Abstract
The antimicrobial activities of myristoyl, palmitoyl, or stearoyl hexoses, which were glucose, mannose, and galactose, coexistent with lysozyme against three Gram-positive bacteria, Bacillus coagulans, Bacillus subtilis, and Bacillus licheniformis, were measured in order to investigate the availability of monoacyl hexose as an antimicrobial co-agent. The lysozyme exhibited an antimicrobial activity against Bacillus subtilis and Bacillus licheniformis, but there was no significant difference between the dependencies of the antimicrobial activity against the two bacteria on the lysozyme concentration. However, the antimicrobial activities of the monoacyl hexoses coexistent with the lysozyme were different between those against the two bacteria. The stearoyl hexose coexistent with the lysozyme exhibited the highest antimicrobial activity against two bacteria. It was indicated that the antimicrobial action of the monoacyl hexose would be exerted parallel with the bacterial lysis of lysozyme. Stearoyl hexose, which has a high hydrophobicity, coexistent with the lysozyme could exhibit a higher antimicrobial activity than only the lysozyme.
INTRODUCTION
Acyl saccharides, which are products from the condensation of the mono- or disaccharide with a fatty acid, are biosurfactants with good emulsifying properties,Citation1–Citation3 and are of much interest for use in the food, cosmetics, and pharmaceuticals industries.Citation4 Many researchers have reported the lipase-catalyzed synthesis of the acyl saccharide by both transesterification and condensation reactions.Citation5–Citation8 Compared to the conventional chemical synthesis, the enzymatic synthesis of acyl saccharides using lipase has some benefits; i.e., the direct use of unmodified substrates, moderate reaction conditions, and high regiospecificity of the enzyme. The authors have also synthesized acyl saccharides, especially acyl hexoses, using an immobilized lipase in a batch or continuous reactor, and examined their surfactant properties.Citation9–Citation11 Generally, many surfactants have an antimicrobial ability. Acyl glycerols and sucroses, which are also called sugar esters, are commonly used to prevent or suppress bacterial spore development in canned soft drinks stored at 50 to 70°C in the cold and cool seasons.Citation12, Citation13 The bacteriostatic activities of monoacyl sugar alcohols with different acyl chains and hydrophilic heads have been reported against some thermophilic spore formers.Citation14 However, the antimicrobial activity of the acyl saccharide, except for acyl glycerols and sugar esters, has not been fully studied.
Lysozyme (EC 3.2.1.17) catalyzes the hydrolytic reaction for the β(1–4) bond between N-acetylmuramic acid and N-acetylglucosamine in polysaccharides present in the cell wall of bacteria, and thus is used as an antimicrobial agent due to its bacterial lytic ability.Citation15 In addition, as lysozyme does not affect food taste, it seems to be appropriate to food reservation. Currently, lysozyme was chemically modified as an antimicrobial macromolecule.Citation16 The lytic activity of lysozyme, however, decreases during the heating process when manufacturing foods due to inactivation by heating. Furthermore, the lysozyme itself could simultaneously be decomposed by the metabolism of microorganisms. Therefore, the addition of co-agents would be useful for suppressing the growth of microorganisms by lysozyme in foods.
In this study, the antimicrobial activities of the monoacyl hexose, which was synthesized through the lipase-catalyzed condensation of glucose, mannose, or galactose, with myristic, palmitic, or stearic acid, coexisting with the lysozyme against several Gram-positive bacteria were evaluated in order to investigate the availability of monoacyl hexose as an antimicrobial co-agent. Bacillus coagulans, Bacillus subtilis, and Bacillus licheniformis were tested as representative Gram-positive bacteria in foods.
MATERIALS AND METHODS
Materials
Bacillus coagulans (NBRC3557), Bacillus subtilis (NBRC3007), and Bacillus licheniformis (NBRC12107) were obtained from the National Institute of Technology and Evaluation (Tokyo, Japan). Lysozyme from chicken egg white was purchased from Sigma (St. Louis, MO, USA). Immobilized lipase from Candida antarctica, Chirazyme® L-2 c.-f. C2, for the syntheses of the monoacyl hexoses was obtained from Roche Molecular Biochemicals (Mannheim, Germany). Peptone, yeast extract, agar medium, D-glucose, D-mannose, D-galactose, and caprylic, capric, lauric, myristic, palmitic, and stearic acids were purchased from Wako Pure Chemical Industries (Osaka, Japan). Filter paper discs, with a diameter of 6 mm and a thickness of 0.7 mm, were purchased from Advantec Toyo (Tokyo, Japan). All other chemicals of analytical grade were purchased from either Wako Pure Chemical Industries or Yoneyama Chemical (Osaka, Japan).
Enzymatic Synthesis and Purification of Monoacyl Hexose
The syntheses of the monoacyl hexoses were carried out according to previous procedures.Citation9, Citation11 D-Glucose, D-mannose, or D-galactose (7.5 mmol), and caprylic, capric, lauric, myristic, palmitic, or stearic acid (37.5 mmol) were weighed in a glass bottle equipped with a screw cap. A 500-mg sample of the immobilized lipase and 150 mL of acetonitrile were then added to the bottle. The bottle was tightly sealed and immersed in a water bath at 60°C with vigorous shaking to commence the condensation reaction. After ca. 48 h, each 6-O-acyl hexose (purity >96%) was isolated from the reaction mixture according to reported methods with a slight modification.Citation7 The immobilized lipase was removed by filtration, and the lipase was sufficiently washed by methanol on the filter. After evaporation of the extract in a vacuum, the concentrate was added to n-hexane to remove the remaining free fatty acid. After filtration, liquid-liquid separation was executed by 2-butanone and distilled water. The organic phase was separated, and the excess solvent was removed by vacuum. This separation was repeated three times. Then, the product was recrystallized in n-hexane at 4°C. The powdered solid was dried in a desiccator containing phosphorus pentaoxide for 1 day.
Antimicrobial Activity of Monoacyl Hexose against Bacillus coagulans in Liquid Medium
The liquid medium was prepared as follows: 10 g of peptone, 2 g of yeast extract, and 1 g of magnesium sulfate heptahydrate was added to distilled water, and 1 L of the liquid medium was prepared. The pH of the mixture was adjusted to 7.0 by the addition of 1.0 mol/L sodium hydroxide. The medium was autoclaved at 121°C for 20 min. A freeze-dried type culture of B. coagulans was rehydrated with the medium, and the inoculum was incubated by slowly shaking in a water bath for 18 h under anaerobic conditions at 37°C. The optical density (OD) at 600 nm of the culture was measured using a spectrophotometer (V-520, JASCO Corporation, Tokyo, Japan), and then the culture was diluted by the medium to adjust the initial OD of the next generation culture to about 0.1. After 20 μL of dimethylsulfoxide containing a specific amount of monoacyl hexose was added to 5 mL of the prepared culture, the culture was incubated at 37°C under anaerobic conditions. At appropriate intervals, the culture was sampled, and the OD was measured at 600 nm. The OD of the culture without monoacyl hexose was evaluated as the control (ODcontrol). The ratio of the OD of the culture with monoacyl hexose to ODcontrol was used as an index for the antimicrobial activity. Each measurement of the antimicrobial activity of monoacyl hexose was done in duplicate, and the mean value was calculated.
Antimicrobial Activity of Monoacyl Hexose Coexistent with Lysozyme by Disc Diffusion Test
The antimicrobial activity of monoacyl hexose coexistent with lysozyme using the disc diffusion test was analyzed using the reported methods.Citation17, Citation18 B. subtilis or B. licheniformis was inoculated into 10 mL of the autoclaved liquid medium, whose pH was adjusted to 7.0 by 1.0 mol/L sodium hydroxide, composed of 10 g/L peptone, 5 g/L sodium chloride, 15 g/L agar, 5 g/L meat extract, and 1 g/L magnesium sulfate heptahydrate, then heat-shocked at 80°C for 10 min. One hundred microliters of the medium was placed on an agar plate, and cultivated at 30°C for 15 h. The Gram-positive bacteria were then cultivated in the liquid medium at about 104 to 106 CFU. One milliliter of the culture was added to 30 mL of the agar medium, and solidified at room temperature. Equal weights of the monoacyl hexose to lysozyme were dissolved in dimethylsulfoxide at concentration from 10 mg/L to 1 g/L. The filter paper disc containing 50 μL of the solution was placed on the agar plate. The bacteria were cultivated at 37°C for 24 h, and the diameter of the zone of growth inhibition was measured. Each growth inhibition of monoacyl hexose coexisting with the lysozyme was measured in duplicate, and the mean value was evaluated.
RESULTS AND DISCUSSION
Relationship Between the Antimicrobial Activity of Monoacyl Hexose and the Concentration or Acyl Chain Length
Figure 1 shows the transient changes in the OD of the culture of B. coagulans with myristoyl galactose at various concentrations. The antimicrobial activity of 4 mg/L myristoyl galactose was not observed. However, at a concentration higher than 40 mg/L, myristoyl galactose suppressed the growth of B. coagulans after 11 h. As shown in the inset in , the growth inhibition by myristoyl galactose at 21 h increased with the increasing concentration. shows the growth inhibition of B. coagulans by monoacyl glucose, mannose, and galactose with various acyl chain lengths from 8 to 18 at the concentration of 40 mg/L. The ratio of the OD for the culture with capryloyl, caproyl, lauroyl, or myristoyl glucose to the ODcontrol did not change with time as shown in , indicating that these esters could not inhibit the growth at this concentration. The palmitoyl and stearoyl glucoses, however, exhibited an antimicrobial activity after 15 h. The myristoyl and stearoyl mannoses also inhibited the growth of B. coagulans, though these esters gradually suppressed the growth from the beginning (). Myristoyl galactose slowly inhibited the growth, but the antimicrobial activity was low (). The OD/ODcontrol value for the culture with palmitoyl or stearoyl galactose more rapidly decreased than that with the myristoyl ester. shows the relationship between the OD/ODcontrol value at 24 h and the acyl chain length of monoacyl glucose, mannose, and galactose. Glucose esters with acyl chain lengths from 8 to 12 hardly exhibited the antimicrobial activity. The myristoyl ester exhibited a very low antimicrobial activity for each hexose, whereas the palmitoyl or stearoyl ester more strongly inhibited the growth. Although the inhibition processes were somewhat different among the monoacyl hexoses as shown in , the antimicrobial activities of the monoacyl hexoses with an acyl chain length from 14 to 18 at 24 h were the same.
Figure 1 The growth inhibition of Bacillus coagulans by myristoyl galactose at the concentration of (○) 4, (□) 20, (▵) 40, and (◊) 200 mg/L, and at 37°C. The closed circle represents the control. The inset shows the relationship between the optical density at 21 h and the concentration of myristoyl galactose. The solid curves were empirically drawn.
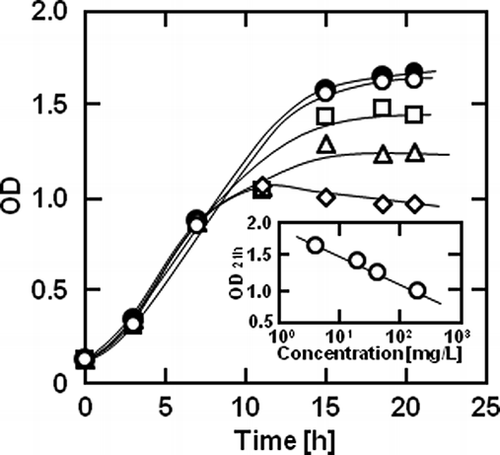
Figure 2 The growth inhibition of Bacillus coagulans by (a) glucose, (b) mannose, and (c) galactose esters condensed with (○) caprylic, (□) capric, (▵) lauric, (•) myristic, (▪) palmitic, or (▴) stearic acid at the concentration of 40 mg/L and 37°C. The solid curves were empirically drawn.
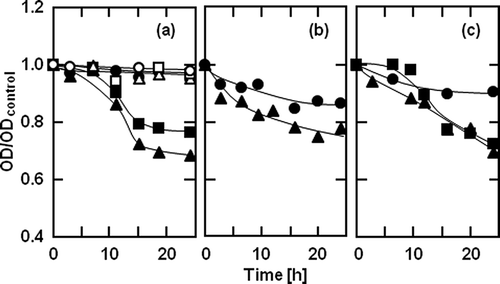
Figure 3 Relationship between the growth inhibition at 24 h and acyl chain length of (○) glucose, (□) mannose, and (▵) galactose esters at the concentration of 40 mg/L and 37°C. The solid curves were empirically drawn.
Many studies about the antimicrobial activity of acyl saccharides have been reported, and most of them involved the acyl sucrose. It was observed that the antimicrobial activity of the monoacyl sucrose was higher than those of the di-, tri-, and tetraacyl sucroses against several mold species from Aspergillus, Penicillium, Cladosporium, and Alternaria.Citation19 For bacteria, the acyl sucrose had an inhibitory effect on the development of spores of Bacillus cereus in the order of lauroyl, palmitoyl, and stearoyl sucrose.Citation20 Ferrer et al. reported that lauroyl sucrose exhibited a high antimicrobial activity against Bacillus sp., whereas it exhibited no activity against Bacillus stearothermophilus.Citation8 It has also been reported that acyl sucrose inhibited the germination and growth of Bacillus coagulans spores, but did not show any antimicrobial activity against Bacillus cereus.Citation21 Furthermore, Tomida and colleagues described that the inhibitory effectiveness of acyl sucrose against Bacillus stearothermophilus was closely related to its hydrophobicity rather than its intrinsic chemical characteristics.Citation22 For the acyl hexose, lauroyl galactose, and fructose, which were mixtures of their positional isomers, the highest growth inhibition was against Streptococcus mutans from among the tested acyl saccharides.Citation23 These reports indicate that the dependency of the antimicrobial activity of the acyl saccharides on the acyl chain length and the saccharide moiety was different between the microorganism species. Results also showed that the antimicrobial activity of the monoacyl hexose depended on its hydrophobicity, though the mechanism of the antimicrobial action of the monoacyl hexose remains unclear. Moriyama and colleagues reported that the antimicrobial activity of acyl sucrose was related to its adsorption on the spore coat proteins of Bacillus cereus.Citation24 On the other hand, it has been postulated that monolauroyl sucrose reorganizes the cellular membrane of Streptococcus mutans by altering its permeability, causing a loss of important metabolites.Citation25 It was also observed that surfactants, such as Triton X-100, cause changes in the cellular morphology of Bacillus subtilis and induce autolysis processes resulting in cell death.Citation26, Citation27 The dependence of the antimicrobial activity of monoacyl hexose on its hydrophobicity would be consistent with the results from its adsorption on bacterial spore-coating proteins.
Antimicrobial Activity of Monoacyl Hexose Coexistent with Lysozyme
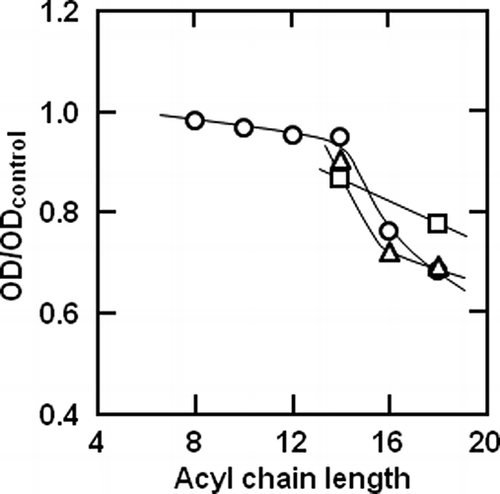
To investigate the availability of monoacyl hexose as a co-agent for the antimicrobial activity, the antimicrobial activity of myristoyl, palmitoyl, or stearoyl hexose coexistent with lysozyme against the two Gram-positive bacteria was measured. and show the effect of the concentrations of monoacyl glucose, mannose or galactose, and lysozyme on the inhibition diameters in the disc diffusion test for B. subtilis and B. licheniformis, respectively. The sole lysozyme exhibited an antimicrobial activity against both Gram-positive bacteria, and there was no significant difference between the dependencies of the antimicrobial activity against the two Gram-positive bacteria on the lysozyme concentration less than 1 g/L of lysozyme. As shown in , the antimicrobial activity of stearoyl hexose coexisting with the lysozyme against B. subtilis was the highest in every hexose ester. Stearoyl hexose coexisting with the lysozyme at 1000 mg/L exhibited a higher antimicrobial activity than the lysozyme alone. The antimicrobial activities of 100 mg/L stearoyl mannose and 10 and 100 mg/L galactose were slightly higher than that of the lysozyme, but each stearoyl hexose hardly suppressed the growth of B. subtilis at 10 mg/L. In addition, the antimicrobial activities of the myristoyl and palmitoyl hexoses coexisting with the lysozyme were lower than that of the lysozyme alone at all the tested concentrations. Therefore, it was found that stearoyl hexose at 1000 mg/L was effective as a co-agent of the lysozyme for the antimicrobial activity against B. subtilis, but the cooperative antimicrobial activity of the monoacyl hexose with thelysozyme against B. subtilis was generally low. It was also shown in that stearoyl hexose coexisting with the lysozyme exhibited the highest antimicrobial activity against B. licheniformis. Similar to the antimicrobial activity against B. subtilis, the activities of the myristoyl and palmitoyl hexoses coexisting with the lysozyme were lower than that of the lysozyme. Compared to the results for B. subtilis, stearoyl glucose and mannose with the lysozyme showed a higher activity against B. licheniformis than the lysozyme at both 10 and 100 mg/L. Stearoyl galactose inhibited the growth of both B. subtilis and B. licheniformis at all the tested concentrations.
Figure 4 Effect of the concentrations of (a) glucose, (b) mannose, and (c) galactose esters with (○) myristic, (□) palmitic, and (▵) stearic acids, and lysozyme on inhibition diameters in disc diffusion test for Bacillus subtilis. Closed square symbols (♦) represent the sole addition of lysozyme. The solid and broken curves were empirically drawn.
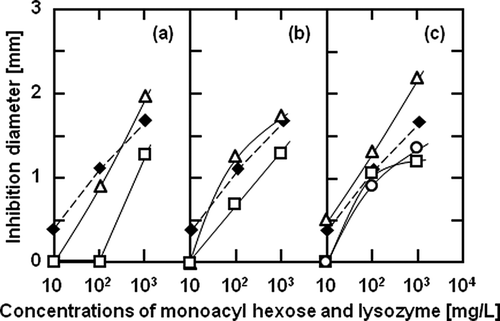
Figure 5 Effect of the concentrations of (a) glucose, (b) mannose, and (c) galactose esters with (○) myristic, (□) palmitic, and (▵) stearic acids, and lysozyme on inhibition diameters in disc diffusion test for Bacillus licheniformis. Closed square symbols (♦) represent the sole addition of lysozyme. The solid and broken curves were empirically drawn.
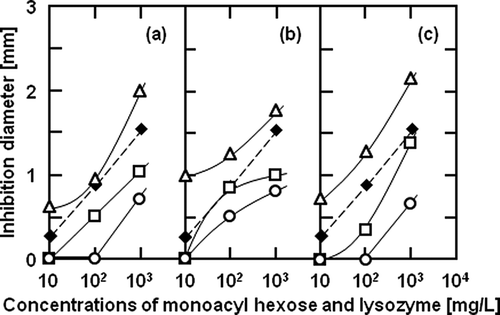
The lysozyme is endo-β-1, 4-N-acetylhexosaminidase and catalyzes the hydrolysis of the β-1, 4-bond between N-acetylglucosamine and N-acetylmuramic acid in the cell wall, resulting in bacterial lysis.Citation28 The reason why the antimicrobial activities of some monoacyl hexoses coexisting with the lysozyme were lower than that of the lysozyme would be due to the inhibition of the lytic action of the lysozyme by the monoacyl hexose. As mentioned above, if the antimicrobial action of the monoacyl hexose is due to its adsorption on spore proteins, this is parallel with the lytic action of lysozyme. Therefore, stearoyl hexose, which had a high hydrophobicity and adsorption ability on spores, coexisting with the lysozyme could exhibit a higher antimicrobial activity than only the lysozyme. The high antimicrobial activity against B. licheniformis by the stearoyl hexose at low concentrations may be due to its high affinity to the spore proteins of B. licheniformis. Stearoyl hexose, especially stearoyl galactose, would be available for the inhibition of some Gram-positive bacteria as an antimicrobial co-agent with the lysozyme.
REFERENCES
- Sarney , D.B. and Vulfson , E.V. 1995 . Application of enzymes to the synthesis of surfactants . Trends in Biotechnology , 13 : 164 – 172 .
- Scheckermann , C. , Schlotterbeck , A. , Schmidt , M. , Wray , V. and Lang , S. 1995 . Enzymatic monoacylation of fructose by two procedures . Enzyme and Microbial Technology , 17 : 157 – 162 .
- Ducret , A. , Giroux , A. , Trani , M. and Lortie , R. 1996 . Characterization of enzymatically prepared biosurfactants . Journal of the American Oil Chemists’ Society , 73 : 109 – 113 .
- Nishikawa , Y. , Yoshimoto , K. , Nishijima , M. , Fukuoka , F. and Ikekawa , T. 1987 . Chemical and biological studies on carbohydrate esters. IX. Antitumor effects of selectively fatty acylated products of maltose . Chemical and Pharmaceutical Bulletin , 29 : 505 – 513 .
- Coulon , D. , Girardin , M. , Rovel , B. and Ghoul , M. 1995 . Comparision of direct esterification and transesterification of fructose by . Candida antartica lipase. Biotechnology Letters , 17 : 183 – 186 .
- Adachi , S. , Nagae , K. and Matsuno , R. 1999 . Lipase-catalysed condensation of erythritol and medium-chain fatty acids in acetonitrile with low water content . Journal of Molecular Catalysis B: Enzymatic , 6 : 21 – 22 .
- Yan , Y. , Bornscheuer , U.T. and Schmid , R.D. 1999 . Lipase-catalyzed synthesis of vitamin C fatty acid esters . Biotechnology Letters , 21 : 1051 – 1054 .
- Ferrer , M. , Soliveri , J. , Plou , F.J. , López-Cortés , N. , Reyes-Duarte , D. , Christensen , M. , Copa-Patino , J.L. and Ballesteros , A. 2005 . Synthesis of sugar esters in solvent mixtures by lipases from Thermomyces lanuginosus and Candida antartica B, and their antimicrobial properties . Enzyme and Microbial Technology , 36 : 391 – 398 .
- Watanabe , Y. , Miyawaki , Y. , Adachi , S. , Nakanishi , K. and Matsuno , R. 2000 . Synthesis of lauroyl saccharides through lipase-catalyzed condensation in microaqueous water-miscible solvents . Journal of Molecular Catalysis B: Enzymatic , 10 : 241 – 247 .
- Watanabe , Y. , Miyawaki , Y. , Adachi , S. , Nakanishi , K. and Matsuno , R. 2001 . Continuous production of acyl mannoses by immobilized lipase using a packed-bed reactor and their surfactant properties . Biochemical Engineering Journal , 8 : 213 – 216 .
- Watanabe , Y. , Miyawaki , Y. , Adachi , S. , Nakanishi , K. and Matsuno , R. 2001 . Equilibrium constant for lipase-catalyzed condensation of mannose and lauric acid in water-miscible organic solvents . Enzyme and Microbial Technology , 29 : 494 – 498 .
- Suwa , N. , Kubota , H. , Takahashi , K. and Machida , H. 1986 . Effects of sucrose fatty acid esters on spoilage of canned coffee caused by thermophilic spore forming bacteria (in Japanese) . Nippon Shokuhin Kogyo Gakkaishi , 33 : 44 – 51 .
- Enda , A. , Ikegami , Y. , Kasetani , M. and Koike , S. 1992 . Antibacterial activity of emulsifier on Clostridium thermohydrosulfuricum (in Japanese) . Kenkyu Houkokusyo-Toyo Shokuhin Kogyo Tanki Daigaku, Toyo Shokuhin Kenkyuusyo , 19 : 167 – 173 .
- Piao , J. , Kawahara-Aoyama , Y. , Inoue , T. and Adachi , S. 2006 . Bacteriostatic activities of monoacyl sugar alcohols against thermophilic sporeformers . Bioscience, Biotechnology, and Biochemistry , 70 : 263 – 265 .
- Onishi , T. 1992 . Properties of naturally occurring antibacterial substances on the food preservation technology (in Japanese) . Seikatsu Eisei , 36 : 179 – 196 .
- Hoq , M.I. , Mitsuno , K. , Tsujino , Y. , Aoki , T. and Ibrahim , H.R. 2008 . Triclosan-lysozyme complex as novel antimicrobial macromolecule: A new potential of lysozyme as phenolic drug-targeting molecule . International Journal of Biological Macromolecules , 42 : 468 – 477 .
- Nostro , A. , Gemanó , M.P , D'Angelo , V. , Marino , A. and Cannatelli , M.A. 2000 . Extraction methods and bioautography for evaluation of medicinal plant antimicrobial activity . Letters in Applied Microbiology , 30 : 379 – 384 .
- Yanagida , T. , Shimizu , N. and Kimura , T. 2005 . Extraction of wax and functional compounds from fresh and dry banana leaves . Japan Journal of Food Engineering , 6 : 79 – 87 .
- Marshall , D.L. and Bullerman , L.B. 1986 . Antimicrobial activity of sucrose fatty acid ester emulsifiers . Journal of Food Science , 51 : 468 – 470 .
- Sugimoto , K. , Tanaka , H. , Moriyama , R. , Nagai , Y. , Ogawa , A. and Makino , S. 1998 . A loss of the antimicrobial activity of sucrose manoesters of fatty acids as caused by esterase released from the germinated spores and vegetative cells of Bacillus cereus . Biocontrol Science , 3 : 17 – 21 .
- Nakayama , M. , Fujimoto , A. , Higuchi , A. , Watanabe , M. , Sadakari , K. , Iio , M. and Miyamoto , T. 2003 . Studies on growth characteristics of Bacillus strains isolated from a liquid seasoning (in Japanese) . Nippon Shokuhin Kagaku Kogaku Kaishi , 50 : 537 – 545 .
- Tomida , M. , Suwa , N. , Machida , H. , Nishimura , A. and Makino , S. 1991 . Inhibition of germination of Bacillus stearothermophilus spores by sucrose monoalkylates and other surfactants . Nippon Shokuhin Kogyo Gakkaishi , 38 : 1044 – 1049 .
- Watanabe , T. , Katayama , S. , Matsubara , M. , Honda , Y. and Kuwahara , M. 2000 . Antibacterial carbohydrate monoesters suppressing cell growth of Streptococcus mutans in the presence of sucrose . Current Microbiology , 41 : 210 – 213 .
- Moriyama , R. , Sugimoto , K. , Miyata , S. , Katsuragi , T. and Makino , S. 1996 . Antimicrobial action of sucrose esters of fatty acids on bacterial spores . Journal of Antibacterial and Antifungal Agents , 24 : 3 – 8 .
- Iwami , Y. , Schachtele , C.F. and Yamada , T. 1995 . Effect of sucrose monolaurate on acid production, levels of glycolytic intermediates, and enzyme activities of Streptococcus mutans NCTC 10449 . Journal of Dental Research , 74 : 1613 – 1617 .
- Cho , H.Y. , Tsuchido , T. , Ono , H. and Takano , M. 1990 . Cell death of Bacillus subtilis caused by surfactants at low concentrations results from induced cell autolysis . Journal of Fermentation Bioengineering , 70 : 11 – 14 .
- Tsuchido , T. , Svarachorn , A. , Soga , H. and Takano , M. 1990 . Lysis and aberrant morphology of Bacillus subtilis cells caused by surfactants and their relation to autolysin activity . Antimicrobial Agents and Chemotherapy , 34 : 781 – 785 .
- Salton , M.R.J. 1952 . Cell wall of Micrococcus lysodeikticus as the substrate of lysozyme . Nature , 170 : 746 – 747 .