Abstract
Polyphenol oxidase enzyme was isolated from Ispir sugar bean by ammonium sulphate precipitation and its biochemical properties were investigated. For this purpose, KM and Vmax values for optimum conditions of pH, temperature, and ionic strength were determined for catechol, catechin, and chlorogenic acid as substrates. Enzyme activities were measured spectrophotometrically at 420 nm using the same substrates at optimum conditions. KM values were found to be 2.4875, 1.3154, and 2.2487 M for catechol, catechin, and chlorogenic acid, respectively. Vmax values were 3.1480, 0.6130, and 0.5039 EU/ml.min for the same substrates, respectively. These results indicated that catechol was used as a subsrate for inhibition studies. For catechol substrate, dithiothreitol, glutathione, thiourea, and L-cysteine chlorid were inhibitors. For these inhibitors, Ki constants were calculated from Lineweaver-Burk plots and inhibiton types were estimated. Moreover, I50 values were also determined. The most effective inhibitor was found to be glutathione.
INTRODUCTION
Polyphenol oxidase (PPO) is a major enzyme responsible for the browning reaction in damaged plant tissues and fruits.[Citation1 Citation2] Enzymatic browning in plants occurs by the oxidation of phenolic compounds, which are oxidized to quinones in the presence of O2 and PPO following the polymerization of quinones.[Citation3 Citation4] In some food products, browning reactions generally result in a good appearance in terms of colour, but they lead to undesirable results with respect to texture, sweetness, and overall flavour. Therefore, inhibition studies have gained more importance for these types of reactions in food and vegetable processing technology.[Citation5] In this regard, PPO has gained more attention in food technology. The agricultural products are also harmed by enzymatic browning reactions during harvesting, storage, and processing. These problems cause low-quality products and economic losses.[Citation1 Citation6 Citation7] Enzymatic browning, catalyzed by PPO, occurs when plant tissues are damaged. This leads to an economic problem for producers, processors, and consumers. The main step in enzymatic browning is the oxidation of phenolic compounds to corresponding quinones by PPO in the presence of oxygen. Then, the quinones condense to form darkened pigments.[Citation8] These reactions, known as enzymatic browning, are not generally desirable for the food industry but can be used for preparation of dark tea. However, the PPO values, especially of the Ispir sugar bean, gain a special interest for study because beans are an important source of nutrients.
Sugar bean grown in many parts of the world is the most important economic species of the genus Phaseolus. The main domestication areas of dry beans are Central and South America, Eastern and Southern Africa, and East Asia.[Citation9 Citation10] It is widely cultivated as an agricultural crop throughout the world both for dry beans and fresh pods. The world leaders in production of dry beans are Brazil, India, and China with 3.17, 3.00, and 1.23 million tonnes, respectively. The total dry bean production in the world and Turkey is 19.70 and 0.20 million tonnes, respectively. In recent years, the dry bean cultivars of ‘Ispir’ have increasingly become more popular, not only in Erzurum, but also in Turkey as a whole because of their high quality seeds.[Citation10 Citation11]
On the other hand, people need to consume a variety of plant foods to obtain all the amino acids necessary for their body to form complete proteins. There have been many researchs on finding a way to optimize their intake of legumes, such as dry bean, chickpea, lentil, and pea, as part of a healthier lifestyle and to make their body stronger by being a part of an alternative treatment against illnesses. Dry bean is a staple food all over the world and one of the best sources of soluble fiber. It is low in fat and high in good quality protein. The soluble fiber in beans helps to lower the level of damaging LDL cholesterol in the blood and so lower the heart-disease risk. Additionally, it fends off unwanted peaks and valleys in blood glucose levels, especially valuable to people with diabetes by slowing down carbohydrate absorption.[Citation12] Dry bean is also highly rich in starch, protein, and dietary fiber. It is also an excellent source of iron, copper, magnesium, potassium, selenium, molybdenum, thiamine, vitamin B6, and folic acid. Furthermore, dry bean is a base meal of the populations indigenous to Central and South America; Central, Eastern, and Southern Africa; and East Asia where it is variously consumed.[Citation13]
There are many works related to PPO from different plants, such as wheat,[Citation14] Allium sp.,[Citation15] wheat flour,[Citation16] Beta vulgaris L.,[Citation17] tea leaf,[Citation18] lettuce,[Citation19] dog-rose,[Citation20] Ferula sp.,[Citation21] peaches,[Citation22] apples,[Citation23 Citation24] grapes,[Citation25 Citation26] rosmarinus officinalis L.,[Citation27] Salvia species,[Citation28] Thymbra spicata L. var. spicata,[Citation29] green bean,[Citation30] dill,[Citation31] and Thymus.[Citation32] In this work, polyphenol oxidase extracted from Ispir sugar bean was characterized to determine the substrate specificity, kinetic parameters, pH stability, inhibition effects of various inhibitors, optimum pH, and temperature.
MATERIALS AND METHODS
Plant Material
Ispir sugar bean has been used as research material in this study. Ispir sugar bean was collected in the spring sessionfrom a field near Ispir, which is a town located in the northeast of Erzurum, Turkey. The sugar bean samples were then stored at 4°C until used in the study.
Extraction and Partial Purification of PPO
Five grams of each Ispir sugar bean sample was frozen in liquid nitrogen and then powdered. The powdered material was added to 100.0 mL of extraction buffer, 0.5 M phosphate buffer, pH 7.3, containing 0.5% polyethylene glycol and 10 mM ascorbic acid. The crude extract samples were centrifuged at 48,000 g for 30 min at 5°C. The supernatant was brought to 80% (NH4)2SO4 saturation with solid (NH4)2SO4. The precipitated PPO was separated by centrifugation at 48,000 g for 30 min. The precipitate was dissolved in a small amount of 5 mM phosphate buffer (pH 5.2) and dialyzed at 4°C in the same buffer for 24 h with four changes of the buffer during dialysis. The dialyzed sample was kept at 4°C and then was used as the PPO enzyme source in the following experiments.
Determination of PPO Activity
PPO activity was determined by measuring the increase in absorbance at 420 nm with a Beckman spectrophotometer (DU 730; Beckman Coulter Inc., Fullerton, CA, USA). The sample cuvette contained 0.10 mL of the enzyme solution and 2.90 mL of substrate solution in various concentrations. The blank sample contained only 3.00 mL of substrate solution. The reaction was carried out at various temperatures and pH values with the substrates mentioned as follows. PPO activity was calculated from the linear portion of the curve.[Citation33] One unit of PPO activity was defined as the amount of enzyme that caused an increase in absorbance of 0.001/min.
Characterization of PPO
Effect of pH
PPO activity was determined with three different substrates (catechol, catechin, and chlorogenic acid) at a concentration of 10 mM. Appropriate buffers (0.1 M citrate/0.2 M phosphate for pH 4.0–5.5, 0.2 M phosphate for 5.5–7.0, and Tris–HCl for 7.0–10.0) were used for the determination of optimum pH for PPO. The optimum pH values obtained from this assay were used in all subsequent experiments. PPO activities were measured in 0.1 M phosphate buffer (pH 5.0–7.0) and 0.1 M Tris–HCl buffer (pH 7.0–8.5) to determine the stability of PPO. The activity measurements were performed every 2 days by using catechol as a substrate under optimum conditions.
Effect of temperature
For determining optimum temperature values of the enzyme, PPO activity was measured at different temperatures in the range 5–85°C using the three different substrates as indicated above. The desired temperatures were provided by using an ice bath for temperatures under 20°C and a constant-temperature circulator for temperatures above 20°C.
Effect of ionic strength
Ionic strength effect on the enzyme was studied at 10.0 mM substrate concentration using different concentrations of buffers.
Enzyme kinetics and substrate specificity
For the determination of Michaelis constant (K M) and maximum velocity (V max) values of the enzyme, PPO activities were measured with three different substrates varying concentrations in the range of 2.0, 4.0, 6.0, 8.0, 10.0, 12.0, and 15.0 mM under optimized conditions of pH, ionic strength, and temperature. K M and V max values of PPO for each substrate, were calculated from a plot of 1/V versus 1/[S] by the method of Lineweaver and Burk.[Citation34] Substrate specificity (V max/K m) was calculated by using the data obtained on the Lineweaver-Burk plot.
Effect of inhibitors
Inhibitor effects on PPO activity were studied by using the following inhibitors: dithiothreitol, glutathione, thiourea, and L-cysteine chlorid at five different concentrations of inhibitors with 10.0 mM catechol as a substrate at pH 5.5. Percent activity graphs were drawn from these results to find both I50 values. Next, PPO activities were measured at three constant inhibitor concentrations with the inhibitors indicated above using five different concentrations of the substrate. 1/V and 1/[S] values obtained from these activity measurements were used for drawing Lineweaver-Burk graphs. Finally, K i constant values were calculated from the graphs.
RESULTS AND DISCUSSION
Extraction and Purification of PPO Enzyme
PPO activity of the precipitate of 0–80% (NH4)2SO4 saturation was found to be the highest, and this saturation point was used for all the extraction processes. Polyethylene glycol was used during extractions to bind the phenols that could inactivate the PPO. It is well documented that oxidation of phenolics by PPO produces quinones that would inhibit PPO.[Citation35] Therefore, ascorbic acid was also used to reduce quinones to phenolic substrates during extraction.
Characterization of Ispir Sugar Bean PPO
The enzyme samples obtained by ammonium sulfate precipitation were used for the characterization of the PPO. Next, the enzyme samples were dialyzed and kept at 4°C. These samples were used as the PPO enzyme source for the following experiments.
Effect of pH
Activity of purified enzyme was measured with three different substrates to determine optimum pH for each substrate (). Optimum pH with catechol substrate was found to be phosphate buffer pH 5.5, Tris-HCl buffer pH 8.0 with (+) catechin, and phosphate buffer pH 7.0 with chlorogenic acid. Most of the plants, vegetables, and fruits generally show maximum activity at or near neutral pH values.[Citation36] Different optimum pH values for PPO obtained from various sources are reported in the literature. For example, it is reported that optimum pH values are 5.5 for strawberry;[Citation37] 6.0 for DeChaunac grape;[Citation38] 7.0 for Amasya apple,[Citation24] aubergine,[Citation39] Yali pear,[Citation40] and Ferula sp.;[Citation21] 7.5 for Allium sp.;[Citation15] and 8.5 for dog-rose[Citation20] using catechol as a substrate. However, when using 4-methylcatechol as a substrate the pH optimum is 4.5 for strawberry, 6.0 for aubergine,[Citation39] 8.5 for dog-rose,[Citation20] and 9.0 for Amasya apple,[Citation24] and 8.5 for dog-rose[Citation20] using dopamine as a substrate and 7.0 for dog-rose[Citation20] and 8.6 for Amasya apple[Citation24] using pyrogallol as a substrate. PPO activity varies with the source of enzyme and substrate within a relatively wide range of pH. Although, in most cases, pH optima have been reported between 4.0 and 7.0. It is noteworthy that the optimum pH can also be affected by the type of buffer and the purity of enzyme.[Citation39]
Table 1 Optimum pH and temperature, and K M and V max and V max/K M values of Ispir sugar bean's PPO
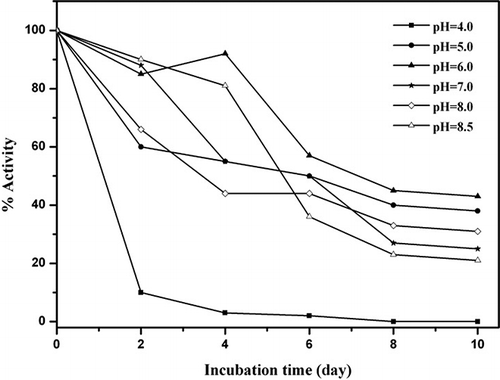
The stability of the enzyme at different pH conditions (4.0–8.5) over a period of 10 days was investigated using catechol as substrate. It was found that PPO activity of Ispir sugar bean was highest at pH 6.0 but decreased at similar rates at each of the pH values studied ().
Effect of temperature
The temperature effects on PPO activity of Ispir sugar bean were studied in the range of 5 and 85°C with each of the three substrates used in the experiments (). As seen in the table, all optimum temperatures are substrate dependent. The optimum temperature is 40°C for catechol, 50°C for catechin, and 20°C for chlorogenic acid. It is reported that the most efficient temperature for PPO is 15°C for Amasya apple,[Citation24] 20°C for DeChaunac grape,[Citation38] 25°C for dog-rose,[Citation20] 30°C for aubergine,[Citation39] 12°C for Ferula sp.,[Citation21] and 40°C for Chinese cabbage[Citation41] using catechol as a substrate; 20°C for dog-rose,[Citation20] 30°C for aubergine,[Citation39] 25°C for Ferula sp.,[Citation21] and 56°C for Amasya apple[Citation24] using 4-methylcatechol as a substrate; and 15°C for dog-rose[Citation20] and 70°C for Amasya apple[Citation24] using pyrogallol as a substrate.
Enzyme kinetics and substrate specificity
In this study, three widely used substrates (catechol, catechin, and chlorogenic acid) were selected for kinetics studies. The K M and V max values were calculated from substrate's Lineweaver-Burk graphs and are shown in . As seen in , the PPO of Ispir sugar bean has a great affinity towards catechin (K M = 1.3154 M) of the three subtrates and the catechol derivatives tested highest, as seen from the high V max/K M ratio (0.021 EU/mmol.s). When the V max values for the three substrates were compared, it was found that the V max for catechol was higher than for the other substrates. Consequently, catechol was used as a substrate in other kinetics studies.[Citation42] This observation was similar to that of the work on PPO from dog-rose,[Citation20] ferula sp.,[Citation21] and Amasya apple.[Citation24] There are a number of compounds, such as dopamine,[Citation20 Citation41] catechol,[Citation20 Citation24 Citation37] chlorogenic acid, L-dopa,[Citation20 Citation24 Citation37 Citation41] pyrogallol,[Citation20 Citation24 Citation37 Citation41] caffeic acid,[Citation37 Citation38] p-cresol,[Citation20 Citation37 Citation41] tyrosine,[Citation20 Citation38] and 4-methylcatechol,[Citation20 Citation24 Citation37] which were used as substrates for polyphenol oxidase in the literature.
Effect of inhibitors
I50 values are shown in for each inhibitor. K i values and inhibition modes for four inhibitors are given in . It was concluded from the Lineweaver-Burk plots that the inhibition modes for all four inhibitors are noncompetitive. The strongest inhibitor was found to be glutathione followed by dithiothreitol, thiourea, and L-cysteine chlorid, respectively. There are a number of inhibitors, such as sodium metabisulphite,[Citation20 Citation38] ascorbic acid,[Citation20 Citation38] glutathione,[Citation27 Citation38] sodium diethyldithiocarbamate,[Citation38] L-cysteine, sodium azide, tannic acid, benzoic acid, and β-mercaptoethanol,[Citation20 Citation27] that were used by researchers to prevent enzymatic browning. L-Cysteine can easily form complexes with quinones and, thereby, inhibiting secondary oxidation and polymerisation reactions.[Citation43] L-Cysteine can also act as a reducing agent.[Citation37] Ascorbic acid reduces quinones to hydroquinones but does not directly inhibit PPO.[Citation44] It will prevent enzymatic browning only as long as it is present in the reduced form. This prevents the formation of key intermediates and inhibits the activity of the oxidase.[Citation45]
Table 2 I50 values for four inhibitors for PPO
Table 3 K i values and inhibition modes for four inhibitors for PPO
CONCLUSION
The charecterization of PPO is important because of the browing reactions, which occur in fruits and vegetables. In this respect, PPO enzyme of Ispir sugar bean was partially purified and PPO activity was measured spectrophotometrically using selected substrates and inhibitors. The best substrate of PPO enzyme was found to be catechol (high V max/K M ratio) while glutathione is the most effective inhibitor. The four inhibitors used in this study caused inhibition effects at a concentration of 10−6 M and can be used safely in the protection of sugar bean.
REFERENCES
- Ricard-Forget , C. and Gauillard , A. 1997 . Oxidation of chlorogenic asid, catechins, and 4 methylcatechol in modal solutions by combinations of pear (Pyrus communis Cv. Williams) polyphenol oxidase and peroxidase: a possible involvement of peroxidase in enzymatic browning . Journal of Agriculture and Food Chemistry , 45 : 2472 – 2476 .
- Singh , H.P. and Ravindanath , S.D. 1994 . Occurrence and distribution of PPO activity in floral organs of some standard and local cultivars of tea . Journal of the Science of Food and Agriculture , 64 ( 1 ) : 117 – 120 .
- Macheix , J-J. , Sapis , J. C. and Fleuriet , A. 1991 . Phenolic compounds and polyphenoloxidase in relation to browning in grapes and wines . Critical Reviews in Food Science and Nutrition , 30 : 441 – 486 .
- Mayer , A.M. and Harel , E. 1991 . “ Phenoloxidases and their significance in fruit and vegetables ” . In Food Enzymology , Edited by: Fox , P.F. 373 – 398 . London , UK : Elsevier Applied Sciences .
- Vamos-Vigyazo , L. 1981 . Polyphenol oxidase and peroxidase in fruits and vegetables . CRC Critical Review in Food Nutrition , 15 : 49 – 127 .
- Eskin , N.A.M. 1990 . “ Biochemistry of food spoilage: Enzymatic browning ” . In Biochemistry of Foods , 2nd , 401 – 432 . New York : Academic Press .
- McEvily , A.J. , Lyengar , R. and Otwell , W.S. 1992 . Inhibition of enzymatic browning in foods and beverages . Critical Reviews in Food Science and Nutrition , 32 : 253 – 273 .
- Gowda , L.R. and Paul , B. 2002 . Diphenol activation of the monophenolase and diphenolase activities of field bean (Dolichos lablab) polyphenol oxidase . Journal of Agricultural and Food Chemistry , 50 : 1608 – 1614 .
- Gepts , P. and Bliss , F.A. 1986 . Phaseolin variability among wild and cultivated common beans (Phaseolus vulgaris) from Colombia . Economic Botany , 40 : 469 – 478 .
- Topcu , Y. , Uzundumlu , A.S. and Yavuz , F. 2010 . Designing the marketing strategies for Ispir sugar bean as a local product using conjoint analysis . Scientific Research and Essays , 5 ( 9 ) : 887 – 896 .
- Ozturk , I. , Kara , M. , Yildiz , C. and Ercisli , S. 2009 . Physico-mechanical seed properties of the common Turkish bean (Phaseolus vulgaris) cultivars ‘Hinis’ and ‘Ispir’ . New Zealand Journal of Crop and Horticultural Science , 37 : 41 – 50 .
- Bazzano , L.A. , He , J. , Ogden , L.G. , Loria , C.M. and Whelton , P.K. 2003 . Dietary fiber intake and reduced risk of coronary heart disease in US men and women: The National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study . Archives of Internal Medicine , 163 : 897 – 904 .
- Queiroz , K.S. , de Oliveira , A.C. and Helbig , E. 2002 . Soaking the common bean in a domestic preparation reduced the contents of refines-type oligosaccharides but did not interfere with nutritive value . Journal of Nutritional Science and Vitaminology , 48 : 283 – 289 .
- Okot-Kotber , M. , Liavoga , A. , Yong , K.J. and Bagorogoza , K. 2002 . Activation of polyphenol oxidase in extracts of bran from several wheat (Triticum aestivum) cultivars using organic solvents, detergents, and chaotropes . Journal of Agricultural and Food Chemistry , 50 ( 8 ) : 2410 – 2417 .
- Arslan , O. , Temur , A. and Tozlu , I. 1997 . Polyphenol oxidase from Allium sp . Journal of Agricultural and Food Chemistry , 45 : 2861 – 2863 .
- Yadav , D.N. , Patki , P.E. , Srihari , S.P. , G.K.Sharma , G.K. and Bawa , A.S. 2010 . Studies on polyphenol oxidase activity of heat stabilized whole wheat flour and its chapatti making quality . International Journal of Food Properties , 13 : 142 – 154 .
- Escribano , J. , Gandia-Herrero , F. , Caballero , N. and Pedreno , M.A. 2002 . Subcellular localization and isoenzyme pattern of peroxidase and polyphenol oxidasein beet root (Beta vulgaris L.) . Journal of Agricultural and Food Chemistry , 50 : 6123 – 6129 .
- Hadler , J. , Tamuli , P. and Bhaduri , A.N. 1998 . Isolation and characterization of polyphenol oxidase from Indian tealeaf (Camellia sinensis) . Journal of Nutritional Biochemistry , 9 : 75 – 80 .
- Chazarra , S. , Cabanes , J. , Escribano , J. and Garcia-Carmona , F. 1996 . Partial purification and characterization of latent polyphenoloxidase in iceberg lettuce (Lactuca sativa L.) . Journal of Agricultural and Food Chemistry , 44 : 984 – 988 .
- Sakiroglu , H. , Küfrevioglu , I.Ö. , Kocacaliskan , I. , Oktay , M. and Onganer , Y. 1996 . Purification and characterization of Dog-rose (Rosa dumalis Rechst.) polyphenol oxidase . Journal of Agricultural and Food Chemistry , 44 : 2982 – 2986 .
- Erat , M. , Sakiroglu , H. and Kufrevioglu , O.I. 2006 . Purification and characterization of polyphenol oxidase from ferula sp . Food Chemistry , 95 : 503 – 508 .
- Flurkey , W.H. and Jen , J.J. 1980 . Purification of peach polyphenol oxidase in the presence of added protease inhibitors . Journal of Food Biochemistry , 4 : 29 – 41 .
- Murata , M. , Tsurutani , M. , Tomita , M. , Homma , S. and Kaneko , K. 1995 . Relationship between apple ripening and browning: Changes in polyphenol content and polyphenol oxidase . Journal of Agricultural and Food Chemistry , 43 : 1115 – 1121 .
- Oktay , M. , Kufrevioglu , I. , Kocacaliskan , I. and Sakiroglu , H. 1995 . Polyphenol oxidase from Amasya apple . Journal of Food Science , 60 : 495 – 499 .
- Lamikanra , O. , Sharon , D.K. and Mitwe , N.M. 1992 . Muscadine grape polyphenol oxidase: Partial purification by high-pressure liquid chromatography and some properties . Journal of Food Science , 57 : 686 – 689 .
- Yilmaz , H. , Sakiroglu , H. and Küfrevioglu , I. 2003 . Polyphenol oxidase from Mazruma Grape (Vitis vinifera L.) . Agrochimica , XLVII ( 1–2 ) : 21 – 27 .
- Aydemir , T. 2010 . Selected kinetic properties of polyphenol oxidase extracted from Rosmarinus Officinalis L . International Journal of Food Properties , 13 : 475 – 485 .
- Gundoğmaz , G. , Doğan , S. and Arslan , O. 2003 . Some kinetic proporties of polyphenol oxsidase obtained from various Salvia species (Salvia viridis L., Salvia virgata Jacq., and Salvia tomentosa Miller) . Food Science and Technology International , 9 ( 4 ) : 309 – 316 .
- Doğan , S. , Turan , P. and Doğan , M. 2006 . Some kinetic properties of polyphenol oxidase from Thymbra spicata L. var. Spicata. Process Biochemistry , 41 ( 12 ) : 2379 – 2385 .
- Guo , L. , Ma , Y. , Shi , J. and Xue , S. 2009 . The purification and characterisation of polyphenol oxidase from green bean (Phaseolus vulgaris L.) . Food Chemistry , 117 ( 1 ) : 143 – 151 .
- Sakiroglu , H. , Ozturk , A.E. , Pepe , A.E. and Erat , M. 2008 . Some kinetic properties of polyphenol oxidase obtained from dill (Anethum graveolens) . Journal of Enzyme Inhibition and Medicinal Chemistry , 23 ( 3 ) : 380 – 385 .
- Dogan , S. and Dogan , M. 2004 . Determination of kinetic proporties of polyphenol oxsidase from Thymus (Thymus longicaulis subsp. Chaubardii var. Chaubardii) . Food Chemistry , 88 ( 1 ) : 69 – 77 .
- Wong , T.C. , Luh , B.S. and Whitaker , J.R. 1971 . Isolation and characterization of polyphenol oxidase isoenzymes of Clingstone peach . Plant Physiology , 48 : 19
- Lineweaver , H. and Burk , D. 1934 . The determination of enzyme dissociation constant . Journal of American Chemists Society , 56 : 658 – 666 .
- Walker , J.R.L. 1964 . Studies on the enzymic browning of apples: Properties of apple polyphenol oxidase . Australian Journal of Biological Sciences , 17 : 360 – 371 .
- Siddiq , M. , Sinha , N.K. and Cash , J.N. 1992 . Characterization of polyphenoloxidase from stanley plums . Journal of Food Science , 57 : 1177 – 1179 .
- Wesche-Ebeling , P. and Montgomery , M.W. 1990 . Strawberry polyphenol oxidase: Extraction and partial characterization . Journal of Food Science , 55 : 1320 – 1325 .
- Lee , C.Y. , Smith , N.L. and Penesi , A.P. 1983 . Polyphenol oxidase from DeChaunac grapes . Journal of the Science of Food and Agriculture , 34 : 987 – 991 .
- Dogan , M. , Arslan , O. and Dogan , S. 2002 . Substrate specificity, heat inactivation and inhibition of polyphenol oxidase from different aubergine cultivars . International Journal of Food Science and Technology , 37 : 415 – 423 .
- Zhou , H. and Feng , X. 1991 . Polyphenol oxidase from Yali pear (Pyrus bretschneiderl) . Journal of the Science of Food and Agriculture , 57 : 307 – 313 .
- Nagai , T. and Suzuki , N. 2001 . Partial purification of polyphenol oxidase from Chinese cabbage (Brassica rapa L) . Journal of Agricultural and Food Chemistry , 49 : 3922 – 3926 .
- Baritaux , O. , Amiot , M.J. , Richard , H. and Nicolas , J.J. 1991 . Enzymatic browning of basil (Ocimum basilicum L.) studies on phenolic compounds and polyphenol oxidase. Sciences des Aliments , 11 : 49 – 62 .
- Davis , R. and Pierpoint , W.S. 1975 . Problem of the reactive species from enzymic and chemical oxidation of o-diphenols: anomalies in the trapping of o-quinonoids with benzenesulfinic acid . Biochemistry Society Transactions , 3 : 671
- Anderson , J.W. 1968 . Extraction of enzymes and subcellular organelles from plant tissues . Phytochemistry , 7 : 1973 – 1988 .
- Schwartz , B. , Olgin , A.K. and Klinman , J.P. 2001 . The role of copper in topa quinone biogenesis and catalysis, as probed by azide inhibition of a copper amine oxidase from yeast . Biochemistry , 40 : 2954 – 2963 .