Abstract
Olive (Olea europaea L.) includes cultivated olive trees (var. europaea) and wild olive trees or oleaster (var. sylvestris) as two botanical varieties. These olive varieties were widely spread in the Mediterranean Region. The aim of this study was to determine fatty acid compositions, sterols, polyphenols, and chlorophylls of oils obtained from 12 wild olive trees from Northern Tunisia. Two dominated oil cultivars in Tunisia (Chétoui and Chemlali) were also used to compare results. The fatty acid methyl ester and the sterol compositions were analyzed using gas-liquid chromatography and thin layer chromatography methods, respectively. The polyphenols and chlorophylls were determined using the calorimetrical method. Results indicated that oils extracted from wild olives displayed good balanced fatty acid compositions, sterols, polyphenols, and chlorophylls. Qualitatively, for wild and cultivated olive oils, the oil has an identical composition, whereas the quantitative variation showed that some wild trees seem to be interesting oil sources as two Tunisian dominated cultivars. The highest oleic acid and polyphenol contents were 71.55% and 537.6 mg/kg of oil found in wild olives (OIch2, OIch1). The β-sitosterol was the major sterolic fraction and ranged from 84.72 to 75.70% according to the wild olives. Consequently, wild olives would be a new future edible olive oil source, as well as commonly cultivated ones.
INTRODUCTION
The olive tree (Olea europaea L.) is the most extensive crop in the Mediterranean Basin. It includes the cultivated (var. europaea) and wild (var. sylvestris) olive trees. Olive tree yields two products, table olives and olive oil, both of which are important commodities in world markets. The Olea europaea L. spreads in the Mediterranean Basin where it is indigenous and in other regions with a Mediterranean climate where it has been introduced, such as in South Africa.[Citation1] The olives cover about 8.7 million ha, supporting a total of almost 750 million olive trees in 33 countries of the world. The Mediterranean Basin accounts for 97% of the olive orchards. In Tunisia, it occupies about 1.6 million ha, representing one-third of arable lands of the country.[Citation2] Olive oil is a natural fruit juice, of fine aroma, and pleasant taste, and has a high nutritional value. It is appreciated for its stability and good characteristics. It is considered as the most useful edible oil in the world due to its nutriment contents and beneficial effects.[Citation3] It was reported that olive oil is free of cholesterol and does not have adverse effects on the human body.
Olive oil is characterized by an oxidative stability enabling long shelf storage, sensory quality, and health properties stemming from a prominent and well balanced chemical composition.[Citation4] Olive oil has a preventive role in cardiovascular and inflammatory diseases; its consumption is associated with a lower coronary risk.[Citation5] Epidemiological studies have reported that the consumption of olive oil is inversely associated with pancreas cancer.[Citation6] In addition, the role of olive oil in the potential prevention of breast cancer has been given attention.[Citation7] The most important natural antioxidants are polyphenols, tocopherols, and pigments, since these compounds delay the oxidation of fatty acids and the production of unpleasant flavors.[Citation8] Despite the large amount of olive oils produced and their confirmed nutritional values, there are no reliable data on the chemical composition of oil from wild olive trees. Little is known about wild olive trees in Tunisia.[Citation9] Several historical reports[Citation10] have pointed out that the wild olive trees were native in Tunisia. The molecular diversity revealed that a few cultivars are issued from the Tunisian wild olive trees based on assignation and admixture analyses.[Citation11] Using nuclear and choroplastic SSR markers, it has been reported that the olive in Tunisia has probably three geographical origins.[Citation12] Therefore, the wild olive trees were important genetic resources deserving to be known, not only its genetic characterization but also its technological potentialities, such as the oil composition. Olive tree genetic improvement is promoted by crosses cultivars to create new combinations of traits. It is used to improve oil composition (low content of saturated fatty acids and high oleic acid content), oil yield, disease resistance, and organoleptic characters of the final products. The knowledge of the extent and the type of genetic variability available and exploitable is essential to a correct layout of breeding programs. The olive resources would be represented, not only by olive cultivars collections but also by wild olive resources. The wild olive trees dispread in Tunisia in natural and agro-ecosystems.[Citation13] Olive oil from cultivated olive trees provides beneficial effects on human health. But little is known about oil extracted from wild olive trees, in particular, oil composition. The present study was conducted to evaluate oil composition of wild olives and the two dominated cultivars oils in Tunisia.
MATERIALS AND METHODS
Plant Material and Oil Extraction
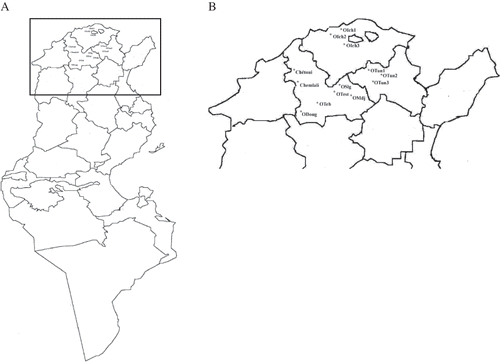
In this study, nine wild olive trees were sampled in a natural ecosystem represented by both parks and forests (Ichkeul, Tunis, Téboursouk, and Dougga). In these localities, the wild olive trees were in natural association with pistachio (Pistacia lentiscus), and they were isolated from all cultural practices. Three wild olive trees were sampled either around orchards in agro-ecosystem (Slouguia, Testoure, and Medjez El Bab) and were in association with prickly pear (Opuntia ficus indica). Two dominated olive cultivars, ‘Chemlali’ and ‘Chétoui’, were also chosen to compare results between wild trees and cultivar olive oils (, ). These two cultivars contribute by 80% of total oil production in Tunisia.
Table 1 List and locations of the studied wild and the two main cultivar olive trees
Figure 1 Geographical sites of wild olive trees and ‘Chétoui’, and ‘Chemlali’, two main cultivars of olives. (a) Tunisia map, and (b) locations details.
Oil extraction was carried out in similar industrial extraction conditions using an oleodosor system. Olives were crushed and slowly mixed for 30 min at 25°C. The paste obtained was centrifuged at 3000 × g. The extracted oils were separated by decantation and stored at 4°C for polyphenols, chlorophylls, and sterols analysis. Total lipids content was determined on dry weight matter following the Soxhlet extraction method. About 40 g of fresh matter was dried at 75°C. Then, the dried matter was ground and extracted in triplicate with 200 mL hexane at 60°C for 6 h. The hexane was removed with a rotary evaporator at 40°C. All experiments were conducted on triplicate from olives harvested at full maturity (December).
Fatty Acid Composition Analysis
Fatty acid methyl ester (FAME) preparation
FAMEs were prepared according to Metcalfe et al.[Citation14] and modified by Lechevallier.[Citation15] An aliquot (0.2 mL) of total lipids was evaporated in a tube of methylation. Fatty acids were esterified with 5 mL of a methanolic sodium hydroxide solution (0.5 N) for 15 min in a boiling water bath at 60°C. As for transmethylation, the mixture was homogenized with 3 mL of a methanolic solution of BF3 (20%, w/v) and the reaction was allowed to proceed for 5 min. FAMEs were extracted twice with 10 mL of petroleum ether (boiling point 20–75°C) and 10 mL of distilled water. The solvent was evaporated and the residues were solubilized in chloroform.
Gas-liquid chromatography
FAMEs were analyzed by gas-liquid chromatography in a Hewlett-Packard HP-4890D (Hewlett-Packard, Wilmington, DE, USA) gas chromatography equipped with a Supelcowax capillary column (Supelco, Bellefonte, PA, USA; 0.25 μm film thickness; 30 m–0.53 mm), operated isothermally at 200°C with an inlet carrier gas (nitrogen) pressure of 0.4 bar. The injector (split-splitless) and the flame ionization detector (FID) were maintained at 230 and 250°C, respectively. Nitrogen was used as the carrier gas at 1 mL/min with split injector system (split ratio 1:100). Fatty acids were expressed in percentage of chromatographic areas. FAMEs were identified by using standards, analyzed in the same experimental conditions.
Total phenol content
Total phenol compounds were quantified calorimetrically.[Citation16] Phenolic compounds were isolated from a solution of oil in hexane (10 g of oil in 25 ml of hexane) by triple-extraction with water-methanol (60:40 V/V). A total of 2.5 mL of the combined extract was mixed with 1.25 mL of Folin-Ciocalteu reagent. After 3 min, 2.5 ml of saturated Na2CO3 solution was added to the mixture followed by the addition of 9 mL of distilled water. The mixture was kept in the dark for 60 min, after which the absorbance read at 725 nm. The blank test was made by 9 mL of distilled water, 5 mL of methanol 60%, and 1.25 mL of Folin-Ciocalteu reagent.
Total phenols were determined with a UV visible spectrophotometer (JENWAY 6505 UV/Vis; JENWAY Ltd., Dunmow, Essex, UK) at 725 nm.
Chlorophylls content
The authors used the Wolf method[Citation17] to measure the chlorophyll content based on absorbance at 630, 670, and 710 nm of olive oil samples. The absorbance was determined using the carbonate tetrachloride (CCl4) as the ‘blank’ measured by spectrophotometer (JENWAY 6505 UV/Vis).
Sterols Content
Saponification of the lipids
In order to separate sterols, oils from wild and cultivated olives were treated with a potassium hydroxide. In fact, 5 g of oil were treated with 50 mL of ethanolic potassium hydroxide solution KOH (2N). The mixture was heated at 60°C for 1.30 h. After cooling, 50 mL of water was added. The unsaponifiable fraction was extracted four times with 50 mL of ethyl ether. The combined ether extract was washed with 50 mL of ethanol-water (1:1). The extracted ether was dried over anhydrous sodium sulphate Na2SO4, filtered and concentrated on a rotary evaporator. The unsaponifiable fraction was dissolved into chloroform for thin layer chromatography (TLC) analysis.[Citation18]
TLC separation and GC analysis
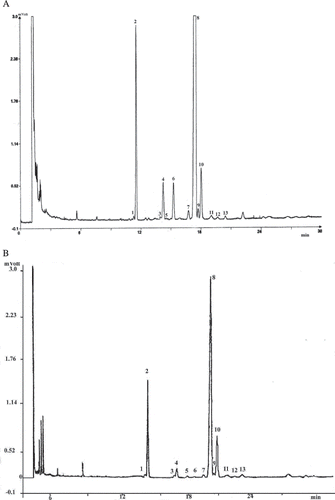
The unsaponifiable matter was separated into sub-fraction on TLC plates coated with ethanolic potassium hydroxide solution KOH-methanol (2 N) impregnated silica gel, previously activated by heating at 100°C for 1 h. The unsaponifiable fraction (5% solution of the unsaponofiable) and internal standards 5-α-cholestanol (0.2%) were spotted on the plates. Elution was performed using hexane/diethyl ether 65:35 (v/v) as the mobile phase. After development, the plate was sprayed with a 0.2% solution of 2,7-dichlorofluorescein in ethanol, and the sterol bands appeared under UV light. Sterol bands were scraped off and dissolved in chloroform (10 mL). The obtained solution was filtered. The chloroform was evaporated by mild heating in a gentle flow of nitrogen. The sterolic fraction was dried in an oven at 105°C for approximately 10 min. The sterolic fraction was treated with a silylation reagent (pyridine/hexamethyldisilazane/trimethylchlorosilane, 9:3:1, v/v/v) at the ratio of 50 μl of reagent for every milligram of sterols for at least 15 min at ambient temperature. Then, this solution was centrifuged for a few minutes. The clear solution is ready for gas chromatography analysis. The trimethylsilyl ethers were analyzed by gas chromatography in a Hewlett-Packard HP-4890D chromatograph equipped with a FID column (30 m × 0.32 mm, 0.25 μm film thickness) with stationary phase of 5% phenyl methyl siloxane, operated isothermally at 280°C, with an inlet carrier gas (helium) giving a column flow of 1.3 mL/min. The injector with a split ratio of 1:15 was maintained at 280°C and the flame ionization detector (FID) at 290°C. Sterols was identified on the basis of retention times and by comparison with mixture of sterol analyzed under the same conditions. Sterols were expressed as percentage of total sterols. The identification of individual peaks was made on the basis of the retention times and by comparison with the mixture of standard sterols analysed under the same conditions ().
Figure 2 Typical chromatograms of sterol composition of (a) standard olive oil and of (b) wild olive oil samples (OSlg). Peaks: (1) Cholesterol; (2) Cholestanol (internal standard); (3) 24-Metilencholesterol; (4) Campesterol; (5) Campestanol; (6) Stigmasterol; (7) Chlerosterol; (8) β-Sitosterol; (9) Sitostanol; (10) Δ5-Avenasterol; (11) Δ5,24-Stigmastadienol; (12) Δ7-Stigmastenol; (13) Δ7-Avenasterol.
Statistical and Chemometric Methods
All analyses were carried out in triplicate and the results were presented as means ± SD (Standard Deviation). Wild and cultivated olive value for each oil compound was compared to the mean of all samples by calculating a confidence interval. Analysis of variance (ANOVA) were used on oil composition (fatty acid composition, sterols, polyphenols, and chlorophylls content) of cultivated olive (cultivars) and wild olive trees based on Duncan's multiple range test using the software Statistica (StaSoft, Johannesburg, ZA).
RESULTS
Total Lipids and Fatty Acid Composition
In wild olive oils, the total lipid ranged from 10.42% (OIch1) to 26.27% (OTest). For the both dominated Tunisian cultivars Chétoui and Chemlali, the total lipids were 59.08 and 51.37%, respectively (). As expected, the oleic acid is the major fatty acid for studied olive oils (both wild and dominated cultivars), followed by linoleic C18:2 and palmitic C16:0 acids. Oleic acid contents for wild olive trees varied from 47.03% (OIch1) to 71.55% (OIch2). For Chétoui and Chemlali, contents are 57.20 and 64.90%, respectively (). Except two wild olive trees (OIch1 and OIch3), the oleic acid content obtained from wild olive oils is higher than the standard values (55%) adopted for extra virgin olive oil.[Citation19] We noticed also that the wild olive trees OIchk2, OTun2, OTun3, OSlg, OTeb, and OMed displayed higher oleic acid contents, compared to Chemlali (64.90%) and Chétoui (57.20%).
Table 2 Fatty acids composition* (% of total fatty acids) of oils obtained from wild and two main cultivars of olives
Monounsaturated fatty acids (MUFA) in the wild oils ranged from 47.37% (OIch1) to 72.06% (OIch2). Chétoui and Chemlali dominated cultivars have 57.80 and 65.22%, respectively. The MUFA average was 63.81% in wild olive tree and 61.30% in cultivars oils. Palmitoleic acid content ranged from 0.16% (OIchk1) to 2.59% (ODoug) in wild tree oils. The Chétoui and Chemlali cultivars have 0.61 and 0.31%, respectively. Except OIch1 and OIch3 (0.27 and 0.16%), all wild olive trees have more palmitoleic acid than the two dominated cultivars oils.
The concentration levels of polyunsaturated fatty acids (PUFA) are given (). Some wild olive oils have higher PUFA levels, such as OTest (21.49%), OIchk1 (37.26%), and OIchk3 (31.75%), than the cultivar Chétoui (20.73%). Five wild olive oils (OTest, OIch1, OIch3, OTun1, and OTun4) have PUFA percentage higher than the Chemlali cultivar (16.80%) (). The linoleic acid is the major polyunsaturated fatty acids in olive oil. This fatty acid percentage varied from 12.22 (OMedj) to 33.56% (OIchk1) in wild olive tree oils; for Chétoui and Chemlali cultivars, contents are 20.08 and 15.96%, respectively. The saturated fatty acids were 21.10% in Chétoui and 17.97% in Chemlali cultivars. However, contents of these saturated fatty acids varied from 13.94% (OIchk2) to 23.62% (OIch1) in wild olive oils. Moreover, we noted 8 out of 12 wild olives have less saturated fatty acids than two cultivars oils. The palmitic acid is the major saturated fatty acid in olive oil, varying from 11.79% (OTun4) to 18.681% (ODoug) in wild olive oils. Contents of the stearic acid are similar in cultivated and wild olive oils. This is in agreement with IOOC criteria.
Phenols Content
The phenolic compounds content was expressed as total phenols. For all wild olive trees, contents ranged from 59.58 mg/kg (OTeb) to 537.6 mg/kg (OIch1) averaging approximately 196 mg/kg of oil. Chétoui and Chemlali cultivars have 490.06 and 214.47 mg/kg, respectively. Consequently, phenols content from wild olive oils are similar to dominated cultivars. However, for OIch1 (537.6 mg/kg), content of phenols are higher than both dominated cultivars. The oils from nine wild olive trees were characterized by their distinct total phenols content pattern (), excepting three OTest, OTun1, and OTeb, which contain less than 100 mg/kg phenolic compounds. Quantitatively, polyphenols content was statistically different (p < 0.05) depending on the wild and cultivated olive trees.
Table 3 Polyphenols and chlorophylls of oils obtained from wild and the two main cultivars of olives
Chlorophylls Content
clearly shows that the OSlg and OTest wild olive oils contain the highest values 12.17 and 13.45 mg/kg of oil, respectively, whereas the mean chlorophyll contents are 7.1 mg/kg for Chétoui and Chemlali oils. Quantitatively, the chlorophylls content was statistically different according the wild olive trees, whereas this difference was not significant according the two olives cultivars.
Sterols Content
Sterols are important constituents of olive oils because they are related to the quality of the oil. The β-Sitosterol is the major sterol, followed by Δ5-Avenasterol and campesterol (, ). The β-Sitosterol varied from 75.7% (OTeb) to 84.72% (OTun4). The Chétoui cultivar has 76.03% and Chemlali cultivar has 77.89%. The Δ5-Avenasterol ranges from 4.86% (OSlg) to 14.49% (OTeb) in wild olive oils. The Δ5-Avenasterol is present in Chétoui and Chemlali cultivars at 18.40 and 13.53%, respectively. Sterols contents are statistically different according the wild and cultivated olive trees (p < 0.05), except for 24-metilencholesterol and chlerosterol.
Table 4 Sterol composition of oil obtained from wild and the two main cultivars of olives
DISCUSSION
The main source of vegetable fats in the Mediterranean diet is olive oil. The composition of this oil differs from other vegetable oils that are currently consumed in many countries. Total lipids for wild olive trees ranged from 10.42% (OIch1) to 26.27% (OTes), averaging 20.57% on dried matter. These values are lesser than those of dominated cultivars (59.08 and 51.37%, respectively, for Chétoui and Chemlali) and in general for Tunisian cultivars.[Citation20] These differences are mainly due to location distribution since the chemical composition of crops varies with the crop cultivars, soil and climatic conditions of the area other than genetic control.[Citation21]
Olive oil contains high amounts of oleic acid and a smaller amount of linoleic acid. Oils extracted from olives having oleic acid higher than 55% are categorized as extra virgin olive.[Citation19] Present findings clearly show that eleven out of twelve of our studied wild olive trees have oleic acid content higher than 55% (). Therefore, they are considered as extra virgin oils in agreement with IOOC norms. As expected, our results showed that the oleic acid is the major fatty acids in wild olive oils as the cultivated olive oils. Consequently, the wild olive oils could be a good source of essential fatty acids required for human health. The PUFA are now well documented to have protective effects against lipid peroxidation.[Citation22] Recently, scientist signaled many species rich lipids and fatty acids composition as pomegranate seeds.[Citation23]
In the present study, a high concentration level of MUFA and polyphenol content were detected in the wild olive oils ( and ). The high range variation of polyphenols content in wild olive oils is in agreement with literature.[Citation24] Polyphenols are important antioxidants that protect the oil against oxygen radicals at the cellular level and due to self oxidation along long shelf storage. Phenols improve olive oil quality due to both organoleptic effect and namely for its sharp bitter taste[Citation25] and they are responsible for fragrance and peculiar flavor of olive oil.[Citation26] Polyphenols content varied according the olive cultivars.[Citation27] It has been reported that the main characteristics of olive oil was the large total phenol content.[Citation28] Oxidative stability was mainly correlated with the concentration of total phenols.[Citation29] The cultivar genotype is the most important factor influencing the antioxidant profile of the olive oil,[Citation29] hence, the wild olive oils subject of this study constitute a new edible oil source characterized by an important natural antioxidant substance, which varied according the oil samples. Particularly, OIch1 phenol content was 537.6 ± 5.9 mg/kg, which was higher than both Chétoui (490.6 ± 4.8 mg/kg) and Chemlali (214.7 ± 2.3 mg/kg). This finding makes Olch1 an attractive candidate as a nutritional supplement for commercial olive oil to prevent oil oxidation.
Olive oil color was the principal result of chlorophylls content. The wild olive oils show statistically significant variation (from 1.2 to 13.4 mg/kg) which was in agreement with high variation (from 2 to 23 mg/kg) observed in olive oil from Spain.[Citation24] Chlorophylls from oils are an important parameter because they are correlated to oil quality. Pigments are involved in autoxidation and photoxidation mechanisms.[Citation30] The research conducted on olive oil chemical composition highlights that the polyphenols are remarkably variable according to the variety, the agronomic conditions, the state of ripeness, and the technology of conservation.[Citation31] Moreover, some authors attribute the variation of polyphenol and chlorophylls contents to the genetic factor.[Citation32, Citation33]
As for sterols, the β-Sitosterol is the major sterol, followed by Δ5-Avenasterol and campesterol which was in agreement with oils extracted from cultivars olives[Citation24, Citation33] and in other vegetable oils.[Citation34] Sterols are important constituents of olive oils because they are related to the quality of the oil. The Δ5-avenasterol content ranged from 4.04% (OTun4) to 14.49% (OTeb) according wild olive trees. Some wild olive trees have high Δ5-avenasterol content (). This compound has been associated with antioxidant activity.[Citation35] The sterols percentages were in agreement with IOOC criteria.[Citation19] Plant sterols are natural dietary components with serum cholesterol-lowering proprieties.[Citation36] This finding resulted in several studies of the cholesterol-lowering effects of plant sterol in humans.[Citation33] The qualitative characterization of wild and two cultivars olive oils was in agreement with the results reported by Casas et al.[Citation37] However, the quantitative characterization was different, which can be explained by the fact of geographical growing area and the olive varieties.[Citation37]
Epidemiological evidence showed a lower incidence of CHD (Coronary Heart Disease) in Mediterranean countries[Citation38] where olive oil is the primary source of fats.[Citation39] The β-sitosterol, the major sterol component, has some nutritional criteria; it reduces cholesterol level of blood and is sometimes used in treating hypercholesterolemia. The β-sitosterol inhibits cholesterol absorption in the intestines.[Citation40] Plant sterols were reported to have antioxidant proprieties.[Citation41] The wild olive oils have β-sitosterol content conformity to IOOC limit permitted.
CONCLUSION
In this study, wild olive trees displayed oil composition in agreement with the International Olive Oil Council (IOOC) norms as extra virgin oil. Therefore, they constitute important olive resources for nutritional oil quality. The wild olive tree is valuable because it provides shelter for diverse birds and wild plants in harsh environments. In this study, we also noticed that the wild olive tree is valued according to its oil composition as fatty acids composition, polyphenols compounds, chlorophylls, and sterols content. The wild olives seem to constitute new nutritional oil sources; therefore, these wild olive trees with valuable oil composition could be experienced for yield, regularity of production, and other agronomic traits and if approved, the wild olive trees constitute new olive cultivars to increase high olive oil quality in Tunisia. Eight wild olives produced fatty acids that are in agreement with IOOC criteria as extra virgin oil. The other criteria, like polyphenols compounds, chlorophylls, and sterols, could be some parameters to qualify the wild olive oil as a valuable nutritional oil source.
REFERENCES
- Costa , C. 1998 . Olive Production in South Africa: A Handbook for Olive Growers , 124 Pretoria : Agricultural Research Council .
- 1996 . DGPA/ONH. L'oléiculture tunisienne. Olivae , 61 : 12 – 20 .
- Visioli , F. , Bellosta , S. and Galli , C. 1997 . Cardioprotective properties of olive-derived polyphenols. Atherosclerosis , 134 : 336
- Jacotot , B. 2001 . Nutritional assets of olive oil. Olivae , 86 : 27 – 29 .
- Trevisan , M. , Krogh , V. , Freudenheim , J. , Blake , A. , Muti , P. , Panico , S. , Farinaro , E. , Mancini , M. , Menotti , A. and Ricci , G. 1990 . “ Consumption of olive oil, butter, and vegetable oils and coronary heart disease risk factors. The Research Group ATS-RF2 of the Italian National Research Council ” . In Journal of the American Medical Association 688 – 692 . 263
- Soler , M. , Chatenoud , L. , La Vecchia , C. , Franceschi , S. and Negri , E. 1998 . “ Diet, alcohol, coffee and pancreatic cancer: Final results from an Italian study ” . In European Journal of Cancer Prevention 455 – 460 . 7
- La Vecchia , C. , Negri , E. , Franceshi , S. , Decarli , A. , Giacosa , A. and Lipworth , L. 1995 . Olive oil, other dietary fats, and the risk of breast cancer (Italy) . Cancer Causes & Control , 6 : 545 – 550 .
- Morales , M.T. , Przybylski , R. , Harwood , J. and Aparicio , R. 2000 . “ Olive oil oxidation ” . In Handbook of Olive Oil: Analysis and Properties , 459 – 490 . Gaithersburg , Maryland : Aspen Publication . InEds
- Hannachi , H. , Sommerlatte , H. , Breton , C. , Msallem , M. , El Gazzah , M. , Ben El Hadj , S. and Bervillé , A. 2009 . “ Oleaster (var Sylvestris) and subsp. cuspidate are suitable genetic resources for improvement of the olive ” . In Olea europaea subsp. europaea var europaea 393 – 403 . Genetic Resources and Crop Evolution56
- Camps-Fabrer , H. 1997 . “ La culture de l'olivier en Afrique du Nord, Evolution et histoire ” . In Encyclopédie Mondial de l'Olivier , 30 – 33 . Madrid , Spain : Espagne . InInternational Olive Oil CouncilEds
- Breton , C. , Tersac , M. and Bervillé , A. 2006 . “ Genetic diversity and gene flow between the, wild olive Olea europaea L. and the olive: Several Plio-Pleistocene refuge zones in the Mediterranean basin suggested by simple sequence repeats analysis ” . In Journal of Biogeography 1916 – 1928 . 33
- Hannachi , H. , Breton , C. , Msallem , M. , Ben El Hadj , S. , El Gazzah , M. and Bervillé , A. 2010 . Genetic relationships between cultivated and wild olive trees 95 – 103 . Olea Europaea L. Var. Europaea and Var. Sylvestrisbased on nuclear and chloroplast SSR markers. Natural Resources1
- Hannachi , H. , Breton , C. , Msallem , M. , Ben El Hadj , S. , El Gazzah , M. and Bervillé , A. 2008 . Are olive cultivars distinguishable from oleaster trees based on morphology of drupes and pits, oil composition and microsatellite polymorphisms? Acta Bot Gallica , 155 ( 4 ) : 531 – 545 .
- Metcalfe , L.D. , Schmitz , A.A. and Pelka , J.R. 1966 . Rapid preparation of fatty acids esters from lipids for gaz-chromatographic analysis . Analytical Chemistry , 38 : 514 – 515 .
- Lechevallier , D. 1966 . Les lipides des Lemnacées, analyse des acides gras des lipides des frondes de Spirodela polyrhiza . Comptes Rendus Biologie , 263 : 1848 – 1852 .
- Vasquez , R.A. , Janer del Valle , C. and Janer del Valle , M.L. 1973 . Determinacion de los polifenoles toatales del aceite de oliva. Grasa y Aceities 350 – 357 . 24
- Wolf , J.P. and Azonlay , A. 1968 . Manuel d'Analyse des Corps Gras Paris Ed
- Nasri , N. , Fady , B. and Triki , S. 2007 . “ Quantification of sterols and aliphatic alcohols in Mediterranean stone pine (Pinus pinea L.) populations ” . In Journal of Agricultural and Food Chemistry 2251 – 2255 . 55
- IOOC . 1992 . “ The international olive oil market ” . In Olivae 9 – 13 . 43
- Abaza , L. , Msallem , M. , Daoud , D. and Zarrouk , M. 2002 . Caractérisation des huiles de sept variétés d'olivier tunisiennes . Oleagineux Corps Gras Lipides , 9 : 174 – 197 .
- Breene , W.M. , Lin , S. , Hardman , L. and Orf , J. 2007 . Protein and oil content of soybeans from different geographic locations . Journal of the American Oil Chemists’. Society , 65 : 927 – 1931 .
- Kratz , M. , Cullen , P. , Kannenberg , F. , Kassner , A. , Fobker , M. , Abuja , P.M. , Assmann , G. and Wahrburg , U. 2002 . Effects of dietary fatty acids on the composition and oxidizability of low-density lipo-protein . European Journal of Clinical Nutrition , 56 : 72 – 81 .
- Elfalleh , W. , Ying , M. , Nasri , N. , Sheng-hua , H. , Guasmi , F. and Ferchichi , A. 2011 . Fatty acids from Tunisian and Chinese pomegranate (Punica granatum L.) seeds . International Journal of Food Science & Nutrition , 62 ( 3 ) : 200 – 206 .
- Salvador , M.D. , Aranda , F. and Fregapane , G. 1998 . Chemical composition of commercial cornicabra virgin oil from 1995/96 and 1996/97 crops . Journal of the American Oil Chemist's Society , 75 : 1305 – 1311 .
- Murkovic , M. , Lechmer , S. , Pietzka , A. , Bratacos , M. and Katzoiamnos , E. 2004 . Analysis of minor components in olive oil . Journal of Biochemical and Biophysical Methods , 61 : 155 – 160 .
- Servili , M. , Selvaggini , R. , Esposto , S. , Taticchi , A. , Montedoro , G. and Morozzi , G. 2004 . Health and sensory properties of virgin olive oil hydrophilic phenols: Agronomic and technological aspects of production that affect their occurrence in the oil . Journal of Chromatography A , 1054 : 113 – 127 .
- Ryan , D. , Prenzler , P.D. , Lavee , S. , Antolovich , M. and Robards , K. 2003 . Quantitative changes in phenolic content during physiocological development of the olive cultivar Hardy's Mamouth . Journal of Agricultural and Food Chemistry , 51 : 2532 – 2538 .
- Cecchi , T. , Passamonti , P. , Alfei , B. and Cecchi , P. 2011 . Monovarietal extra virgin olive oils from the Marche Region, Italy: Analytical and sensory characterization . International Journal of Food Properties , 14 ( 3 ) : 483 – 495 .
- Tura , D. , Gigliotti , C. , Pedò , S. , Failla , O. , Bassi , D. and Serraiocco , A. 2007 . “ Influence of cultivar and site of cultivation on levels of lipophilic and hydrophilic antioxidants in virgin olive oils (Olea europea L.) and correlations with oxidative stability ” . In Scientia Horticulturae 108 – 119 . 112
- Gandul , B. and Minguez , M.I. 1996 . Chlorophylls and carotenoid composition in virgin olive oils from various Spanish olive varieties . Journal of the Science of Food Agriculture , 72 : 31 – 39 .
- Krichene , D. , Taamalli , W. , Daoud , D. , Salvador , M.D. , Fregapane , G.D. and Zarrouk , M. 2007 . Phenolic compounds, tocopherols and other minor components in virgin olive oils of some Tunisian varieties . Journal of Food Biochemistry , 31 : 194 – 197 .
- Ceci , L.N. and Carelli , A.A. 2007 . Characterization of monovarietal Argentinian olive oils from new productive zones . Journal of the American Oil Chemist's Society , 84 : 1125 – 1136 .
- Sakouhi , F. , Absalon , Ch. , Flamini , G. , Cioni , P.L. , Kallel , H. and Boukhchina , S. 2010 . “ Lipid components of olive oil from Tunisian Cv. Sayali: Characterization and authenticity ” . In Comptes Rendus Biologie 642 – 648 . 33
- Alvarez-Chávez , L.M. , Valdivia-Lόpez , M.L.A. , Aburto-Juárez , M.L.A. and Tecante , A. 2008 . Chemical characterization of the lipid fraction of Mexican Chia seed (Salvia hispanica L.) . International Journal of Food Properties , 11 ( 3 ) : 687 – 697 .
- Weststrate , J.A. and Meijer , G.W. 1998 . Plant sterol-enriched margarines and reduction of plasma total- and LDL-cholesterol concentrations in normocholesterolemic and mildy hypercholesterolemic subjects . European Journal of Clinical Nutrition , 52 : 334 – 343 .
- Perez-Jimenez , F. , Espino , A. , Lopez-Segura , F. , Blanco , J. , Ruiz-Gutierrez , V. , Prada , J.L. , Lopez-Miranda , J. , Jimenez-Pereperez , J. and Ordovas , J.M. 1995 . Lipoprotein concentrations in normolipidemic males consuming oleic acid-rich diets from two different sources: Olive oil and oleic-rich sunflower . The American Journal of Clinical Nutrition , 62 : 769 – 775 .
- Casas , J.S. , Bueno , E.O. , García , A.M. and Cano , M.M. 2004 . Sterol and erythrodiol + uvaol content of virgin olive oils from cultivars of Extremadura (Spain) . Food Chemistry , 87 : 225 – 230 .
- Aravanis , C. , Corcondilas , A. , Dontas , A.S. , Lekos , D. and Keys , A. 1970 . Coronary heart disease in seven countries . IX. The Greek islands of Cret and Corfu. Circulation , 41 : 188 – 100 .
- Stark , A.H. and Madar , Z. 2002 . Olive oil as a functional food: Epidemiology and nutritional approaches . Nutrition Reviews , 60 : 170 – 176 .
- Matsuoka , K. , Nakazawa , T. , Nakamura , A. , Honda , C. , Endo , K. and Tsukada , M. 2008 . Study of thermodynamic parameters of solubilization of plant sterol and stanol in bile salt micelles . Chemistry and Physics of Lipids , 154 : 87 – 93 .
- Berger , A. , Jones , P.J. and Abumweis , S.S. 2004 . Plant sterols: Factors affecting their efficacy and safety as functional food ingredients . Lipids in Health and Disease , 3 : 5