Abstract
Three different biochemical test systems were chosen based on their solubility to study the antioxidant activity of ginger extracts. Reducing power and DPPH. scavenging activity tests were considered to produce hydrophilic environments and the H2O2 test was considered as creating a lipophilic environment. The average yields were 10.23 ± 1.02% and 0.48 ± 0.19% for oleoresin and essential oil, respectively. The content of total phenols was 67.6 ± 1.08 mg GAE/g of dry extract. In terms of EC50, in hydrophilic environment standards, it showed the highest effects compared to ginger extracts, with oleoresin presenting more activity than essential oil. In contrast, except for quercetin, essential oil showed the best scavenging activity in inhibiting H2O2 compared to all other antioxidants. The present work demonstrated that, when using reducing power, DPPH· free radical scavenging and H2O2 scavenging assays, the same ginger extracts exhibit different antioxidant activities, which were affected not only by the extract itself but also by the chemical environment (hydrophilic/lipophilic).
INTRODUCTION
Oxidative stress remains a major health problem for which several therapies are adopted and where medicinal plants offer a promising alternative. The World Health Organization (WHO) has estimated that up to 80% of the world's population relies on plant preparations as medicines to meet their health needs.Citation[1] In particular, a number of aromatic plants and spices have gained the interest of many research groups.Citation2–5 Citation Citation Citation5]
Ginger, the rhizome of Zingiber officinale, has a long history of medicinal use; it is one of the most widely used species of the ginger family.Citation6–8 Citation Citation8] The distinct yellow, pungent, aromatic rhizome is the plant's organ that confers its value to the spice and the source of oleoresin and the essential oil. The oleoresin contains the phenolic pungent principles of ginger, which represent 5–8% of the dry weight.Citation[9] The essential oil produced by Zingiber officinale rhizomes is pale yellow to light amber and can be extracted with yields ranging approximately from 1.5 to 3.0% depending on the quality of the crop.Citation[10] Both oil and oleoresin are used in various foods,Citation[11] beverages, such as soft drinks, and many types of medicinal products.Citation[12]
Ginger has been extensively studied for its biological activities.Citation[7] The antioxidative and free radical scavenging properties of the ginger extracts have been well established.Citation[12, Citation13] On the other hand, comparative studies of oleoresin and essential oil performed concomitantly, under the same conditions, have not yet been reported. It is well recognized that ginger's essential oil is much more lipophilic than the oleoresin. Therefore, the lipophilic or hydrophilic environment of a test could be one of the parameters in the variation of plant antioxidative effects. Thus, the aim of the present study was to compare antioxidant activity of ginger extracts (essential oil and oleoresin) using three different assays on the basis of their chemical environment: reducing power, DPPH· free radical scavenging activity (hydrophilic environments), and H2O2 scavenging assay (lipophilic environment).
MATERIALS AND METHODS
Plant Materials
The air-dried roots of ginger, of Chinese origin, were purchased from a local spice store in Bejaia, Algeria in January 2009. Mature and healthy rhizomes were ground using a mortar and a pestle. The essential oil was extracted directly from the mortar and pestle crushed tissues. For the extraction of the oleoresin, the tissues were further ground into a fine powder (500-μm particles) with an electric mill (Ika Labortechnik, Staufen, Germany). All chemicals were purchased from Sigma (represented by Algerian Chemical Society, Setif, Algeria). The percentage moisture content was calculated by expressing the loss in weight according to Balladin's formula.Citation[14]
Plant Extraction
Extraction of the essential oil
Essential oil was extracted by a hydrodistillation process. Light yellow-colored oil, with a pleasant odor, was recovered from the top of the aqueous distillate and dried with anhydrous sodium sulphate. The essential oil was kept in air-tight sealed glass vials, covered with aluminum foil, and stored at 4°C until studied.
Extraction of the oleoresin
Oleoresin compounds were extracted from dry ginger powder by the Soxhlet apparatus method as explained by Tejasari.Citation[15] Briefly, the samples weighing about 10 g were packaged in filter paper, tied, and soaked in methanol at 70°C for 4–8 h. The methanol extracts were evaporated by a rotary evaporator to yield the oleoresin. Each weighed dry oleoresin sample was then reconstituted in 10 ml of methanol and stored in the dark at a low temperature (4°C) until tested.
Total phenolic compounds determination
Total phenolic compounds were estimated using a slightly modified Folin-Ciocalteu method previously described.Citation[16] Briefly, aliquots (200 μl) of appropriately diluted oleoresin extracts or standard solutions of gallic acid (20, 40, 60, 80, and 100 μg/l), used to establish the calibration curve, were added to 500 μl of Folin-Ciocalteu reagent (10%). The reagents were thoroughly mixed by shaking. The mixture was incubated at room temperature for 5 min, before 1500 μl of Na2CO3(7.5%) were added. All the reaction mixtures were then shaken and incubated for 30 min. The absorbance of blue mixtures was recorded at 765 nm using UV-1601 PC UV visible spectrometer (Shimadzu Corporation, Japan). Total phenolic contents were expressed as mg gallic acid equivalents (GAE)/g of crude extract from the gallic acid calibration curve using the equation: y = 0.0094x + 0.0299 (R 2 = 0.998). All samples were analyzed in triplicate.
Antioxidant Activity
Reducing power
The Fe3+ reducing power of ginger extracts was determined by the method of Yen and DuhCitation[17] with some modifications. Different concentrations of oleoresin or essential oil were mixed with 2.5 ml of phosphate buffer (0.2 M, pH 6.6) and 2.5 ml of 1% potassium ferricyanide. The mixtures were incubated for 20 min at 50°C. After incubation, 2.5 ml of 10% trichloroacetic acid was added to the mixtures, followed by centrifugation at 3000 rpm for 10 min. The upper layer (1 ml) was mixed with 1 ml of distilled water and 0.5 ml of 0.1% ferric chloride. The absorbance of the obtained solution was measured at 700 nm. A higher absorbance indicates a higher reducing power. EC50 value (mg/ml) was the effective concentration at which the absorbance was 0.5 for reducing power and was obtained by interpolation from a linear regression analysis. Quercetin, ascorbic acid, and butylated hydroxyanisole (BHA) were used as controls and all tests were carried out in triplicate.
DPPH radical-scavenging activity
The capacity to scavenge the “stable” free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) was assessed according to the method described previously.Citation[18] Two ml of various concentrations of ginger essential oil and oleoresin were added to 0.4 ml solution of DPPH radical in methanol (final concentration of DPPH was 0.5 mM). The mixture was shaken and kept in the dark for 30 min; the absorbance of the resulting solution was measured at 517 nm. Inhibition of free radical DPPH in percent (DPPH I%) was calculated as follows:
where Ablank represents the absorbance of the control reaction (containing all reagents except the tested compound), and Asample represents the absorbance of the tested compound. Extract concentration providing 50% inhibition (EC50) was calculated. Synthetic antioxidants reagents (gallic acid, quercetin, and ascorbic acid) were used as positive controls.
H2O2 scavenging activity
The H2O2 scavenging activity was determined according to a previously described method.Citation[19] A solution of hydrogen peroxide (40 mM) was prepared in phosphate buffer (pH 7.4). Different concentrations of ginger extract were added to a hydrogen peroxide solution (0.6 ml, 40 mM). Absorbance of hydrogen peroxide at 230 nm was determined after 10 min. The percentage scavenging of hydrogen peroxide by extracts and standard compounds (gallic acid, quercetin, and ascorbic acid) was calculated using the following formula:
where A 0 represents the absorbance of the control and A 1 represents the absorbance in the presence of the extracts and standards. EC50 was the effective concentration at which 50% of hydrogen peroxide was scavenged.
Statistical Analysis
All experiments in the present study were repeated at least three times, and data were expressed as means ± SD. Statistical analysis was performed with the analysis of factorial variance (ANOVA) using Statistica Software (version 5.5, Statsoft, France); a correlation coefficient was calculated. Values were considered significant when P < 0.05.
RESULTS AND DISCUSSION
Extraction Yields
Essential oil
The analysis of the dried roots from Z. officinale revealed a yield of water content of 11%, which is in conformity with its commercialization norm.Citation[20] The hydrodistillation of dried roots of Z. officinale resulted in an average oil yield of 0.48 ± 0.19% (w/w) based on the weight of the plant. The oil yield from this study was much lower compared to those obtained either by Sultan et al.Citation[21] or Tewtrakul and Subhadhirasakul,Citation[22] where the yields were 0.98, 1.58, and 0.7%, respectively. These differences are probably due to the crop season and country origin. Sultan and coworkersCitation[21] reported a higher percentage of essential oil in ginger originated from Thailand (1.58%) compared to that from China (0.98%).
Oleoresin
It is well established that the effectiveness of the extraction is dependent not only on the extraction method but also on the solvent of extraction.Citation[23] Various solvents were used for the extraction of the active phenolic compounds (methanol, ethanol, acetone, water, ether, etc.).Citation[24] In this study, methanol was used as the solvent. This latter has been reported to extract the maximum of polyphenolsCitation[25] and has the advantage of being easily removed from the fraction under vacuum; moreover, it has the capacity to dissolve the lipophilic compounds and the majority of the polar ones. The average yield of the crude extract is 10.23 ± 1.02%. This result is in agreement with those reported by TejasariCitation[15] (10.2%), Tewtrakul and SubhadhirasakulCitation[22] (11.4% and 10.4%), and Ahui et al.Citation[26] (11%). However, it is higher than those obtained by Mascolo et al.Citation[6] (2.8%), Alfaro et al.Citation[27] (3.6, 3.2, and 0.27%), and Goyal and KadnurCitation[28] (4.6 and 4.3%).
Total phenolics determination
Results of the colorimetric analysis of total phenols showed an amount of 67.6 ± 1.08 mg GAE/g of dry extract. The content of total phenolic compounds observed was significantly higher than that reported elsewhereCitation[16] (1.57 ± 0.18 mg GAE/g).
Comparative Study of the Antioxidant Activity
It is well established that the antioxidant activity of plant extracts containing polyphenols components is due to their redox properties that act as donors of hydrogen atoms or electrons and allow the capture of free radicals, quenching singlet and triplet oxygen, or decomposing peroxides.Citation[12] In the present work, the antioxidant activity was evaluated by the measurement of the reducing power, the capacity of trapping the DPPH free radical, and finally, the H2O2 scavenging activity. The various tests were carried out to compare oleoresin and essential oil to standards.
Reducing power
Reducing power measures the ability of an antioxidant to donate an electron.Citation[29] These antioxidants trap the free radicals, act on certain peroxide precursors, and prevent the peroxidation chain reaction.Citation[30] shows a significant increase (p < 0.05) of the reducing power in relation to standards (quercetin, ascorbic acid, and BHA) and ginger extracts (oleoresin and essential oil) concentrations.
Figure 1 Reducing power of different concentrations of oleoresin and essential oil from Zingiber officinale Roscoe, quercetin, ascorbic acid, and BHA by the spectrophotometer detection of the Fe3+–Fe2+ transformations. Values are expressed as mean ± standard deviation (n = 3), p < 0.05.
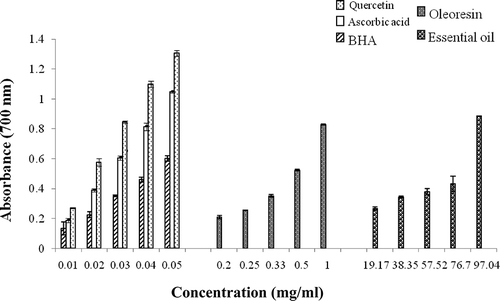
The standards were tested at the same concentrations (0.01 to 0.05 mg/ml); the resulting power varies from 0.27 ± 0.004 to 1.3 ± 0.02 for quercetin, from 0.18 ± 0.01 to 1.046 ± 0.004 for the ascorbic acid, and from 0.13 ± 0.04 to 0.60 ± 0.01 for the BHA. The reducing power of the studied extracts varies from 0.21 ± 0.009 to 0.83 ± 0.003 for oleoresin with concentrations ranging from 0.2 to 1 mg/ml. For essential oil, this capacity varies from 0.26 ± 0.009 to 0.88 ± 0.002 with concentrations ranging from 19.17 to 97.04 mg/ml. Therefore, it appears that the reducing power is sensibly affected by the concentration of the extracts. Indeed, we noted a strong coefficient of correlation (r) for oleoresin (r = 0.99) and essential oil (r = 0.86). These findings corroborate previous studies for other plants.Citation[31]
The reducing power of the oleoresin is clearly higher than that of the oil, which is probably due to the high reducing potential of polyphenols present in the oleoresin. In addition, this general chelating ability of phenolics is probably related to the high nucleophilic character of the aromatic rings rather than to specific chelating groups within the molecule.Citation[32, Citation33] However, the studied extracts present weak reducing power compared to the standards. Quercetin showed the strongest reducing potential compared to the ascorbic acid and to BHA. Thus, the reducing potentials observed are classified in the following decreasing order: quercetin > ascorbic acid > BHA > oleoresin > essential oil.
DPPH radical-scavenging activity
DPPH analysis is one of the tests used to prove the ability of antioxidant components to act as donors of hydrogen atoms.Citation[31, Citation34, Citation35] It has been established that the bleaching of the DPPH solution increased regularly with increasing the amount of polyphenols.Citation[29] illustrates the results of the antiradical activity expressed as a percentage inhibition of DPPH· The scavenging effect increases significantly (p < 0.05) with the increasing of the concentration of standard substances (gallic acid, quercetin, and ascorbic acid) and ginger extracts (oleoresin and essential oil). Others, by studying this same plant reached similar results, also demonstrating that scavenging activity is a concentration dependent effect.Citation[12, Citation13]
Figure 2 Free radical scavenging activity of different concentrations of ginger extracts (oleoresin and essential oil), gallic acid, quercetin, and ascorbic acid by 2,2′-diphenyl-1-picrylhydrazyl radical. Values are expressed as mean ± SD (n = 3), p < 0.05.
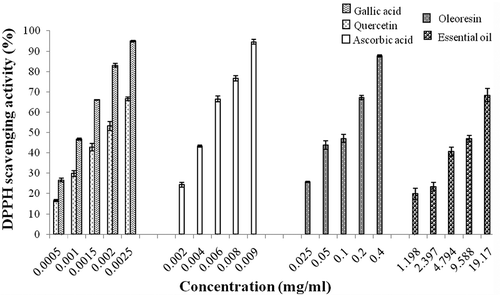
For concentrations of gallic acid and quercetin ranging from 0.0005 to 0.0025 mg/ml, the scavenging activity obtained evolved from 23.10 ± 0.91% to 91.49 ± 0.41% and from 13.07 ± 0.55% to 62.99 ± 0.93%, respectively. For ascorbic acid with concentrations ranging from 0.002 to 0.009 mg/ml, the scavenging activity evolved from 24.36 ± 1.14% to 94.51 ± 1.16%. The scavenger effect of the studied extracts varies from 25.83 ± 0.30% to 87.65 ± 0.46% for oleoresin with concentrations ranging from 0.025 to 0.4 mg/ml and from 19.92 ± 2.80% to 68.31 ± 3.32% for essential oil with concentrations ranging from 1.2 to 19.17 mg/ml. Thus, this scavenging activity is significantly affected by the extracts concentration with a strong coefficient of correlation (r = 0.96 for oleoresin and r = 0.97 for essential oil).
It also appears that as for the reducing power, the scavenging activity is observed at low concentrations for oleoresin compared to essential oil; however, ginger extracts showed less antioxidant activity than the standards. Therefore, the scavenging effects observed are classified in the following decreasing order: gallic acid > quercetin > ascorbic acid > oleoresin > essential oil.
H2O2 Scavenging Activity
H2O2 is a non-radical reactive oxygen species and the most stable intermediate in the four-electron reduction of O2 to water.Citation[36] Since H2O2 is uncharged, it easily passes through cell membranes by diffusion, and when inside the cells it can react with transition metals generating hydroxyl radicals (HO.).Citation[37] At high concentrations, these radicals induce peroxidation of lipids and proteins, affecting cell integrity.Citation[37] Thus, exploring ginger extracts' behavior in the presence of H2O2 remains of high interest.
The effect of the ginger extracts and standards on H2O2 is represented in . The classification of their scavenging activity in the decreasing order of strength is as follows: quercetin > essential oil > gallic acid > oleoresin > ascorbic acid. Except for quercetin, essential oil showed a stronger scavenging activity in inhibiting H2O2 compared to the other antioxidants (gallic acid, ascorbic acid, and oleoresin). EC50 values of reducing power, scavenging activity against DPPH radical, and H2O2 scavenging activity showed significant variation among the ginger extracts and synthetic standards. The calculated EC50 are listed in . In the reducing power test, the lowest EC50 corresponds to the strongest reducing capacity.Citation[38] The values in show that the EC50 of essential oil is greatly higher than that of the oleoresin. Therefore, oleoresin exerts greater reducing power than oil. Likewise, in the DPPH test, the lowest EC50 indicates the highest scavenging capacity of free radicals.Citation[29] The required concentrations (mg/ml) for the neutralization of 50% of the concentration of the DPPH are: 0.13 ± 0.02 for the oleoresin and 11.68 ± 0.39 for essential oil. This confirms the results presented above where a superiority of the oleoresin was observed. However, the required concentrations (mg/ml) for the inactivation of 50% of the H2O2 are: 0.118 ± 0.005 for essential oil and 0.458 ± 0.017 for oleoresin.
Table 1 EC50 values (mg/ml) of ginger extracts and standard antioxidants in reducing power, DPPH scavenging, and H2O2 scavenging assays
Figure 3 Hydrogen peroxide scavenging activity against increasing concentrations of ginger extracts (oleoresin and essential oil), gallic acid, quercetin, and ascorbic acid. Values are expressed as mean ± SD (n = 3), p < 0.05.
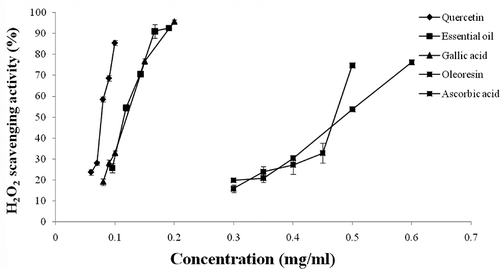
As it has been stated previously, various methods are necessary for the evaluation of antioxidant activity.Citation[39] The weak reducing power of essential oil does not necessarily mean a weak antioxidant activity because in this test one uses an aqueous environment that is unfavorable to non-polar ginger extracts.Citation[40, Citation41] Grassmann et al.Citation[42] and Kulisic et al.Citation[39] using other tests than those used in the present work (the Fenton System, the xanthine oxidase assay, the copper-induced oxidation of low-density lipoprotein (LDL), β-carotene bleaching (BCB) test, and thiobarbituric acid reactive species (TBARS) assay), found that if oils were tested in an aqueous environment, they showed either less or moderated antioxidant activity, but in a lipophilic medium, these same oils showed a strong antioxidant activity. Moreover, the importance of the amphipathic nature of antioxidants has been well demonstrated with α-tocopherol and ascorbic acid; lipophilic α-tocopherol preferentially inhibits the oxidative injury occurring in membranes and lipoproteins, whereas ascorbic acid effectively suppresses oxidative stress in the aqueous phase.Citation[43] This might be a key determinant of the studied extracts showing a strong scavenging activity against the H2O2 in particular by essential oil.
CONCLUSION
The objective of the present work was to study the antioxidant activities of ginger extracts using tests with hydrophilic or lipophilic chemical environments. When using reducing power and DPPH· scavenging assays, the oleoresin showed higher antioxidant activity compared to essential oil. Thus, EC50 of reducing power and DPPH values were 0.56 ± 0.03 versus 63.23 ± 1.59 and 0.137 ± 0.02 versus 11.68 ± 0.39 for oleoresin and essential oil respectively. However, when using the H2O2, scavenging test essential oil showed significantly greater antioxidant activity, and in fact, the EC50 value for essential oil was lower than that of the oleoresin with values of 0.118 ± 0.005 and 0.458 ± 0.017, respectively. In conclusion, the authors' results showed that the same extract exhibits different antioxidant activities depending not only on the concentration but also on the solubility. This highlights the importance of testing the antioxidant properties of Zingiber officinale Roscoe extracts (or other plant extracts) by different tests. However, further experimental work is needed to check whether the effect of solubility observed in the in vitro tests is present in cellular models, such as in red blood cells, epithelial cells, or endothelial cells, following their exposure to oxidative stress.
REFERENCES
- WHO . March 22 1991 . “ Traditional medicine and modern health care: progress report by the director general document A 44(10) ” . In World Health Organization, Geneva March 22 ,
- Szabo , M.R. , Idiţoiu , C. , Chambree , D. and Lupea , A.X. 2008 . Antiradical activity evaluation of some plants used in the Romanian cuisine . International Journal of Food Properties , 11 ( 2 ) : 330 – 338 .
- Kaban , G. 2010 . Volatile compounds of traditional turkish dry fermented sausage (Sucuk) . International Journal of Food Properties , 13 ( 3 ) : 525 – 534 .
- Szabo , M.R. , Radu , D. , Gavrilas , S. , Chambre , D. and Idiţoiu , C. 2010 . Antioxidant and antimicrobial properties of selected spice extracts . International Journal of Food Properties , 13 ( 3 ) : 535 – 545 .
- Jang , J. , Yang , Y.C. , Zhang , G.H. , Chen , H. , Lu , J.L. , Du , Y.Y. , Ye , J.H. , Ye , Q. , Borthakur , D. , Zheng , X.Q. and Liang , Y.R. 2010 . Effect of ultra-violet B on release of volatiles in tea leaf . International Journal of Food Properties , 13 ( 3 ) : 608 – 617 .
- Mascolo , N. , Jain , R. , Jain , S.C. and Capasso , F. 1989 . Ethnopharmacologie investigation of ginger (Zingiber officinale) . Journal of Ethnopharmacology , 27 ( 1–2 ) : 129 – 140 .
- Ali , A. and Gilani , A.H. 2007 . Medicinal value of ginger with focus on its use in nausea and vomiting of pregnancy . International Journal of Food Properties , 10 ( 2 ) : 269 – 278 .
- Ali , B.H. , Blunden , G. , Tanira , M.O. and Nemmar , A. 2008 . Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research . Food and Chemical Toxicology , 46 ( 2 ) : 409 – 420 .
- Zarate , R. and Yeoman , M.M. 1996 . Changes in the amounts of [6]-gingerol and derivatives during a culture cycle of ginger, Zingiber officinale . Plant Science , 121 ( 1 ) : 115 – 122 .
- Rani , K. 1999 . Cyclisation of farnesyl pyrophosphate into sesquiterpenoids in ginger rhizomes (Zingiber officinale) . Fitoterapia , 70 ( 6 ) : 568 – 574 .
- Kayaardi , S. , Durak , F. , Kayacier , A. and Kayaardi , M. 2005 . Chemical characteristics of kavurma with selected condiments . International Journal of Food Properties , 8 ( 3 ) : 513 – 520 .
- Stoilova , I. , Krastanov , A. , Stoyanova , A. , Denev , P. and Gargova , S. 2007 . Antioxidant activity of a ginger extract (Zingiber officinale) . Food Chemistry , 102 ( 3 ) : 764 – 770 .
- Singh , G. , Kapoor , I.P.S. , Singh , P. , de Heluani , C.S. , de Lampasona , M.P. and Catalan , C.A.N. 2008 . Chemistry, antioxidant and antimicrobial investigations on essential oil and oleoresins of Zingiber officinale . Food and Chemical Toxicology , 46 ( 10 ) : 3295 – 3302 .
- Balladin , D.A. , Yen , I.C. , McGaw , D.R. and Headley , O. 1996 . Solar drying of west Indian ginger (Zingiber officinale Roscoe) rhizome using a wire basket dryer . Renewable Energy , 7 ( 4 ) : 409 – 418 .
- Tejasari , N.S. 2007 . Evaluation of ginger (Zingiber officinale Roscoe) bioactive compounds in increasing the ratio of T-cell surface molecules of CD3+CD4+:CD3+CD8+ in-vitro . Malaysian Journal of Nutrition , 13 ( 2 ) : 161 – 170 .
- Chan , E.W.C. , Lim , Y.Y. , Wong , L.F. , Lianto , F.S. , Wong , S.K. , Lim , K.K. , Joe , C.E. and Lim , T.Y. 2008 . Antioxidant and tyrosinase inhibition properties of leaves and rhizomes of ginger species . Food Chemistry , 109 ( 3 ) : 477 – 483 .
- Yen , G.-C. and Duh , P.-D. 1993 . Antioxidative properties of methanolic extracts from peanut hulls . Journal of the American Oil Chemists' Society , 70 ( 4 ) : 383 – 386 .
- Tien , Y.-Y. , Ng , C.-C. , Chang , C.-C. , Tseng , W.-S. , Kotwal , S. and Shyu , Y.-T. 2005 . Studies on the lactic-fermentation of sugar apple (Annona squamosa L.) puree . Journal of Food and Drug Analysis , 13 ( 4 ) : 377 – 381 .
- Kumar , S. , Kumar , D. , Singh , N. and Vasisht , B.D. 2007 . In vitro free radicals scavenging and antioxidant activity of moringa oleifera pods . Journal of Herbal Medicine and Toxicology , 1 ( 2 ) : 17 – 22 .
- Kalyanaraman , K. 1998 . “ Quality specifications for spices and spice products in Europe ” . In Quality Requirements of Spices for Export , Edited by: Sivadasan , C.R. and Kurup, M.P. , C.R. Cochin : Spices Board India .
- Sultan , M. , Bhatti , H.N. and Iqbal , Z. 2005 . Chemical analysis of essential oil of ginger (Zingiber officinale) . Pakistan Journal of Biological Sciences , 8 ( 11 ) : 1576 – 1578 .
- Tewtrakul , S. and Subhadhirasakul , S. 2007 . Anti-allergic activity of some selected plants in the Zingiberaceae family . Journal of Ethnopharmacology , 109 ( 3 ) : 535 – 538 .
- Lapornik , B. , Prosek , M. and Wondra , A.G. 2005 . Comparison of extracts prepared from plant by-products using different solvents and extraction time . Journal of Food Engineering , 71 ( 2 ) : 214 – 222 .
- Cowan , M.M. 1999 . Plant products as antimicrobial agents . Clinical Microbiology Reviews , 12 ( 4 ) : 564 – 582 .
- Kallithraka , S. , Garcia-Viguera , C. , Bridle , P. and Bakker , J. 1994 . Survey of solvents for the extraction of grape seed phenolics . Phytochemical Analysis , 6 ( 5 ) : 265 – 267 .
- Ahui , M.L.B. , Champy , P. , Ramadan , A. , Van , L.P. , Araujo , L. , André , K.B. , Diem , S. , Damotte , D. , Kati-Coulibaly , S. , Offoumou , M.A. , Dy , M. , Thieblemont , N. and Herbelin , A. 2008 . Ginger prevents Th2-mediated immune responses in a mouse model of airway inflammation . International Immunopharmacology , 8 ( 12 ) : 1626 – 1632 .
- Alfaro , M.J. , Bélangera , J.M.R. , Padilla , F.C. and Paré , J.R.J. 2003 . Influence of solvent, matrix dielectric properties, and applied power on the liquid-phase microwave-assisted processes (MAPTM)1 extraction of ginger (Zingiber officinale) . Food Research International , 36 ( 5 ) : 499 – 504 .
- Goyal , R.K. and Kadnur , S.V. 2006 . Beneficial effects of Zingiber officinale on gold thioglucose induced obesity . Fitoterapia , 77 ( 3 ) : 160 – 163 .
- Lim , T.T. and Tee , J.J. 2007 . Antioxidant properties of several tropical fruits: A comparative study . Food Chemistry , 103 ( 3 ) : 1003 – 1008 .
- Frankel , E.N. 1991 . Recent advances in lipid oxidation . Journal of the Sciences and Food Agriculture , 54 ( 4 ) : 495 – 511 .
- Duh , P.-D. , Yen , W.J. , Du , P.-C. and Yen , G.C. 1997 . Antioxidant activity of mung bean hulls . Journal of the American Oil Chemists' Society , 74 ( 9 ) : 1059 – 1063 .
- Becana , M. and Klucas , R.V. 1992 . Transition metals in legume root nodules; iron-dependent free radical production increases during nodule senescence . Proceedings of the National Academy of Science USA , 89 ( 19 ) : 8958 – 8962 .
- Shon , M.-Y. , Kim , T.-H. and Sung , N.-J. 2003 . Antioxidants and free radical scavenging activity of phellinus baumii (Phellinus of Hymenochaetaceae) extracts . Food Chemistry , 82 ( 4 ) : 593 – 597 .
- Yamaguchi , T. , Takamura , H. , Matoba , T. and Terao , J. 1998 . HPLC method for evaluation of the free radical-scavenging activity of foods by using 1,1-diphenyl-2-picrylhydrazyl . Bioscience, Biotechnology, and Biochemistry , 62 ( 6 ) : 1201 – 1204 .
- Lo Scalzo , R. 2008 . Organic acids influence on DPPH scavenging by ascorbic acid . Food Chemistry , 107 ( 1 ) : 40 – 43 .
- da Rosa , C.E. , Bianchini , A. and Monserrat , J.M. 2008 . Antioxidant responses of laeonereis acuta (Polychaeta) after exposure to hydrogen peroxide . Brazilian Journal of Medical and Biological Research , 41 ( 2 ) : 117 – 121 .
- Abele-Oeschger , D. , Sartoris , F.J. and Pörtner , H.O. 1997 . Hydrogen peroxide causes a decrease in aerobic metabolic rate and in intracellular ph in the shrimp Crangon crangon . Comparative Biochemistry and Physiology , 117 ( 2 ) : 123 – 129 .
- Chang , H.-C. , Huang , G.-J. , Agrawal , D.C. , Kuo , C.-L. , Wu , C.-R. and Tsay , H.-S. 2007 . Antioxidant activities and polyphenol contents of six folk medicinal ferns used as “Gusuibu” Botanical Studies , 48 : 397 – 406 .
- Kulisic , T. , Radonic , A. , Katalinic , V. and Milos , M. 2004 . Use of different methods for testing antioxidative activity of oregano essential oil . Food Chemistry , 85 ( 4 ) : 633 – 640 .
- Nagoshi , C. , Shiota , S. , Kuroda , T. , Hatano , T. , Yoshida , T. , Kariyama , R. and Tsuchiya , T. 2006 . Synergistic effect of [10]-gingerol and aminoglycosides against vancomycin-resistant enterococci (VRE) . Biological & Pharmaceutical Bulletin , 29 ( 3 ) : 443 – 447 .
- Schnitzler , P. , Koch , C. and Reichling , J. 2007 . Susceptibility of drug-resistant clinical herpes simplex virus type 1 strains to essential oils of ginger, thyme, hyssop, and sandalwood . Antimicrobial Agents and Chemotherapy , 51 ( 5 ) : 1859 – 1862 .
- Grassmann , J. , Hippeli , S. , Vollmann , R. and Elstner , E.F. 2003 . Antioxidative properties of the essential oil from Pinus mugo . Journal of Agricultural and Food Chemistry , 51 ( 26 ) : 7576 – 7582 .
- Niki , E. 1987 . Antioxidants in relation to lipid peroxidation . Chemistry and Physics of Lipids , 44 ( 2–4 ) : 227 – 253 .