Abstract
Asphodelus aestivus Brot. is a common spring-flowering geophyte encountered on the Marmara, Aegean, and Mediterrenean coasts of Turkey, which has been utilized traditionally for culinary and medicinal purposes. In the present study, different antioxidant tests were employed in order to evaluate the antioxidant activities of methanolic and acetone extracts of A. aestivus Brot. leaves. In addition, the results were compared with natural and synthetic antioxidants. The levels of total flavonoid, total chlorophyll, and total carotenoid contents of the extracts were also determined. Both extracts showed good total flavonoid content, inhibited lipid peroxidation, and showed radical scavenging activities.
INTRODUCTION
Plants and their products are rich sources of phytochemicals, and they possess a variety of biological activities including antioxidant potential. Natural antioxidants are in high demand for applications, such as nutraceuticals, bio-pharmaceuticals, as well as food additive because of consumer preference.[Citation1] Currently, there is a great interest in finding antioxidants from natural sources to minimize oxidative damage to cells. Oxidative damage is caused by free radicals and reactive oxygen species, mostly generated endogenously. They are recognized to be involved in the pathogenesis of various diseases, such as atherosclerosis, cancer, diabetes mellitus, and reperfusion disorder. Researchers have demonstrated that appropriate consumption of foods containing antioxidants, such as herbs and vegetables, can prevent such deleterious effects of free radicals and reactive oxygen species.[Citation2,Citation3]
The genus Asphodelus (Asphodelaceae or Liliaceae) is a circum-Mediterranean genus, which includes five sections and it is presented by 16 species. A. aestivus Brot. is a common spring-flowering geophyte encountered on the Marmara, Aegean, and Mediterrenean coasts of Turkey that has been utilized traditionally for culinary and medicinal purposes.[Citation4,Citation5] A new class of anthraquinone-anthrone-C-glycosides has been isolated from A. ramosus. Sesquiterpene lactones, flavonoids, and anthraquinones have been reported from A. aestivus, A. globifera, A. anatolica, and A. damascene, whereas arylcoumarins, anthraquinones, and glycosides have been isolated from A. microcarpus. The bulbs and roots of A. microcarpus are used as an antimicrobial agent.[Citation6–11 Citation Citation Citation Citation Citation11 The leaves of A. aestivus Brot. are commonly consumed cooked as a vegetable dish in Turkey, where it is known as “ciris otu.” In traditional medicine, the tuber and the roots of this plant are used against hemorrhoids, nephritis, burns, and wounds.[Citation12,Citation13] Extracts of A. aestivus roots were reported to have gastroprotective effect against ethanol-induced lesions.[Citation14] To the authors' knowledge, there are no published reports on the antioxidant activity of the extracts of A. aestivus Brot.
The aim of this study was to investigate the influence of the methanolic and acetone extracts of leaves of A. aestivus Brot. on stable radicals. Using different chemical reaction-based assays, the reductive potential, β-carotene bleaching effect, total antioxidant, and scavenging activities of these extracts were evaluated. Their total flavonoid, total chlorophyll, and total carotenoid contents were also studied. These antioxidant activities were compared to those of standard synthetic antioxidants, such as BHA, BHT, Trolox, epicatechin, ascorbic acid, and α-tocopherol.
MATERIALS AND METHODS
Chemicals
2,2′-Azino-bis(3-ethybenzothiazoline-6-sulfonic acid) (ABTS), nicotinamide adenine dinucleotide (NADH), 6-hydroxy-2,5,7,8-tetramethyl-chroman-2-carboxylic acid (Trolox), nitroblue tetrazolium (NBT), phenazine methosulphate (PMS), the free 1,1-diphenyl-2-picryl-hydrazyl (DPPH•) radical, linoleic acid, polyoxyethylenesorbitan monolaurate (Tween-20), and trichloroacetic acid (TCA) were obtained from Sigma-Aldrich (GmbH, Steinheim, Germany). Butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) were provided from Fluka (Buchs, Switzerland). Ammonium thiocyanate was purchased from Merck (Darmstadt, Germany). All other chemicals were of analytical grade and obtained from either Sigma-Aldrich, Fluka, or Merck.
Plant Material and Extraction Procedure
The leaves of A. aestivus Brot. were purchased in May from a local market in Istanbul, Turkey and identified by Associate Professor Dr. Tamer Ozcan (Institute of Botany, Department of Biology, Istanbul University). Plant materials were washed with deionized water and dried at room temperature. The dried parts were put in a plastic bag and stored at −20°C until used. The extracts were prepared by refluxing 10 g of the dried leaves with 100 ml of methanol or 100 ml of acetone for 2 h at Soxhlet apparatus and filtering through linen cloth at room temperature. The filtrates were evaporated to dryness under reduced pressure and controlled temperature (40–50°C) in a rotary evaporator, then they were weighed to determine the total extractable compounds. The crude extracts were kept at −20°C and used for the assessment of antioxidant activity.
Determination of Total Flavonoid
The total flavonoid contents of the extracts were determined according to colorimetric method.[Citation15] Briefly, 0.25 mL of the extract (1000 μg/mL) or (+)-catechin standard solution (2–20 μg/mL) was mixed with 1.25 mL of distilled water in a test tube, followed by the addition of 75 μL of a 5% (w/v) sodium nitrite solution. After 6 min, 150 μL of a 10% (w/v) aluminium chloride solution was added and the mixture was allowed to stand for a further 5 min before 0.5 mL of 1 M sodium hydroxide was added. The mixture was brought to 2.5 mL with distilled water and mixed well. The absorbance was measured immediately at 510 nm using a spectrophotometer. The amount of total flavonoids was calculated as μg of (+)-catechin equivalents from the calibration curve of (+)-catechin standard solution (covering the concentration range between 2–20 μg/ml) and expressed as μg catechin/mg of extract. Data were presented as the average of triplicate analyses.
Determination of Total Chlorophyll and Total Carotenoid
Total chlorophyll and total carotenoid content were calculated using the modified equations of Lichtenthaler and Wellburn.[Citation16] A total of 10 mg of each extract of leaves was dissolved with 10 ml of distilled water. The absorbance of the sample was measured at 450, 645, and 663 nm in the UV-Vis light spectrophotometer. The total chlorophyll and total carotenoid content were calculated using the equations as follows:
β-Carotene Bleaching Test
Approximately 10 mg of trans-β-carotene was dissolved in 10 mL of chloroform, and 0.2 mL of the solution was placed in a boiling flask containing 20 mg of linoleic acid and 200 mg of Tween-40. After removal of the chloroform, 50 mL of distilled water was added to the flask and vigorously shaken. Then, 5 mL of this emulsion were added to tubes containing the 0.2 mL of A. aestivus Brot. leaf extracts. The extracts were dissolved in distilled water (2 mg/mL). The tubes were stopped and placed in a water bath at 50°C. Spectrophotometric readings at 470 nm were taken after 60 and 120 min of incubation. BHA (2 mg/mL) was used for comparative purposes. A control solution containing 0.2 mL of distilled water and 5 mL of the above emulsion was prepared. Relative antioxidant activities (RAA) were calculated with the following formula:
Reducing Power Assay
The reducing power of the acetone and methanolic extracts were measured according to the method of Oyaizu.[Citation18] Various concentrations of extracts (20–100 μg) in 1 ml of distilled water were mixed with 2.5 ml of phosphate buffer (0.2 M, pH 6.6) and 2.5 ml potassium ferricyanide [K3Fe(CN)6] (1%, w/v), and the mixture was incubated at 50°C for 30 min. Afterwards, 2.5 ml of trichloroacetic acid (10%, w/v) was added to the mixture and centrifuged at 3000 rpm for 10 min. Finally, 2.5 ml of upper-layer solution was mixed with 2.5 ml distilled water and 0.5 ml FeCl3 (0.1%, w/v), and the absorbance was measured at 700 nm. α-Tocopherol, BHA, and BHT were used as standard antioxidants. Higher absorbance of the reaction mixture indicated greater reducing power.
DPPH Radical-Scavenging Assay
The free radical scavenging activity of the acetone and methanolic extracts were measured with 1,1-diphenyl-2-picryl-hydrazil (DPPH•) using the slightly modified method.[Citation19] Briefly, 20 mg/L DPPH• solution in methanol was prepared and 1.5 ml of this solution was added to 0.75 ml of the sample, BHA, BHT, α-tocopherol, and ascorbic acid solution (100–500 μg/ml). The mixture was shaken vigorously and kept at room temperature for 30 min. Then the absorbance of the mixture was measured at 517 nm. Water (0.75 ml) in place of the plant extract was used as the control. The decrease in the absorbance indicates an increase in DPPH radical scavenging activity. This activity was calculated by the following equation:
where A0 is the absorbance of the control reaction and A1 is the absorbance in the presence of the extracts or standards. The extract concentration providing 50% inhibition (IC50) was calculated from the graph of inhibition percentage plotted against extract concentration.
ABTS•+ Assay
For ABTS cation radical scavenging assay, the procedure followed the method of Arnao et al.[Citation20] with some modifications. The stock solutions included 7.4 mM ABTS solution and 2.6 mM potassium persulfate solution. The working solution was then prepared by mixing the two stock solutions in equal quantities and incubating them for 12 h at room temperature in the dark. The solution was then diluted by mixing 1 ml ABTS•+ solution with 60 ml methanol to obtain an absorbance of 1.1 ± 0.02 units at 734 nm using the spectrophotometer. Fresh ABTS•+ was prepared for each assay. Plant extracts (150 μl) were allowed to react with 2850 μl of the ABTS•+ solution for 2 h in the dark. Then the absorbance was measured at 734 nm using the spectrophotometer. The standard curve was linear between 100 and 500 μM Trolox. Results are shown as μM Trolox equivalents (TE)/μg extract.
Inhibition on Linoleic Acid Peroxidation
The antioxidant activity was determined according to the thiocyanate method with slight modifications.[Citation21] For the stock solution, 10 mg of extracts were dissolved in 10 ml of water. Then, the different amounts of stock solution or standards samples (20–100 μg) prepared in 2.5 ml of potassium phosphate buffer (0.04 M, pH 7.0) was added to 2.5 ml of linoleic acid emulsion. Linoleic acid emulsion contains Tween-20 (175 μg), linoleic acid (155 μl), and potassium phosphate buffer (0.04 M, pH 7.0). In addition, a solution containing 2.5 ml of linoleic acid emulsion and 2.5 ml of potassium phosphate buffer (0.04 M, pH 7.0) was prepared as the control. Each solution was then incubated at 37°C in a glass flask in the dark. At 24-h intervals during incubation, 0.1 ml of this incubation solution was added to 4.7 ml of 75% (v/v) ethanol and 0.1 ml of 30% (w/v) ammonium thiocyanate. Precisely 3 min after the addition of 0.1 ml of 0.02 M FeCl2 in 3.5% (w/v) HCl to the reaction mixture, the absorbance of the red colour was measured at 500 nm in a spectrophotometer. The solutions without added extracts or standards were used as a control. The inhibition of lipid peroxidation in percentage was calculated by the following equation:
where A0 is the absorbance of the control reaction and A1 is the absorbance in the presence of the extracts or standards.
Superoxide Radical Scavenging Activity
Measurement of superoxide anion scavenging activity of acetone and methanolic extracts of A. aestivus Brot. were based on the method described by Liu et al.[Citation22] Superoxide anions were generated in a non-enzymatic phenazine methosulfate-nicotinamide adenine dinucleotide (PMS-NADH) system by oxidation of NADH and assayed by reduction of nitroblue tetrazolium (NBT). In this experiment, the superoxide anion was generated in 3 ml of Tris-HCl buffer (16 mM, pH 8.0) containing 1 ml of NBT (50 μM) solution, 1 ml of NADH (78 μM) solution, and different concentrations (0.1–1.0 mg/ml) of sample solution. The reaction was started by adding 1 ml of PMS solution (10 μM) to the mixture. The reaction mixture was incubated at 25°C for 5 min and absorbance at 560 nm was recorded against blank samples in a spectrophotometer. Epicatechin, BHA, and Trolox were used as standard samples (0.1–1.0 mg/ml). The inhibition of superoxide radical generation (%) was calculated by the following equation:
where A0 is the absorbance of the control reaction and A1 is the absorbance in the presence of the extracts or standards.
Statistical Analysis
The experimental results were given as mean ± standard deviation of three parallel measurements. Statistical comparisons were performed using the Student's t-test. Differences were considered significant at p < 0.05.[Citation23]
RESULTS AND DISCUSSION
Determination of Total Flavonoid
Flavonoids are a main class of polyphenols in plants. They are known as antioxidants and free radical scavengers.[Citation24] The antioxidant activity of plants has been correlated to the total flavonoid content. Total flavonoid contents of methanolic and acetone extracts from A. aestivus Brot. leaves were shown in . The results were expressed as catechin equivalent of flavonoids per mg of sample extract. The total flavonoid content of acetone extract (17.74 ± 0.46 μg catechin/mg of extract) was nearly eight-fold higher than methanolic extract (2.30 ± 0.02 μg catechin/mg of extract). The method using acetone showed a greater efficiency in the extraction of flavonoids than that with methanol. However, the extraction with acetone resulted in lower yields of extractable compounds. The yield for methanol and acetone extraction were 3.52 g (70%) and 1.09 g (22%), respectively. The authors' results are higher than the work of Sun et al.,[Citation25] who reported that the methanolic extract (0.9 ± 0.4 mg rutin/g dry weight) and acetone extract (1.6 ± 0.6 mg rutin/g dry weight) of broccoli also had flavonoid contents.
Table 1 The total flavonoid, total chlorophyll, and total carotenoid contents of extracts from Asphodelus aestivus Brot. leaves
Determination of Total Chlorophyll and Total Carotenoid
The major group of the pigments, chlorophyll a and b, and carotenoids are the non-polyphenolic compounds of plants. It has been known that all these pigments exhibit significant antioxidant activities. Total chlorophyll and carotenoid contents of extracts of A. aestivus Brot. were shown in . Acetone extract of the plant exhibited significantly higher total chlorophyll and carotenoid content compared to methanolic extract. Therefore, flavonoid, chlorophyll a and b, and carotenoid as non-polyphenolic compounds might also contribute to antioxidant activity of the acetone extract.
β-Carotene Bleaching Test
β-Carotene has a strong biological activity and it is a physiologically important compound. Two important properties of β-carotene are its ability to trap certain organic free radicals and to deactivate the excited molecules, particularly excited or singlet oxygen.
In the β-carotene bleaching test, the methanol and acetone extracts of A. aestivus Brot. exhibited higher activity than the positive control BHA at 60 min (1.77 ± 0.05 and 1.66 ± 0.04, respectively) and this activity was only slightly reduced after 120 min (1.24 ± 0.02 and 1.24 ± 0.03) of incubation (). The result obtained with the methanolic and acetone extract of A. aestivus Brot. leaves was higher than that obtained from the methanolic extracts of Cistus ladanifer (0.66 ± 0.01) and Cupressus lusitanica leaves (0.44 ± 0.04) at 120 min.[Citation26] Thus, it is apparent that A. aestivus extracts have strong effects against the discoloration of β-carotene.
Table 2 β-Carotene bleaching test.Footnote a BHA was used as reference antioxidant
Reducing Power Assay
In the reducing power assay, the presence of antioxidants in the extracts results in the reduction of the Fe3+/ferricyanide complex to its ferrous form. shows the extent of the reduction, in terms of absorbance values at 700 nm, for the extracts ranging in concentration from 20–100 μg/ml. From a comparison of the absorbance at 700 nm, the reducing power of acetone extract (0.218 ± 0.011) was found to be significantly similar to those of BHT (0.224 ± 0.009) and α-tocopherol (0.203 ± 0.019) at 20 μg/ml concentration. The reducing power of the acetone extract, BHT, α-tocopherol, and BHA was increased with increasing concentration. The methanol extract, at all concentrations, showed weaker reducing power than the other extract and the standards.
Figure 1 Reducing power of the extracts from Asphodelus aestivus Brot. leaves. α-Tocopherol, BHA, and BHT were used as reference antioxidants. Values are means ± SD (n = 3).
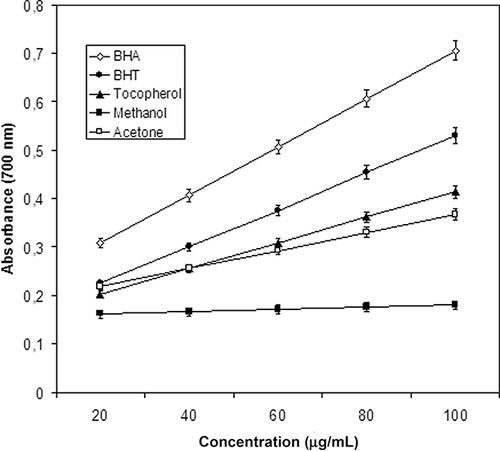
These results revealed that methanolic and acetone extracts of A. aestivus Brot. leaves were moderate electron and hydrogen donors and could terminate the radical chain reaction, converting free radicals to more stable products. The authors' results were in accordance with other investigators who have also reported that antioxidant properties are concomitant with the development of reducing power.[Citation27]
DPPH Radical-Scavenging Assay
Antioxidant properties, especially radical scavenging activities, are very important due to the deleterious role of free radicals in foods and in biological systems. Excessive formation of free radicals accelerates the oxidation of lipids in foods and decreases food quality and consumer acceptance.[Citation28] Hydrogen-donating ability is an index of the primary antioxidants. These antioxidants donate hydrogen to free radicals, leading to non-toxic species and therefore to inhibition of the propagation phase of lipid oxidation. DPPH assay evaluates the ability of antioxidants to scavenge free radicals. The DPPH radical scavenging effects of A. aestivus extracts were presented in . All the extracts showed antiradical activity by inhibiting DPPH radical in a concentration-dependent manner. The free radical scavenging activity of the methanolic extract was higher than acetone extract at all concentrations. The acetone extract exhibited a poor activity of 34.34 ± 0.01% at a concentration of 0.5 mg/ml. The methanol extract showed an inhibition of 78.72 ± 0.23 at a concentration of 0.5 mg/ml. However, when compared to reference antioxidants, BHA, BHT, α-tocopherol, and ascorbic acid, all of the tested extracts showed significantly (p < 0.05) lower DPPH radical scavenging activity.
Figure 2 DPPH radical scavenging activity of the extracts from Asphodelus aestivus Brot. leaves. α-Tocopherol, ascorbic acid, BHA, and BHT were used as reference antioxidants. Values are means ± SD (n = 3).
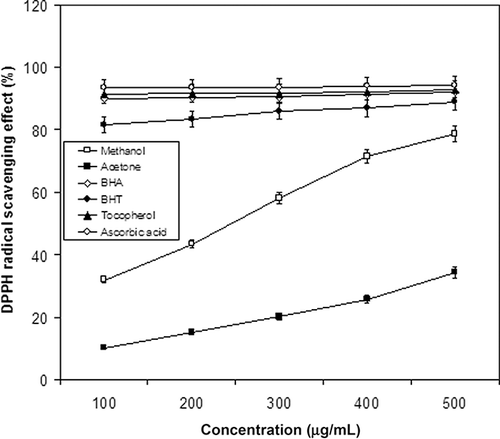
The DPPH scavenging activities of the extracts, expressed as an IC50 value (the inhibitory concentration at which the DPPH radicals were scavenged by 50%), ranged from 0.16 ± 0.009 to 0.50 ± 0.015 mg/ml. IC50 value of methanolic extract (0.16 ± 0.009 mg/ml) was higher than that of acetone (0.50 ± 0.015 mg/ml) extract. A higher DPPH radical scavenging activity is associated with a lower IC50 value. It was evident that the extracts did show the hydrogen donating ability to act as antioxidants. IC50 values, in scavenging abilities on DPPH radicals, were significantly different (p < 0.05) from the IC50 values obtained for ascorbic acid (53.57 μg/ml), α-tocopherol (54.77 μg/ml), BHA (55.59 μg/ml), and BHT (61.19 μg/ml).
ABTS•+ Assay
One of the most commonly used organic radicals for the evaluation of antioxidant efficiency of pure compounds and complex mixtures is the radical cation derived from 2,2′-azinobis-3-ethyl-benzothiazoline-6-sulfonic acid (ABTS). These radical cations could be generated by enzymatic, chemical, and electrochemical means. The extent and the rapidity with which polyphenols quench the ABTS radical cation chromophores are the criteria that are used to assess their relative antioxidant capacity compared to a standard antioxidant, Trolox. In these ABTS•+-based methods, it is assumed that the antioxidants simply reduce the radicals back to the parent substrate, ABTS.[Citation29] The antioxidant activity measurements of methanolic and acetone extracts from A. aestivus Brot. are expressed as Trolox equivalent antioxidant capacity and were presented in . All extracts showed antioxidant activities proving their capacity to scavenge the ABTS•+ radical cation. ABTS•+ scavenging ability of methanolic and acetone extracts of A. aestivus Brot. leaves were 318.90 ± 0.68 and 318.26 ± 1.56 μmol Trolox equivalent at 100 μg/ml, respectively. These results showed that the methanolic and acetone extracts of the plant exhibited a higher ABTS•+ scavenging activity.
Table 3 ABTS•+ scavenging ability of the extracts from Asphodelus aestivus Brot. leaves. The results were expressed as μmol Trolox equivalent at 20, 40, 60, 80, and 100 μg/ml of extract, respectively
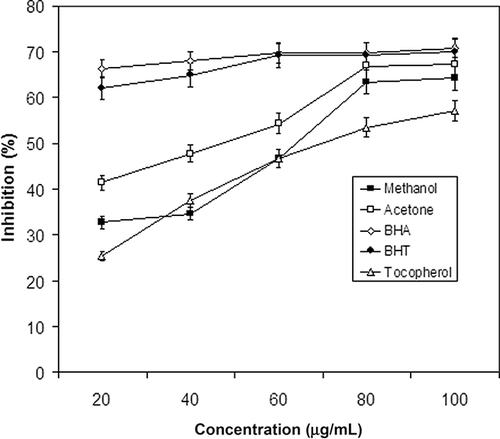
Inhibition on Linoleic Acid Peroxidation
Total antioxidant activity of A. aestivus Brot. extracts were determined by the thiocyanate method in linoleic acid emulsion. The amount of peroxide in the initial stages of lipid oxidation was measured every 24 h, over a period of 6 days. The effect of A. aestivus extracts at different concentration on peroxidation of linoleic acid emulsion was shown in . The antioxidant activity of A. aestivus methanolic and acetone extract was compared with commercial antioxidants, such as α-tocopherol, BHT, and BHA. The extracts showed maximum antioxidant activity at 80 and 100 μg/ml concentrations. Total antioxidant activity of the acetone (67.24 ± 0.01%) and the methanol extract (64.31 ± 0.05%) at 100 μg/ml did not show any significant difference (p > 0.05) from that of BHA (70.82 ± 0.08%) and BHT (69.98 ± 0.56%) at the same concentration. The acetone and methanol extracts, at higher concentrations (80 and 100 μg/ml), exhibited higher activities than the well-known standard α-tocopherol at 100 μg/ml (57.17 ± 0.23%). These results showed that the A. aestivus Brot. methanolic and acetone extracts have a strong total antioxidant activity compared to α-tocopherol.
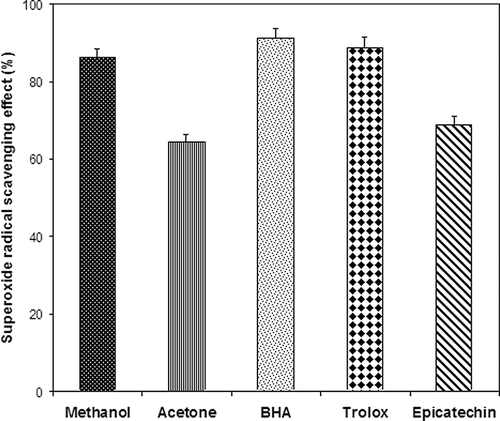
Superoxide Radical Scavenging Activity
The superoxide anions are well-recognized free radical species and they are generated continuously by several cellular processes, including the microsomal and mitochondrial electron transport systems. Although the superoxide anion is limited in activity, its combination with other reactive species, such as nitric oxide that is produced by macrophages, might yield more reactive species. The results () showed that the methanolic extract had a higher superoxide radical scavenging ability than the acetone extract. At a concentration of 1.0 mg/ml, the inhibition activities were 86.11 ± 0.51% and 64.44 ± 0.41% for the methanolic and acetone extract, respectively. The superoxide radical scavenging activity by 1.0 mg/mL of BHA, Trolox, and epicatechin was found to be 91.11 ± 0.55%, 88.88 ± 0.31%, and 68.88 ± 0.42%, respectively. Methanol extract exhibited significantly higher superoxide anion scavenging activity than epicatechin (p < 0.05). The superoxide radical scavenging activity of those samples were in the following order BHA > Trolox > methanolic extract > epicatechin > acetone extract. These results showed that methanolic extract from the leaves has a scavenging effect on superoxide radicals.
CONCLUSION
In this study, the antioxidant and radical scavenging activities of the extracts of Asphodelus aestivus Brot. leaves growing in Turkey were demonstrated. The authors' results showed that the plant has a powerful antioxidant activity at various antioxidant systems in vitro. Both of the extracts were potent radical scavengers, and their antioxidant capacities seem to be related to their chemical compositions. The reducing power and total antioxidant activities of the acetone extract were higher than the methanolic extract whereas the methanol extract exhibited higher free radical and superoxide anion radical scavenging ability compared to acetone extract. Therefore, active extracts of A. aestivus Brot. might be an alternative to more toxic synthetic antioxidants as additives in food, pharmaceutical, and cosmetic preparations.
ACKNOWLEDGMENT
The authors would like to thank Associate Professor Dr. Tamer Ozcan (Istanbul University, Department of Biology, Institute of Botany) for the identification of the plant.
REFERENCES
- Verma , A.R. , Vijayakumar , M. , Rao , C.V. and Mathela , C.S. 2010 . In vitro and in vivo antioxidant properties and DNA damage protective activity of green fruit of Ficus glomerata . Food and Chemical Toxicology , 48 : 704 – 709 .
- Abraham , L.C.N. , Masakuni , T. , Isao , H. and Hajime , T. 2008 . Antioxidant flavonoid glycosides from the leaves of Ficus pumila L . Food Chemistry , 109 : 415 – 420 .
- Yildiz , G. , Vatan , O. , Celikler , S. and Dere , S. 2011 . Determination of the phenolic compounds and antioxidative capacity in red algae Gracilaria bursa-pastoris . International Journal of Food Properties , 14 : 496 – 502 .
- Matthews , V.A. and Asphodelus , L. 1984 . Flora of Turkey and the East Aegean Islands , Edited by: Davis , P.H. Vol. 8 , 85 – 86 . Edinburg : University Press .
- Baytop , T. 1999 . Therapy with Medicinal Plants in Turkey (Past and Present) , 362 – 365 . Istanbul , , Turkey : Nobel Presshouse .
- Rizk , A.M. , Hammouda , F.M. and Abdel-Gawad , M. 1972 . Anthraquinones of Asphodelus microcarpus . Phytochemistry , 11 : 2122 – 2125 .
- Adinolfi , M. , Corsaro , M.M. , Lanzetta , R. , Parrilli , M. and Scopa , A. 1989 . A bianthrone C-glycoside from Asphodelus ramosus tubers . Phytochemistry , 28 : 284 – 288 .
- Adinolfi , M. , Lanzetta , R. , Marciano , C.E. , Parrilli , M. and De Giulio , A. 1991 . A new class of anthraquinone-anthrone-C-glycosides from Asphodelus ramosus tubers . Tetrahedron , 47 : 4435 – 4440 .
- Van Wyk , B.E. , Yenesew , A. and Dagne , E. 1995 . Chemotaxonomic significance of anthraquinones in the roots of Asphodeloideae (Asphodelaceae) . Biochemical Systematics and Ecology , 23 : 277 – 281 .
- Calis , I. , Birincioglu , S.S. , Kirmizibekmez , H. , Pfeiffer , B. and Heilmann , J. 2006 . Secondary metabolites from Asphodelus aestivus . Zeitschrift für Naturforschung B: Chemical Sciences , 61 : 1304 – 1310 .
- El-Seedi , H.R. 2007 . Antimicrobial arylcoumarins from Asphodelus microcarpus . Journal of Natural Products , 70 : 118 – 120 .
- Tuzlaci , E. and Aymaz-Eryasar , P. 2001 . Turkish folk medicinal plants, Part IV: Gönen (Balıkesir) . Fitoterapia , 72 : 323 – 343 .
- Ugurlu , E. and Secmen , O. 2008 . Medicinal plants popularly used in the villages of Yunt Mountain (Manisa-Turkey) . Fitoterapia , 79 : 126 – 131 .
- Gurbuz , I. , Ustun , O. , Yesilada , E. , Sezik , E. and Akyurek , N. 2002 . In vivo gastroprotective effects of five Turkish folk remedies against ethanol-induced lesions . Journal of Ethnopharmacology , 283 : 241 – 244 .
- Zhishen , J. , Mengcheng , T. and Jianming , W. 1999 . The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals . Food Chemistry , 64 : 555 – 559 .
- Lichtenthaler , H.K. and Wellburn , A.R. 1983 . Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents . Biochemical Society Transactions , 11 : 591 – 592 .
- Bruni , R. , Muzzoli , M. , Ballero , M. , Loi , M.C. , Fantin , G. , Poli , E. and Sacchetti , G. 2004 . Tocopherols, fatty acids and sterols in seeds of four Sardinian wild Euphorbia species . Fitoterapia , 75 : 50 – 61 .
- Oyaizu , M. 1986 . Studies on products of browning reaction prepared from glucose amine . Japanese Journal of Nutrition , 44 : 307 – 315 .
- Brand-Williams , W. , Cuvelier , M.E. and Berset , C. 1995 . Use of a free radical method to evaluate antioxidant activity . Food Science and Technology–Lebensmittel Wissenschaft und Technologie , 28 : 25 – 30 .
- Arnao , M.B. , Cano , A. and Acosta , M. 2001 . The hydrophilic and lipophilic contribution to total antioxidant activity . Food Chemistry , 73 : 239 – 244 .
- Osawa , T. and Namiki , N. 1981 . A novel type of antioxidant isolated from leaf wax of Eucalpyptus leaves . Agricultural and Biological Chemistry , 45 : 735 – 739 .
- Liu , F. , Ooi , V.F.C. and Chang , S.T. 1997 . Free radical scavenging activity of mushroom polysaccharide extracts . Life Sciences , 60 : 763 – 771 .
- Hintze, J.L. Number Cruncher Statistical System. Kaysville, UT, USA, NCSS, 1986
- Scalbert , A. , Manach , C. , Morand , C. and Remesy , C. 2005 . Dietary polyphenols and the prevention of diseases . Critical Reviews in Food Science and Nutrition , 45 : 287 – 306 .
- Sun , T. , Powers , J.R. and Tang , J. 2007 . Evaluation of the antioxidant activity of asparagus, broccoli and their juices . Food Chemistry , 105 : 101 – 106 .
- Guimaraes , R. , Sousa , M.J. and Ferreira , I.C.F.R. 2010 . Contribution of essential oils and phenolics to the antioxidant properties of aromatic plant . Industrial Crops and Products , 32 : 152 – 156 .
- Andarwulan , N. , Batari , R. , Sandrasari , D.A. , Bolling , B. and Wijaya , H. 2010 . Flavonoid content and antioxidant activity of vegetables from Indonesia . Food Chemistry , 121 : 1231 – 1235 .
- Min , D.B. 1998 . “ Lipid oxidation of edible oil ” . In Food Lipids Chemistry, Nutrition, and Biotechnology , Edited by: Akoh , C.C. and Min , D.B. NewYork : Marcel Decker .
- Osman , A.M. , Wong , K.K.Y. and Fernyhough , A. 2006 . ABTS radical driven-oxidation of polyphenols: Isolation and elucidation of covalent adducts . Biochemical and Biophysical Research Communications , 346 : 321 – 329 .