Abstract
Nutritional composition, total phenolic content, total flavonoid, antioxidant capacity, and antioxidant vitamins of bilimbi (Averrhoa bilimbi) and carambola (Averrhoa carambola) were determined and compared in this study. Bilimbi was found to contain higher moisture, ash, carbohydrate, protein, fat, and dietary fiber compared to carambola. Total phenolic content was higher in carambola although bilimbi yielded more total flavonoid. Vitamins A, C, and E contents of bilimbi were also higher than carambola. Antioxidant and scavenging activity as determined by β-carotene bleaching assay and DPPH radical scavenging assay of carambola were significantly (p < 0.05) higher than bilimbi. These results suggested that carambola was a potent natural antioxidant food and that contribution of phenolic compounds to its antioxidant capacity was greater than that of antioxidant vitamins.
INTRODUCTION
Lately, much attention has been focused on the positive effects of antioxidants in numerous degenerative diseases, such as cancer, cardiovascular disease, aging, and cataracts.[Citation1] Noting the abundant beneficial effects of antioxidants to human health, various research and epidemiology studies have been done. Fruits, in particular, are best known to be rich sources of antioxidants, such as vitamin A, vitamin C, vitamin E, polyphenolic compounds, and flavonoids.[Citation2] Hence, risk of developing chronic diseases could be minimized with the intake of natural dietary antioxidants from fruits.
Bilimbi (Averrhoa bilimbi) and carambola (Averrhoa carambola) are among the underutilized fruits commonly consumed and widely cultivated in Malaysia. They belong to the family Oxalidaceae. Bilimbi is commonly known as belimbing buluh or belimbing asam among the locals. Traditionally, bilimbi has been widely used as a medicine for the treatment of cough, cold, itches, boils, rheumatism, syphilis, diabetes, whooping cough, and hypertension.[Citation3] On the other hand, carambola is popularly known as belimbing besi or belimbing manis among the locals. It is distinguishable from bilimbi through its attractive star shape. Its juice is one of the popular beverages used as thirst-quenchers. Carambola was reported to be a good source of antioxidants[Citation4] and very high in phenolics.[Citation5]
Antioxidants play important roles in neutralizing free radicals, quenching singlet and triplet oxygen, decomposing peroxides,[Citation6] donating hydrogen, and chelating metal ion.[Citation7] These protective properties enable antioxidants to decrease DNA damage, reduce lipid peroxidation, and inhibit malignant transformation or cell proliferation.[Citation8]
In view of the importance of naturally occurring antioxidants to human health and the scarcity of data for antioxidant content in these fruits, this study was undertaken. This study focuses on the determination of nutritional composition, total phenolic, total flavonoid, antioxidant capacity, and antioxidant vitamins (vitamins A, C, and E) of these locally consumed fruits. Comparison between these two varieties as well as a correlation test was also carried out.
MATERIALS AND METHODS
Reagents and Chemicals
All chemicals and reagents used for proximate and spectrophotometric analysis were of analytical grade; absolute ethanol, Folin-Ciocalteu reagent, and sodium carbonate were purchased from Merck (Darmstadt, Germany); chloroform and aluminium chloride hexahydrate were from Fischer Scientific (Loughborough, UK); total dietary fiber assay kit, gallic acid, aluminium chloride hexahydrate (AlCl3.6H20), rutin, β-carotene, linoleic acid, Tween 20, butylated hydroxytoulene (BHT), ascorbic acid, and 2,2-diphenyl-2-picrylhydrazyl (DPPH) were from Sigma Chemical Co. (St. Louis, MO, USA). All chemicals and reagents used for HPLC analysis were from Merck (Darmstadt, Germany) and were HPLC grade; tetrahydrofuran, methanol, petroleum ether, acatonitrile, dichloromethane, chloroform, hexane and isopropanol except BHT, ammonium acetate, metaphospheric acid, potassium dihydrogen phosphate, sodium sulphate, and potassium hydroxide were from Sigma Chemical Co. (St. Louis, MO, USA).
Sample Preparation
Fresh bilimbi and carambola were purchased from Pasar Tani FAMA, Serdang, Selangor, Malaysia. Following purchase, samples were cleaned and washed with excess pipe water and pointed ends were removed. Edible portions (100 g) of the fruit were cut into small pieces and homogenized using a blender for 2 min. Samples were then put in a freezer at –80°C overnight and were freeze-dried for 3 days. Then, samples were ground using a dry grinder and fine powders were obtained using a fine mesh sieve. These were stored at –20°C before proximate analyses and extraction.
Proximate Analyses
Fine powders were analyzed for nutritional composition by the standard AOAC[Citation9] method for moisture and ash contents. The Clegg Anthrone method[Citation10] was used to determine total available carbohydrates. Total protein was estimated by the Kjeldahl method,[Citation11] while total fat was determined using the Soxhlet method.[Citation11] Total dietary fiber was analyzed using a total dietary fiber assay kit (Sigma Chemical Co., St. Louis, MO, USA).
Sample Extraction
Fine powders (2 g) were transferred into a 50-ml volumetric flask and 70% ethanol was added up to the mark. The mixture was shaken using a shaking incubator at 200 rpm for 120 min at 50°C. The mixture was then centrifuged at 3000 rpm for 15 min at room temperature and the supernatant saved was stored at –20°C. This supernatant was used for total phenolic content, total flavanoid, β-carotene bleaching, and DPPH radical scavenging assays. The absorbance was measured by UV-Vis spectrophotometer (SECOMAM CE, France) using 70% ethanol as the blank. Triplicate measurements were carried out and all tests were performed within a week.
Determination of Total Phenolic Content (TPC)
Determination of total phenolic content was carried out using a method as described by Djeridane et al.[Citation6] with slight modifications. Briefly, 100 μl of supernatant extracts were added in 1500 μl (1/10 dilution) of Folin–Ciocalteu reagent. The solution was mixed and incubated at room temperature for 1 min. After 1 min, 1500 μl of 60 g/L sodium carbonate (Na2CO3) solution was added. The mixture was then shaken and incubated for 90 min in the dark at room temperature. Finally, absorbance was measured at 725 nm. Phenolic content of the extracts was compared against a gallic acid standard calibration curve, which was plotted at 0.02, 0.04, 0.06, 0.08, and 0.10 mg/ml. Total phenolic content was expressed as milligram of gallic acid equivalents (GAE) per 100 g of dry weight of fruit.
Determination of Total Flavonoid (TF)
Determination of TF was carried out using a method as described by Quettier-Deleu et al.[Citation12] with slight modifications. Briefly, 1 ml of 70% ethanolic extract solution was added to 1 ml of 2% ethanolic aluminium chloride hexahydrate (AlCl3.6H2O). The mixture was incubated for 10 min at room temperature. Then, absorbance was measured at 430 nm. Flavonoid content of the extracts was compared against the rutin standard calibration curve, which was plotted at 0.02, 0.04, 0.06, 0.08, and 0.10 mg/ml. Total flavonoid was expressed as milligram of rutin per 100 g of dry weight of fruit.
Determination of Antioxidant Capacity
β-Carotene bleaching activity
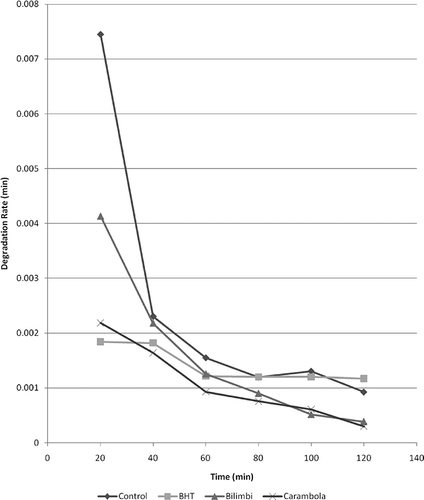
Determination of antioxidant activity by β-carotene bleaching method was carried out using a method as described by Velioglu et al.[Citation13] with slight modifications. β-Carotene solution (1 ml of 0.2 mg/ml in chloroform) was added to a 50-ml round-bottom flask containing 20 μl of linoleic acid and 200 μl of Tween 20. Each mixture was then added with 200 μl of sample extract. For the control, the extract was replaced by 200 μl of 70% ethanol, whereas for the standard, extract was replaced by 200 μl 40 g/l BHT. After evaporation to dryness under a vacuum at 40°C, 100 ml of distilled water was added and the mixture was shaken. The samples were then placed in water bath at 45°C. The absorbance of the solution at 470 nm was monitored by taking measurements at 20 min intervals for 120 min in a dark room to avoid ß-carotene oxidation. Antioxidant activity (AOX) was calculated as percent inhibition relative to control using the following equation:
DPPH Radical Scavenging Activity
Determination of antioxidant activity by the DPPH radical scavenging method was carried out using a method as described by Lim et al.[Citation14] with slight modifications. Sample extracts (200 μl) in various concentrations (1, 2, 4, 8 mg/ml in 70% ethanol) or ascorbic acid (standard) (10, 20, 40, 80 μg/ml) were mixed with 1 ml of 100 μM DPPH previously prepared in 70% ethanol. The mixture was shaken vigorously and left to stand for 30 min at room temperature in a dark room. Absorbance was read at 517 nm. The scavenging effect on the DPPH radical was calculated using the following equation:
Determination of Antioxidant Vitamins
Extraction of vitamin A (β-carotene)
The method of Hart and Scott[Citation15] for the analysis of vitamin A was used. Fine powders (100 μg) were mixed with 50 ml tetrahydrofuran–methanol (1:1) and homogenized for 1 min. The extract was filtered through Whatman No. 1 filter paper and filtrates were transferred to a separating funnel. Petroleum ether (50 ml of 40–60°C fraction containing 0.1% BHT) and 50 ml of 10% sodium chloride solution were added and mixed. The lower THF/MeOH/aqueous phase was drawn off and the upper petroleum ether phase was transferred to a 250-ml evaporating flask. The THF/MeOH/aqueous phase was extracted two more times with 50-ml aliquots of petroleum ether. The petroleum ether phases were combined in the flask and evaporated at 35°C in a rotary evaporator to dryness. The residue was redissolved to a final volume of 2 ml in HPLC-grade dichloromethane.
Extraction of vitamin C
The method of Abushita et al.[Citation16] for the analysis of vitamin C was used. Fine powders (1 g) were mixed with 50 ml of 2% metaphospheric acid and transferred to a conical flask. The mixture was mechanically shaken with orbital shaker for 15 min and then filtered through a Whatman No. 1 filter paper to obtain clear extracts.
Extraction of vitamin E (α-tocopherol)
The method of Abushita et al.[Citation16] for the analysis of vitamin E was used. Fine powders (500 μg) were mixed with 60 ml of chloroform (CCl4)–methanol (3:1) and mechanically shaken for 20 min. The CCl4 fraction was separated from the aqueous phase in a separatory funnel and dried over anhydrous Na2SO4. The filtrate was evaporated to dryness using a rotary evaporator at 40°C. The extracted lipid fraction was refluxed with 4 ml of 30% methanolic KOH in a water bath for 30 min at the boiling point of methanol in the presence of 0.5 g ascorbic acid. After cooling the flask, 15 ml of salted water were added and extracted twice with 40 ml of petroleum ether in a separatory funnel. Ether fractions were collected, washed twice with distilled water, and dried over anhydrous Na2SO4. The solvent was evaporated using a rotary evaporator at 30°C and the residues were redissolved in 2 ml of HPLC-grade hexane.
HPLC Analysis
A HPLC system (Agilent 1100, Palo Alto, CA, USA) with diode array detector (DAD) was used. The column system consisted of a reversed-phase C18 column (Nova-Pak, 150 mm × 4 mm, 5 μm; Waters, Milford, MA, USA). All reagents were from Merck (Darmstadt, Germany) and were HPLC grade except BHT, ammonium acetate, potassium dihydrogen phosphate, sodium sulphate, and potassium hydroxide from Sigma Chemical Co. (St. Louis, MO, USA). The prepared mobile phase was degassed using ultrasonic agitation and filtered under a vacuum through a 0.45-μm nylon membrane filter before analysis. Separation conditions for the antioxidant vitamins are listed in .
Table 1 Conditions for HPLC separation of antioxidant vitamins
Statistical Analysis
Data are presented as mean ± standard deviation. Statistical analysis was done using SPSS (SPSS Inc., Chicago, IL, USA) version 16.0 for Windows. An independent-samples t-test was used to test for significant difference and Pearson's correlation coefficient was used to determine correlation among tests. The significant values were considered at the level of p < 0.05.
RESULTS AND DISCUSSION
Nutritional Composition
In this study, both bilimbi and carambola were freeze-dried and proximate analyses as well as antioxidant assays were carried out using freeze-dried samples. Ruzainah et al.[Citation17] claimed that high moisture content could diminish fruit quality and its shelf-life. Therefore, samples in the present study were freeze-dried in order to control microbial activity. Apart from that, both samples are a type of seasonal fruit; thus, fresh fruits are not always available for analysis.
Table 2 Nutritional composition of bilimbi and carambola.Footnote a
Besides, to the best of our knowledge, there has been no study done on proximate analyses of bilimbi and carambola based on their freeze-dried forms. Hence, the present study was done in order to provide more insight on the proximate composition of these freeze-dried fruit powders. For proximate analyses, both samples were analyzed for moisture, ash, carbohydrate, protein, fat, and total dietary fiber. Results are presented in .
In the present study, moisture content of bilimbi (16.84 ± 0.36%) was not significantly different (p > 0.05) from carambola (12.04 ± 0.05%). Although there have been reports suggesting its limitation,[Citation18 Citation19] this method remains commonly utilized due to its time- and cost-efficiency. Ash content of bilimbi (4.62 ± 0.01%) was slightly higher than carambola (3.87 ± 0.01%). However, the result of statistical analysis revealed that there was no significant difference (p > 0.05) in the ash content of both samples. This implied that mineral content of bilimbi was comparable to carambola as total mineral content of food is indicated by its ash content.[Citation20]
Similarly, total available carbohydrate of bilimbi (32.02 ± 3.15%) was not significantly different (p > 0.05) from carambola (24.22 ± 2.91%). Meanwhile, Tee et al.[Citation21] reported that carbohydrate content was 2.8% in bilimbi and 5.0% in carambola in the Malaysian Food Composition Database. For both samples, carbohydrate content was reported to be higher in the present study as compared to the study by Tee et al.[Citation21] This could be due to the utilization of a difference method in both studies, whereby Tee et al.[Citation21] estimated carbohydrate content using a difference method by subtracting other components (moisture, ash, protein, fat, and dietary fiber) from 100. Drawbacks related to the difference method were highlighted by Cummings and Stephen.[Citation22] In their review, they stated that total carbohydrate calculated with the difference method includes noncarbohydrate constituents, such as organic acids, tannins, lignin, and waxes. Therefore, this might contribute to errors.
Table 3 Total phenolic content, total flavonoid, antioxidant capacity, and antioxidant vitamins of bilimbi and carambola.Footnote a
Protein content of bilimbi (11.50 ± 0.36%) was also not significantly different (p > 0.05) from carambola (7.88 ± 0.18%). Although Kjeldahl method has its limitations, it is still used universally because of the high precision and reproducibility. A major drawback of the method is that it does not measure true protein content of a sample. This is because non-protein compounds containing nitrogen will be measured as well.[Citation23] Meanwhile, fat content of bilimbi (3.38 ± 2.43%) was significantly higher (p < 0.05) than carambola (1.36 ± 0.03%). The Soxhlet method offers few advantages, such as requiring no filtration and the sample is continuously extracted with fresh portions of solvent, thus elevating motion of analyte from the matrix.[Citation24] Likewise, bilimbi (15.31 ± 2.40%) contained significantly higher (p < 0.05) total dietary fiber as compared to carambola (7.89 ± 0.92%). These results indicated that soluble and insoluble fiber of bilimbi was higher than that of carambola. Overall, in all analyses, bilimbi exhibited higher nutritional composition as compared to carambola.
Total Phenolic Content (TPC)
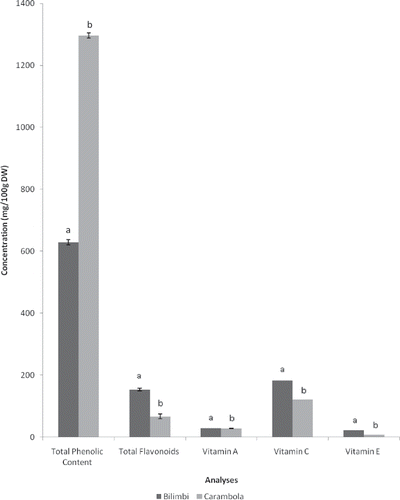
Folin-Ciocalteu assay is based on the redox reaction between Folin-Ciocalteu reagent with all or most phenolics in the sample. TPC values summarized in are quantified based on the linear equation obtained from a gallic acid standard calibration curve, which was determined to be y = 4.7072x + 0.0979 (R 2 = 0.9964) where y is the absorbance at 725 nm and x is the concentration of gallic acid. Thus, TPC values are expressed as gallic acid equivalent (mg GAE/100 g samples) and are presented in . Statistical analysis indicated that TPC of bilimbi (629.17 ± 14.38 mg GAE/100 g DW) was significantly lower (p < 0.05) and approximately half of carambola (1296.25 ± 14.74 mg GAE/100 g DW). Therefore, these suggested that total reducing activity of carambola were significantly higher than bilimbi. Besides, reducing power of carambola was 30 times greater than that of freeze-dried tomatoes as reported by Chang et al.[Citation25]
Figure 1 Total phenolic content, total flavonoid, vitamin A, vitamin C, and vitamin E contents of bilimbi and carambola. Different letters in the same column were significantly different at the level of p < 0.05.
Djeridane et al.[Citation6] reported that non-phenolic substances, such as water, fat, sugars, proteins, and pigments, might interfere with the evaluation of TPC. Therefore, samples in this study were lyophilized to remove water in an attempt to reduce interruption. This was supported by a finding from Chang et al.[Citation25] whereby they reported that there was a significant increase of TPC in tomatoes upon lyophilization as compared with its fresh forms. However, it is important to note that this method has its limitations. Apart from phenolic compounds, reducing agents, such as ascorbic acid, might be involved in the redox reaction with Folin-Ciocalteu reagent too, therefore, this could contribute to an overestimation of TPC values.[Citation26]
Total Flavonoid (TF)
Quantitative determination of TF is performed using the aluminum chloride colorimetric method and is expressed in terms of rutin equivalent. The TF values summarized in are quantified based on the linear equation obtained from rutin standard calibration curve, which was determined to be y = 7.854x + 0.0452 (R 2 = 0.9975) where y is the absorbance at 430 nm and x is the concentration of rutin. Thus, TF values are expressed as rutin equivalent (mg rutin/100 g DW) and are presented in .
Statistical analysis indicated that TF of bilimbi (153.38 ± 8.02 mg rutin/100 g DW) was significantly higher (p < 0.05) than carambola (66.64 ± 13.41 mg rutin/100 g DW). However, as discussed earlier, TPC of bilimbi was significantly lower (p < 0.05) than carambola. These results suggested that high TPC does not necessarily exhibit high TF and vice versa. It was in agreement with a study by Miliauskas et al.[Citation27] whereby they demonstrated that there was only low correlation (r = 0.43) between total phenolic content and total flavonoid in the studied sample. However, this method is only specific for flavones and flavonols as shown in a study by Chang et al.[Citation28] They reported that the real content of total flavonoid must be the sum of flavonoid contents determined by the aluminum chloride method and by the 2,4-dinitrophenylhydrazine method that is specific for flavanones. In comparison, freeze-dried tomatoes, as reported by Chang et al.,[Citation25] showed that total flavonoid was approximately 20 times lower than bilimbi and 8 times lower than carambola.
Antioxidant Capacity
β-Carotene bleaching activity (AOX)
β-Carotene bleaching assay is based on the ability of antioxidant in sample extracts in neutralizing free radicals. In this study, butylated hydroxytoluene (BHT) was used as a standard whereas 70% ethanol, which contained no antioxidant components, was used as a control for comparison with samples extracts.
The antioxidant values summarized in are quantified as percent inhibition relative to control. Statistical analysis indicated that antioxidant activity of bilimbi (28.41 ± 5.31%) was significantly (p < 0.05) lower than carambola (47.73 ± 5.54%). Both extracts had a lower antioxidant activity than BHT (). These showed that carambola was more capable of retarding β-carotene bleaching by free radicals generated during peroxidation of linoleic acid. To validate the antioxidant capacity of these sample extracts, a second assay was performed. Generally, oxidation is initiated by free radical attack. Therefore, assays to evaluate the radical scavenging activity are representative of the capability of an extract to suppress oxidation. Thus, DPPH radical scavenging assay was chosen.
DPPH radical scavenging activity (IC50)
This assay measures the capability of an extract in donating an electron to reduce DPPH free radicals, thereby bleaching its typical purple color. An extract with the highest IC50 value exhibits the lowest scavenging activity and vice versa. In this study, ascorbic acid was used as a standard at various concentrations (10, 20, 40, and 80 μg/ml). Likewise, sample extracts were prepared at various concentrations of 1, 2, 4, and 8 mg/ml in 70% ethanol.
Statistical analysis indicated that IC50 of bilimbi (6.93 ± 0.25 mg/ml) was significantly higher than carambola (1.88 ± 0.62 mg/ml). However, as mentioned earlier, extract with the highest IC50 value exhibited the lowest scavenging activity. Therefore, carambola was a better radical scavenger as compared to bilimbi. It was in agreement with a study by Lim et al.,[Citation14] whereby they reported that carambola was a very potent radical scavenger. Likewise, Leong and Shui[Citation29] classified carambola as high in antioxidant capacity based on its ability to scavenge DPPH free radicals.
Antioxidant Vitamins
Vitamin A (β-carotene)
Determination of vitamin A was performed using the HPLC method. Vitamin A values summarized in are quantified based on the linear equation obtained from the β-carotene standard calibration curve, which was determined to be y = 9.6944x – 157.59 (R 2 = 0.9966) where y is area (mAU*s) and x is the concentration of β-carotene. Vitamin A contents are expressed in mg/100 g DW and are presented in .
Statistical analysis revealed that vitamin A content of bilimbi (28.99 ± 0.12 mg/100 g DW) was significantly higher (p < 0.05) than carambola (27.61 ± 0.90 mg/100 g DW). This could be due to fat content in bilimbi that was significantly higher than carambola, as vitamin A is a fat soluble vitamin. On the other hand, previous reports[Citation8 Citation30 Citation31] showed far much lower vitamin A content in carambola as compared to the present study. A few factors could have contributed to the varying levels of vitamin A in these studies. For instance, heat and light play a role in the formation of free radical and oxidation of carotenoids.[Citation32] Apart from that, storage at low temperatures might also cause the loss of carotenoids compounds due to oxidative degradation.[Citation33]
Vitamin C
Determination of vitamin C was performed using the HPLC method. Vitamin C values summarized in are quantified based on the linear equation obtained from the ascorbic acid standard calibration curve, which was determined to be y = 54.319x + 9.5507 (R 2 = 0.9953), where y is area (mAU*s) and x is the concentration of ascorbic acid. Vitamin C contents are expressed in mg/100 g DW and are presented in .
Statistical analysis revealed that vitamin C content of bilimbi (182.98 ± 0.42 mg/100 g DW) was significantly higher (p < 0.05) than carambola (120.74 ± 0.46 mg/100 g DW). In contrast, vitamin C content of bilimbi as reported in the Malaysian Food Composition Database[Citation21] was lower than carambola. This could be attributed to the agronomic conditions and varieties in cultivar. In addition, environmental factors, such as climate, soils, and light exposure, play important roles in influencing vitamin C content. They might have loss during sample preparation or extraction as vitamin C is very susceptible to light, heat, and air. In addition, a variation could be due to the different stages of maturity or the effect of ripeness in fruits.[Citation34]
Vitamin E (α-tocopherol)
Determination of vitamin E was performed using the HPLC method. Vitamin E values summarized in are quantified based on the linear equation obtained from α-tocopherol standard calibration curve, which was determined to be y = 20.32x – 15.936 (R 2 = 0.9954), where y is area (mAU*s) and x is the concentration of α-tocopherol. Vitamin E contents are expressed in mg/100 g DW and are presented in .
Statistical analysis revealed that vitamin E content of bilimbi (21.48 ± 0.04 mg/100 g DW) was significantly higher (p < 0.05) than carambola (7.34 ± 0.01 mg/100 g DW). Similar to vitamin A, vitamin E is also a fat soluble vitamin. Therefore, it was observed that vitamin E content of bilimbi, which contained higher fat, was higher than carambola. In contrast, vitamin E content in bilimbi was reported to be much lower in a study by Ling and Mohamed[Citation35] as compared to the present study. This could be due to the different drying methods whereby Ling and Mohamed[Citation35] utilized an oven-drying method, while the present study utilized a freeze-drying method. An oven-drying process could have an impact on vitamin E content because the vitamin is easily oxidized and destroyed during processing with high temperatures. Moreover, differences in analytical methods, postharvest handling, maturity, growing practices, and cultivars could have contributed to the variations of vitamin E content in both studies.[Citation36]
Correlations Between TPC, TF, Antioxidant Capacity, and Antioxidant Vitamins
Pearson's correlation coefficient was used to determine a correlation between TPC, TF, antioxidant capacity (β-carotene bleaching activity and DPPH radical scavenging activity), and antioxidant vitamins (vitamins A, C, and E). Results are presented in . Statistical analysis revealed that there was a positive strong correlation between AOX and TPC (r = 0.971, p < 0.01). In another way, AOX is correlated negatively strong with IC50 (r = −0.898, p < 0.01) and vitamin A (r = −0.809, p < 0.01). However, there was only negative moderate correlation between AOX and vitamin C (r = −0.714, p < 0.05). Also, no correlation was found between AOX with TF (r = 0.508) and vitamin E (r = −0.522).
Table 4 Correlation between TPC, TF, antioxidant capacity, and antioxidant vitamins.a
In contrast, IC50 was correlated positively strong with vitamin A (r = 0.847, p < 0.01) but negatively strong with TPC (r = −0.905, p < 0.01). There was a positive moderate correlation between IC50 and vitamin C (r = 0.685, p < 0.05) and negative moderate correlation with TF (r = −0.584, p < 0.05). However, there was no correlation between IC50 and vitamin E (r = 0.443). Hence, results from the correlation test suggested that samples with high total phenolic content exhibited high antioxidant activity. In other words, they were capable of neutralizing free radicals. It was also well documented in previous studies that antioxidant activity of foods was positively related to polyphenol content.[Citation37 Citation38] Conversely, high total phenolic content was found to be inversely related to IC50. This implied that samples with high phenolic content were excellent radical scavengers and possessed high hydrogen atom donating abilities. It was consistent with the results reported previously[Citation39] that higher total phenolic content yielded higher scavenging activity.
Contradictory, vitamin A was found to have conflicting antioxidant potential as compared with phenolic compounds. Correlation tests revealed that samples with high vitamin A did not possess high antioxidant activity. In addition, they were not a potent radical scavenger. Likewise, no correlation was found between vitamin E and antioxidant activity as well as its IC50 value. This explained that regardless of its concentration, vitamin E was unable to neutralize free radicals or act as a hydrogen donor. Interestingly, vitamin C was found to be inversely correlated with antioxidant activity and scavenging activity. It was evidenced in a study by Sharique and Seerat[Citation40] whereby they demonstrated that vitamin C and antioxidants were not statistically significant and poorly correlated. Likewise, Kalt et al.[Citation41] showed that antioxidant capacity and ascorbate content of strawberry, raspberry, and high- and lowbush blueberry were negatively correlated.
Correlation between antioxidant activity and scavenging activity was also demonstrated. Results revealed that samples with high antioxidant activity were also strong radical scavengers. Eventually, this study showed that contribution of phenolic compounds to antioxidants was much greater than those of antioxidant vitamins. This was supported by recent studies that reported that phenolic compounds are stronger antioxidants than antioxidant vitamins.[Citation42]
CONCLUSION
Moisture, ash, carbohydrate, and protein content of bilimbi were comparable to carambola, while fat and total dietary fiber of bilimbi were significantly higher than carambola. High antioxidant and scavenging activity were observed in carambola as compared to bilimbi, although bilimbi contained significantly higher antioxidant vitamins. The free radicals neutralizing and scavenging effect of carambola was attributed to its high total phenolic content. Thus, carambola is a potent natural antioxidant food and contribution of phenolic compounds to its antioxidant capacity is greater than that of antioxidant vitamins.
ACKNOWLEDGMENT
The authors would like to acknowledge the assistance rendered by the laboratory staff from the Department of Nutrition and Dietetics, Universiti Putra Malaysia and also Fundamental Research Grant Scheme (Vote No. 5523622) for providing financial support throughout the completion of this study.
REFERENCES
- Arcan , I. and Yemenicioglu , A. 2009 . Antioxidant activity and phenolic content of fresh and dry nuts with or without the seed coat . Journal of Food Composition and Analysis , 22 : 184 – 188 .
- Diplock , A.T. , Charuleux , J.L. , Crozier-Willi , G. , Kok , F.J. , Rice-Evans , C. , Roberfroid , M. , Stahl , W. and Vina-Ribes , J. 1998 . Functional food science and defence against reactive oxidative species . British Journal of Nutrition , 80 : S77 – S112 .
- Goh , S.H. , Chuah , C.H. , Mok , J.S.L. and Soepadmo , E. 1995 . Malaysian Medicinal Plants for the Treatment of Cardiovascular Diseases , Malaysia : Pelanduk .
- Shui , G. and Leong , L.P. 2004 . Analysis of polyphenolic antioxidants in star fruit using liquid chromatography and mass spectrometry . Journal of Chromatography A , 1022 : 67 – 75 .
- Mahattanatawee , K. , Manthey , J.A. , Luzio , G. , Talcott , S.T. , Goodner , K. and Baldwin , E.A. 2006 . Total antioxidant activity and fiber content of select Florida-grown tropical fruits . Journal of Agricultural and Food Chemistry , 54 : 7355 – 7363 .
- Djeridane , A. , Yousfi , M. , Nadjemi , B. , Boutassouna , D. , Stocker , P. and Vidal , N. 2006 . Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds . Food Chemistry , 97 : 654 – 660 .
- Ikram , E.H.K. , Eng , K.H. , Jalil , A.M.M. , Ismail , A. , Idris , S. , Azlan , A. , Nazri , H.S.M. , Diton , N.A.M. and Mokhtar , R.A.M. 2009 . “ Antioxidant capacity and total phenolic content of Malaysian underutilized fruits ” . In Journal of Food Composition and Analysis 388 – 393 . 22
- Charoensiri , R. , Kongkachuichai , R. , Suknicom , S. and Sungpuag , P. Beta-carotene . 2009 . lycopene, and alpha-tocopherol contents of selected Thai fruits . Food Chemistry , 113 : 202 – 207 .
- AOAC . 1990 . Official Methods of Analysis , MD : 16th ed.; AOAC International: Gaithersburg .
- Clegg , K.M. 1955 . The application of the anthrone reagent to the estimation of starch in cereals . Journal of the Science of Food and Agriculture , 7 : 40 – 44 .
- Tee , E.S. , Rajam , K. , Young , S.I. , Khor , S.C. and Zakiyah , H.O. 1996 . “ Division of Human Nutrition ” . In Laboratory Procedures in Nutrient Analysis of Foods , 4 – 10 . Kuala Lumpur : Institute for the Medical Research .
- Quettier-Deleu , C. , Gressier , B. , Vasseur , J. , Dine , T. , Brunet , C. , Luyckx , M. , Cazin , M. , Cazin , J-C. , Bailleul , F. and Trotin , F. 2000 . Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour . Journal of Ethnopharmacology , 72 : 35 – 42 .
- Velioglu , Y.S. , Mazza , G. , Gao , L. and Oomah , B.D. 1998 . Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products . Journal of Agricultural and Food Chemistry , 46 : 4113 – 4117 .
- Lim , Y.Y. , Lim , T.T. and Tee , J.J. 2007 . Antioxidant properties of several tropical fruits: A comparative study . Food Chemistry , 103 : 1003 – 1008 .
- Hart , D.J. and Scott , K.J. 1995 . Development and evaluation of an HPLC method for the analysis of carotenoids in foods, and the measurement of the carotenoid content of vegetables and fruits commonly consumed in the UK . Food Chemistry , 54 : 101 – 111 .
- Abushita , A.A. , Hebshi , E.A. , Daood , H.G. and Biacs , P.A. 1997 . Determination of antioxidant vitamins in tomatoes . Food Chemistry , 60 : 207 – 212 .
- Ruzainah , A.J. , Ahmad , R.B.A.H. , NorZaini , C.M. and Vasudevan , R. 2009 . Proximate analysis of dragon fruit (Hylecereus polyhizus) . American Journal of Applied Sciences , 6 : 1341 – 1346 .
- Isengard , H.-D. 2001 . Water content, one of the most important properties of food . Food Control , 12 : 395 – 400 .
- Ruckold , S. , Grobecker , K.H. and Isengard , H.D. 2000 . Determination of the contents of water and moisture in milk powder . Fresenius’ Journal of Analytical Chemistry , 368 : 522 – 527 .
- Hasnah , H. , Amin , I. , Azrina , A. , Suzana , S and Loh , S.P. 2009 . Daidzein and genestein contents in tempeh and selected soy products . Food Chemistry , 115 : 1350 – 1356 .
- Tee , E.S. , Mohd Ismail , N. , Mohd Nasir , A. and Idris , K. 1997 . “ Division of Human Nutrition ” . In Nutrient Composition of Malaysian Foods , 66 Kuala Lumpur : Institute for the Medical Research .
- Cummings , J.H. and Stephen , A.M. 2007 . Carbohydrate terminology and classification . European Journal of Clinical Nutrition , 61 (Suppl. 1), S5–8.
- Skalicka-Wozniak , K. , Najda , A. , Głowniak , K. and Wolski , T. 2009 . Protein content in fruits of Peucedanum alsaticum and Peucedanum cervaria . Annales UMCS, Pharmacia , 22 : 155 – 158 .
- Luque-Garcia , J.L. , Luque and de Castro , M.D. 2004 . Focused microwave-assisted Soxhlet extraction: Devices and applications . Talanta , 64 : 571 – 577 .
- Chang , C.H. , Lin , H.Y. , Chang , C.Y. and Liu , Y.C. 2006 . Comparisons on the antioxidant properties of fresh, freeze-dried and hot-air-dried tomatoes . Journal of Food Engineering , 77 : 478 – 485 .
- Deepa , N. , Kaur , C. , Singh , B. and Kapoor , H.C. 2006 . Antioxidant activity in some red sweet pepper cultivars . Journal of Food Composition and Analysis , 19 : 572 – 578 .
- Miliauskas , G. , Venskutonis , P.R. and van Beek , T.A. 2004 . Screening of radical scavenging activity of some medicinal and aromatic plant extracts . Food Chemistry , 85 : 231 – 237 .
- Chang , C.C. , Yang , M.H. , Wen , H.M. and Chern , J.C. 2002 . Estimation of total flavonoid content in propolis by two complementary colorimetric methods . Journal of Food and Drug Analysis , 10 : 178 – 182 .
- Leong , L.P. and Shui , G. 2002 . An investigation of antioxidant capacity of fruits in Singapore markets . Food Chemistry , 76 : 69 – 75 .
- Setiawan , B. , Sulaeman , A. , Giraud , D.W. and Driskell , J.A. 2001 . Carotenoid content of selected Indonesian fruits . Journal of Food Composition and Analysis , 14 : 169 – 176 .
- Tee , E.S. and Lim , C.L. 1991 . Carotenoid composition and content of Malaysian vegetables and fruits by the AOAC and HPLC methods . Food Chemistry , 41 : 309 – 339 .
- Chandler , L.A. and Schwartz , S.J. 1988 . Isomerization and losses of trans-beta-carotene in sweet potatoes as affected by processing treatments . Journal of Agricultural and Food Chemistry , 36 : 129 – 133 .
- Lin , C.H. and Chen , B.H. 2005 . Stability of carotenoids in tomato juice during storage . Food Chemistry , 90 : 837 – 846 .
- Taylor , J.E. , Seymour , G.B. , Taylor , J.E. and Tucker , G.A. 1993 . “ Exotics ” . In Biochemistry of Fruit Ripening , 163 – 165 . Boca Raton , FL : Chapman and Hall . InEds
- Ling , S.C. and Mohamed , S. 2001 . Alpha-tocopherol content in 62 edible tropical plants . Journal of Agricultural and Food Chemistry , 49 : 3101 – 3105 .
- Mozafar , A. 1994 . Plant Vitamins—Agronomic, Physiological and Nutritional Aspects , Boca Raton: CRC Press: Boca Raton, FL .
- Zujko , M.E. and Witkowska , A.M. 2011 . Antioxidant potential and polyphenol content of selected food . International Journal of Food Properties , 14 : 300 – 308 .
- Najjaa , H. , Zerria , K. , Fattouch , S. , Ammar , E. and Neffati , M. 2011 . “ Antioxidant and antimicrobial activities of Allium roseum L. lazoul, a wild edible endemic species in North America ” . In International Journal of Food Properties Vol. 14 , 371 – 380 .
- Yan , S.W. and Asmah , R. 2010 . Comparison of total phenolic contents and antioxidant activities of turmeric leaf, pandan leaf and torch ginger flower . International Food Research Journal , 17 : 417 – 423 .
- Sharique , A. and Seerat , H.B. 2009 . Ascorbic acid, carotenoids, total phenolic content and antioxidant activity of various genotypes of Brassica oleracea encephala . Journal of Medical and Biological Sciences , 3 : 1 – 8 .
- Kalt , W. , Forney , C.F. , Martin , A. and Prior , R.L. 1999 . Antioxidant capacity, vitamin C, phenolics, and anthocyanins after fresh storage of small fruits . Journal of Agricultural and Food Chemistry , 47 : 4638 – 4644 .
- Gil , M.I. , Tomas-Barberan , F.A. , Hess-Pierce , B. and Kader , A.A. 2002 . Antioxidant capacities, phenolic compounds, carotenoids, and vitamin C contents of nectarine, peach, and plum cultivars from California . Journal of Agricultural and Food Chemistry , 50 : 4976 – 4982 .