) and defatted foxtail millet flour (FFM and FFMp) (107 CFU/g) in a polyethylene bag (140 mm × 200 mm) and vacuum sealed, and then solid-state fermentation was performed for 48 h at 37°C. Fermented foxtail millet extracts were prepared according to the method described by Ye et al.[Citation18] Five grams of fermented foxtail millet were mixed with 50 mL of distilled water, homogenized for 1 min and incubated at 37°C for 60 min. The incubated mixture was centrifuged at 9600 rpm for 2 min using a D-3756 Osterode AM Harz Model 4515 Centrifuge (Hamburg, Germany) and the residue was washed with 20 mL of distilled water, centrifuged again at the same speed and time, and the combined supernatant was freeze-dried and stored at –20°C until further use. The freeze-dried extracts were weighed and the yield was calculated based on the weight of foxtail millet bran and flour.
Chemical Analysis
Crude protein content (N = 6.25) was determined using the micro-Kjeldahl method.[Citation19] For total sugar determination, a 500-mg portion of sample was extracted three times with 20 mL of hot 80% ethanol and the extract was evaporated and made up to 10 mL. An aliquot was assayed for total soluble sugars by the phenol-sulphuric acid method[Citation20] using glucose as a standard. in vitro protein digestibility (IVPD) was carried out according to the method described by Elkhalil et al.,[Citation21] with slight modifications. Twenty milligrams of fermented foxtail millet extract (flour and bran) samples were digested in triplicate in 10 mL of trypsin (0.2 mg/mL in 100 mM Tris-HCl buffer, pH 7.6). The suspension was incubated at 37°C for 2 h. Reaction was stopped by adding 5 mL of 50% trichloroacetic acid (TCA). The mixture was allowed to stand for 30–35 min at 4°C and was then centrifuged at 5000 rpm for 5 min using a D-3756 Osterode AM Harz Model 4515 Centrifuge (Hamburg, Germany). The resultant precipitate was dissolved in 5 mL of NaOH and protein concentrate was measured using the Kjeldahl method. Digestibility was calculated as follows:
where A is total protein content (mg) in the sample and B is total protein content (mg) in TCA precipitate.
Molecular Weight Determination
The samples were determined using a Waters™ 600E Advanced Protein Purification System (Waters Corporation, Milford, MA, USA). A TSK gel, 2000SWXL (7.8 × 300 mm) column was used with 10% acetonitrile + 0.1% TFA in HPLC grade water as the mobile phase. The calibration curve was obtained by running bovine carbonic anhydrase (29,000 Da), horse heart cytochrome C (12,400 Da), bovine insulin (5800 kDa), bacitracin (1450 Da), Gly-Gly-Tyr-Arg (451 kDa), and Gly-Gly-Gly (189 Da). The total surface area of the chromatograms was integrated and separated into eight ranges, expressed as a percentage of the total area.
Amino Acid Analysis and Protein Quality Evaluation
The freeze-dried samples were digested with HCl (6 M) at 110°C for 24 h under nitrogen atmosphere. Reversed phase high performance liquid chromatography (RP-HPLC) analysis was carried out in an Agilent 1100 (Agilent Technologies, Palo Alto, CA, USA) assembly system after precolumn derivatization with o-phthaldialdehyde (OPA). Each sample (1 μL) was injected on a Zorbax 80 A C18 column (i.d. 4.6 × 180 mm; Agilent Technologies, Palo Alto, CA, USA) at 40°C with detection at 338 nm. Mobile phase A was 7.35 mmol/L sodium acetate/triethylamine/tetrahydrofuran (500:0.12:2.5, v/v/v), adjusted to pH 7.2 with acetic acid, while mobile phase B (pH 7.2) was 7.35 mmol/L sodium acetate/methanol/acetonitrile (1:2:2, v/v/v). The amino acid composition was expressed as g of amino acid per 100 g of protein.
Total hydrophobic amino acids (THAA) and hydrophobic indices (HΦ) was calculated according to Adler-Nissen.[Citation22] The estimated predicted protein efficiency ratio (PER) values of fermented foxtail millet extracts were carried out in accordance with Alsmeyer et al.,[Citation23] using three regression equations. The FAO/WHO reference pattern of essential amino acid requirements (g/100 g of protein) (FAO/WHO[Citation24]) was used as the standard.
DPPH Radicals Scavenging Activity Assay
The scavenging effect of fermented foxtail millet extracts on DPPH free radical was measured according to the method of Shimada et al.[Citation25] with little modification. Two milliliters of each sample solution (3, 5, 7, and 10 mg/mL) were added to 2 mL of 0.1 mM DPPH dissolved in 95% ethanol. The mixture was shaken and left for 30 min at room temperature, and the absorbance of resulting solution was read at 517 nm. A lower absorbance represents a higher DPPH scavenging activity. The scavenging effect was expressed as shown in the following equation:
Reducing Power
The reducing power of fermented foxtail millet extracts were measured according to Wu et al.[Citation26] The sample (0, 1, 1.5, 2, and 5 mg/mL) was added to 2 mL of 0.2 M phosphate buffer (pH 6.6) and 2 mL of 1% (w/v) potassium ferricyanide. The mixture was incubated at 50°C for 20 min, and then 2 mL of 10% (w/v) trichloroacetic acid (TCA) added. The mixture was centrifuged for 10 min at 3000× g, and 2 mL of the supernatant was mixed with 2 mL of distilled water and 0.4 mL of 0.1% (w/v) FeCl3. After reaction for 10 min, the absorbance of the solution was read at 700 nm. Increase in the absorbance of the reaction mixture indicated increased reducing power.
Antimicrobial Activity
Antibacterial activity was determined by the disc diffusion method.[Citation27] The suitable nutrient agar media was prepared. Pure S. aureus, E. coli, and S. typhi culture were added separately to sterile petri plates. Sterilized media was poured into the sterile petri plates. After the media had cooled and solidified, wells (8 mm in diameter) were made in the agar and 50 μL of fermented foxtail millet extract was loaded in the wells. Gentamycin was used as a positive control. The plates were incubated for 24 h at 37°C. After incubation, the zone of inhibition was observed and the diameter was measured.
Statistical Analysis
All experiments were conducted at least in triplicate. Analysis of variance (ANOVA) was performed and significant difference in mean values were evaluated by a Tukey HSD multiple range test at (P < 0.05) using SPSS version 17.0 (SPSS, Chicago, IL, USA).
RESULTS AND DISCUSSION
Chemical Analysis
Both fermented foxtail millet flour with and without added protease revealed appreciable amounts of the protein (FFMp: 21.04%; FFM: 20.54%). Fermented foxtail millet bran with and without added protease exhibited an insignificant increase in protein content, likewise with FFM and FFMp; however, these results affected their nutritional values (). A previous study reported influence of fermentation on nutrients composition using Lactobacillus plantarum.[Citation28]
Table 1 Chemical composition of fermented foxtail millet extracts
Little increase was observed in total soluble sugar content during fermentation after the addition of protease and this implies that foxtail millet flour or bran were utilized as the main energy source for L. paracasei Fn032 activity. shows an insignificant increase (P < 0.05) between fermented samples (FFM: 3.58%; FFMB: 2.07%) and that of samples with added protease during the fermentation (FFMp: 3.79%; FFMBp: 2.13%). The entire samples showed a significant difference (P < 0.05) in vitro trypsin digestibility above 89% (). As expected, fermented foxtail millet flour had higher digestibility, sugar solubility, and more protein content than FFMB extracts. However, the yield of fermented foxtail millet bran extract was the higher (30.22%) with significant difference (P < 0.05) compared to FFM flour. Our data corroborated with the work reported by Chavan et al.[Citation29] and Dharmaraj et al.[Citation5]
Table 2 Radical scavenging activity of fermented foxtail millet extracts
DPPH Radical-Scavenging Activity
The DPPH radicals were widely used to investigate the scavenging activity of some natural compounds. DPPH free radicals are stable in ethanol and show maximum absorbance at 517 nm. When DPPH radicals encounter a proton-donating substance, such as an antioxidant, the radicals would be scavenged and the absorbance is reduced.[Citation26] Results shown in revealed that FFMp exhibited the highest DPPH radical-scavenging activity (91.40% at 3 mg/mL concentration). However, fermented foxtail millet bran extracts (FFMB and FFMBp) showed significant (P < 0.05) radical scavenging activity inversely proportional with the concentrations, whereas fermented foxtail millet flour extracts (FFM and FFMp) showed direct correlation. Similar observation was reported by Lee et al.[Citation11] and Amadou et al.[Citation10] on the water extracts from fermented soybeans. Both fermented foxtail millet bran and flour extracts showed significant scavenging abilities on DPPH radicals and that may be explained considering that the extracts containing bioactive peptides, which were electron donors and could react with free radicals to convert them to more stable products and terminate the radical chain reaction.
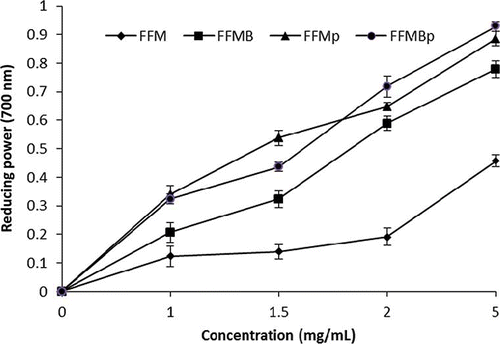
Reducing Power
Duh et al.[Citation30] and Zhu et al.[Citation31] reported earlier that antioxidant activity and reducing power were directly related. In this assay, the presence of antioxidants caused the reduction of the Fe3+/ferricyanide complex to the ferrous form, and the yellow color of the test solution changed to various shades of green and blue depending on the reducing power of all compounds. The reducing power of fermented foxtail millet extracts increased with increasing concentrations and it was observed that the addition of protease enhanced the reducing power at 1 to 5 mg/mL (). The results of this research showed that the addition of protease has an effect on the reducing power, FFMBp and FFMp extracts were higher than that of FFMB and FFM at 1 to 5 mg/mL concentrations, as shown in . The result indicated that fermented foxtail millet extracts are capable of donating electrons that can react with free radicals to convert them to stable products and strongly inhibiting radical chain reaction.[Citation11 Citation32]
Antimicrobial Activity
The foxtail millet extracts obtained from L. paracasei Fn032 fermentation were examined for their antimicrobial () properties. The zones of inhibition for the fermented foxtail millet flour, without and with protease were the bigger (16.27 and 15.10 mm, respectively; see ). It is shown in that the FFMp extract exhibited significant inhibition (P < 0.05) against S. Aureus; moreover, the extracts from fermented flour, with and without protease, revealed stronger inhibition than their counterparts from the fermented bran. Since the fermented foxtail millet extract exhibited the highest antioxidant activity, it can be inferred that the peptides derived from L. paracasei Fn032 activity under the medium of foxtail millet flour are responsible for the microbial inhibition. It was obvious that all extracts showed lower inhibition than the controlled samples. The fermented foxtail millet flour extracts exhibited higher inhibitory response than the fermented foxtail millet bran due to higher activity of L. paracasei Fn032 in the flour. Generally, it is reported that L. paracasei Fn032 have the property of inhibiting the proliferation of other microorganisms but the information on the foxtail millet peptides extracts constituents with respect to their inhibitory activity is scanty.[Citation13 Citation33 Citation34]
Table 3 Antimicrobial activity of fermented foxtail millet extracts
Amino Acids Content and Protein Quality
The total amino acid composition of the foxtail millet extracts obtained from L. paracasei Fn032 fermentation, are shown in , along with the hydrophobic amino acid index and estimated predicted protein efficiency ratio (PER).[Citation24] showed a significant difference (P < 0.05) in their content of essential amino acids as well as non-essential amino acids. FFMp revealed the highest while FFM had the lowest content of hydrophobic and essential amino acids. The solid state fermentation with L. paracasei Fn032 in combination with protease was achieved owing to the difference in the content of hydrophobic amino acid, which makes up the peptides. Furthermore, aspartic acid, glutamic acid, leucine, and proline were found to be abundant as expected in most fermented cereals.[Citation13 Citation28] For both fermented foxtail millet bran and flour, water extracts showed significant balance (P < 0.05) in amino acid composition and most of them were at a higher level than FAO/WHO/UNU protein and amino acid requirements in human nutrition.[Citation24] Conversely, FFMp with the highest antioxidative activity and relatively higher antimicrobial inhibition ability did correlate well with total hydrophobic amino acids (THAA) and hydrophobic indices (HΦ), respectively, for 51.39 g/100 g and 8.47 Kj/mol AAR.
Table 4 Amino acid composition (g/100 g protein), hydrophobic index, and estimated predicted protein efficiency ratio
Both the amount and quality of protein provided by a food are important in the human diet. Many benefits are attributed to fermentation. It preserves and enriches food, improves digestibility, and enhances the taste and flavor of foods.[Citation4 Citation10] The protein quality, also known as the nutritional or nutritive value of a food, depends on its amino acid content and on the physiological utilization of specific amino acids after digestion, absorption, assimilation, and minimal obligatory rates of oxidation. Because direct assessment of protein nutritional value in human subjects is impractical for regulatory purposes, methods based on in vitro (chemical) and animal bioassays for assessment of protein quality have been developed.[Citation4] Good quality protein should exceed 2.00 predicted PER values.[Citation35] FFMp has the highest PER value compared to FFM, FFMB, and FFMBp (). Furthermore, the PER values of FFMp surpassed significantly the required level of 2.00 PER values and that of standard casein PER of 2.5.[Citation35] Solid state fermentation of foxtail millet using L. paracasei Fn032, not only improved the physiochemical quality but also enhanced the nutritional values.
Molecular Weight Distributions
The molecular weight distributions of the various extracts were determined by SE-HPLC. The molecular weights for all samples were calculated according to the standard equation below: Log Mol 6.77–0.217 T (R 2 = 0.992). The size of peptides is known to be a significant factor in the overall antioxidant activity of hydrolyzed proteins. Results in showed that the molecular weight distribution of different extracts (FFM, FFMB, FFMp, and FFMBp), have different molecular weight (MW) distributions. Fermentation with L. paracasei Fn032 have the ability to produce low molecular weight distributions peptides with bioactivity, such as antimicrobial and antioxidative properties, compared with a previous investigation by Kamara et al.[Citation14] There was significant (P < 0.05) influence of the protease in combination with L. paracasei Fn032 on the extracts' characteristics.[Citation13 Citation28] The MW distributions ranged below 180 Da to 5000 Da above (). These findings are in agreement with observations from other studies and support the fact that antioxidative, antimicrobial peptides properties are highly influenced by their molecular mass.[Citation11 Citation28 Citation29]
Table 5 Molecular weight distribution of fermented foxtail millet extracts
CONCLUSION
From the results obtained, it may be inferred that the fermented foxtail millet is a good source of bioactive peptides with signifilcantly higher antioxidant and antimicrobial activities. Thus, solid state fermentation of foxtail millet using L. paracasei Fn032, in combination with protease, not only improves the physiochemical quality but also enhanced the nutritional values of the meal. Remarkable antioxidant activity and protein quality exhibited by all the extracts could be associated with the potential activity of L. paracasei Fn032 during the bioprocessing. Further investigation in this direction may be helpful in the purification and utilization of the specific peptides constituents of the fermented foxtail millet bran or flour extracts as food preservatives.
ACKNOWLEDGMENTS
This research was supported by the National Natural Science Foundation of China (No. 30671525), the National High Technology Research and Development Program (“863” Program) of China (No. 2007, AA10Z325), 111 project-B07029. The authors also wish to express their deep gratitude to Dr. Sun Jin and Dr. Mohamed Beva Kelfala Foh.
REFERENCES
- Prashant , S.H. , Namakkal , S.R. and Chandra , T.S. 2005 . Effect of the antioxidant properties of millet species on oxidative stress and glycemic status in alloxan-induced rats . Nutrition Research , 25 : 1109 – 1120 .
- Xue , Y.Y. , Li , P. and Lin , Q.B. 2008 . Research evolution on chemical component and physical character of foxtail millet . Journal of Chinese Cereals Oils Association , 22 : 51 – 56 .
- En , H. , Pang , Z.H. and Xiong , B.H. 2008 . Comparative analysis of composition and nutritive value of millet bran feed . China Feed , 18 : 39 – 41 .
- Motarjemi , Y. 2002 . Impact of small scale fermentation technology on food safety in developing countries . International Journal of Food Microbiology , 75 ( 3 ) : 213 – 229 .
- Dharmaraj , U. , Ravi , R. and Malleshi , N.G. Physicochemical and textural characteristics of expanded finger millet . International Journal of Food Properties , [First posted on January 19, 2011 (iFirst)] doi: 10.1080/10942912.2010.487626
- Campbell-Platt , G. 1994 . Fermented foods a world perspective . Food Research International , 27 : 253 – 257 .
- Hwang , P.M. and Vogel , H.J. 1998 . Structure-function relationships of antimicrobial peptides . Biochemistry Cell Biology , 76 : 235 – 246 .
- Sookkhee , S. , Chulasiri , M. and Prachyabrued , W. 2001 . Lactic acid bacteria from healthy oral cavity of Thai volunteers: Inhibition of oral pathogens . Journal of Applied Microbiology , 90 : 172 – 179 .
- Kehrer , J.P. 1993 . Free radicals as mediators of tissue injury and disease . Critical Review and Toxicology , 23 : 21 – 48 .
- Amadou , I. , Guo-Wei , L. , Yong-Hui , S. and Sun , J. Reducing . 2011 . radical scavenging, and chelation properties of fermented soy protein meal hydrolysate by Lactobacillus plantarum Lp6 . International Journal of Food Properties , 14 ( 3 ) : 654 – 665 .
- Lee , Y.L. , Yang , J.H. and Mau , J.L. 2008 . Antioxidant properties of water extracts from Monascus fermented soybeans . Food Chemistry , 106 : 1128 – 1137 .
- Carroll , I.M. , Andrus , J.M. , Bruno-Bárcena , J.M. , Klaenhammer , T.R. , Hassan , H.M. and Threadgill , D.S. 2007 . Anti-inflammatory properties of Lactobacillus gasseri expressing manganese superoxide dismutase using the interleukin 10-deficient mouse model of colitis . American Journal of Physiology-Gastrointestinal and Liver Physiology , 293 : G729 – G738 .
- Sun , J. , Hu , X.L. , Le , G.W. and Shi , Y.H. 2010 . Lactobacilli prevent hydroxyl radical production and inhibit Escherichia coli and Enterococcus growth in system mimicking colon fermentation . Letter of Applied Microbiology , 50 : 264 – 269 .
- Kamara , M.T. , Zhu , K. , Amadou , I. , Tarawalie , F. and Zhou , H. Functionality . 2009 . in vitro digestibility and physicochemical properties of two varieties of defatted foxtail millet protein concentrates . International Journal of Molecular Sciences , 10 : 5224 – 5238 .
- Kamara , M.T. , Amadou , I. , Tarawalie , F. and Zhou , H. 2010 . Effect of enzymatic hydrolysis on the functional properties of foxtail millet (Setaria italica L.) proteins . International Journal of Food Science and Technology , 45 : 1175 – 1183 .
- Liang , S. , Yang , G. and Ma , Y. 2010 . Chemical characteristics and fatty acid profile of foxtail millet bran oil . Journal of American Oil Chemist's Society , 87 : 63 – 67 .
- Durojaiye , A.A. , Falade , K.O. and Akingbala , J.O. 2010 . Chemical composition and storage properties of Fura from pearl millet (Pennisetum americanum) . Journal of Food Processing and Preservation , 34 : 820 – 830 .
- Ye , Y.T. , Xue , M. , Lin , S.M. , Wang , Y.H. , Luo , L. and Tian , J.S. 2003 . Enzymolysis kinetics of digestive enzyme from intestine and hepatopancreas in grass carp to four kinds of raw feed materials . Journal of Fishery Science China , 10 ( 2 ) : 470 – 473 . (in Chinese with English abstract).
- Tkachuk , R. 1969 . Nitrogen-to-protein conversion factors for cereals and oilseed meals . Cereal Chemistry , 46 : 419 – 424 .
- Dubois , M. , Gilles , D.A. , Hamilton , J.K. , Rebers , P.A and Smith , F. 1956 . Colorimetric method for the determination of sugars and related substances . Analytical Chemistry , 28 : 350 – 356 .
- Elkhalil , E.A.J. , El-Tinay , A.H. , Mohamed , B.E. and Elshseikh , E.A.E. 2001 . Effect of malt pretreatment on phytic acid and in vitro protein digestibility of sorghum flour . Food Chemistry , 72 : 29 – 32 .
- Adler-Nissen , J. 1986 . Enzymic Hydrolysis of Food Proteins; New York: Elsevier Applied Science Publishers: New York , : 57 – 109 .
- Alsmeyer , R.H. , Cunigham , A.E. and Happieh , M.L. 1974 . Equations predicted (PER) from amino acid analysis . Food Technology , 28 : 34 – 40 .
- FAO . 2007 . Protein and amino acid requirements in human nutrition. Report of a joint WHO/FAO/UNU expert consultation (WHO Technical Report Series, No. 935) Geneva , Switzerland
- Shimada , K. , Fujikawa , K. , Yahara , K. and Nakamura , T. 1992 . Antioxidative properties of xanthan on the antioxidation of soybean oil in cyclodextrin emulsion . Journal of Agricultural and Food Chemistry , 40 : 945 – 948 .
- Wu , H.C. , Chen , H.M. and Shiau , C.Y. 2003 . Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus) . Food Research International , 36 : 949 – 957 .
- Collin , C.H. , Lyne , D.M. and Grange , J.M. 1995 . Microbiological Methods , 7th , 178 Butterworth, Heineman, Oxford Publishers, London .
- Amadou , I. , Le , G.W. , Shi , Y.H. , Gbadamosi , O.S. , Kamara , M.T. and Sun , J. 2011 . Optimized Lactobacillus plantarum Lp6. Solid-state fermentation and proteolytic hydrolysis improve some nutritional attributes of soybean protein meal . Journal of Food Biochemistry , doi: 10.1111/j.1745-4514.2010.00493.x
- Chavan , U.D. , Chavan , J.K. and Kadam , S.S. 1988 . Effect of fermentation on soluble protein and in vitro protein digestibility of sorghum, green gram and sorghum green blends . Journal of Food Science , 53 : 1574 – 1578 .
- Duh , P.D. , Tu , Y.Y. and Yen , G.C. 1999 . Antioxidant activity of water extract of Harng Jyur (Chrysanthemum morifolium Ramat) . LWT–Food Science and Technology , 32 : 269 – 277 .
- Zhu , Y.P. , Cheng , Y.Q. , Wang , L.J. , Fan , J.F. and Li , L.T. 2008 . Enhanced antioxidative activityof Chinese traditionally fermented Okara (Meitauza) prepared with various microorganism . International Journal of Food Properties , 11 ( 3 ) : 519 – 529 .
- Rao , A.S.V.C. , Reddy , S.G. , Babu , P.P. and Reddy , A.R. 2010 . The antioxidant and antiproliferative activities of methanolic extracts from Njavara rice bran . BMC Complementary and Alternative Medicine , 10 ( 4 ) doi: 10.1186/1472-6882-10-4
- Chuang , L.C. , Huang , C.S. , Ou-Yang , L.W. and Lin , S.Y. 2010 . Probiotic Lactobacillus paracasei effect on carciogenic bacterial flora . Clinical Oral Investigations , Published online: May 26 doi: DOI 10.1007/s00784-010-0423-9
- Viswanath , V. , Urooj , A. and Malleshi , N.G. 2009 . Evaluation of antioxidant and antimicrobial properties of finger millet polyphenols (Eleusine coracana) . Food Chemistry , 114 : 340 – 346 .
- Friedman , M. 1996 . Nutritional value of proteins from different food sources . A review. Journal of Agriculture and Food Chemistry , 44 : 6 – 29 .