Abstract
Wild sage seed is a small, rounded, and mucilaginous seed, which comes from Salvia macrosiphon. The viscoelastic behavior of sage seed gum, at different concentrations (0.5–2%, w/w), was examined by measuring the transient (in-shear structural recovery and creep/recovery tests) and dynamic (stress and frequency sweeps) rheological properties. The mechanical spectra showed typical weak gel behavior at all concentrations, with storage modulus higher than loss modulus, and little variation with frequency. Both moduli greatly increased with increasing the concentration, and the concentration dependency was well described by the power-law model. The loss tangent was increased slightly with increasing the frequency in the range of 0.25–0.67, although it was not affected by an increase in gum concentration. Moreover, the complex viscosity was found to increase with the increase of sage seed gum concentration and to decrease linearly with the increase of frequency. All samples showed typical viscoelastic response to stress in creep/recovery tests, with recoverable strain increasing in direct proportion to sage seed gum concentration. Creep curves were adequately fitted with a Burger model of four parameters. The elastic and viscous contributions to the general viscoelastic behavior were analyzed through the obtained parameters. The concentration had no specific effect on the in-shear recovery properties of sage seed gum gels, and the gel structure was highly recovered after applying shear. The results of this article indicated that sage seed gum may offer an excellent alternative for commercial gums as a thickening/gelling agent.
INTRODUCTION
Hydrocolloids as thickener, gelling, and stabilizer agents can help to enhance the texture and taste of foods. The global hydrocolloid market was worth about $19.0 billion in 2006, growing at about 3–4% per year between 2006 and 2011, and valued at $22.5 billion by 2011.Citation[1] Factors feeding demand include increases in processed/prepared foods and growing consumer interest in healthier and more nutritional products. The volume share of food ingredients depends on the security of their supply, quality, and price. Hydrocolloids from plants have the advantage over those from animals because of their friendly image towards consumers.Citation[2] In the last decade, significant efforts have been dedicated to find new structure-functionality issues, in order to maximize hydrocolloids’ effectiveness and to produce novel formulations. However, there is still a place in the hydrocolloid market for new sources of plant hydrocolloids to meet the demand for ingredients with more specific functionality in food and chemical systems. Some plants, growing in different regions of Iran, have valuable polysaccharides. Seeds extract from these plants can be used as novel food hydrocolloid sources.Citation[3] For example, the seeds of plants known as Alyssum homolocarpum (Qodume Shirazi),Citation Citation Citation[4–6] Lallemantia royleana (Balangu),Citation Citation Citation[7–9] Lepidium perfoliatum (Qodume Shahri),Citation[10] Lepidium sativum (Shahi),Citation[11,12] Ocimum basilicum L. (Reihan),Citation Citation Citation[13–15] Plantago major L. (Barhang),Citation[16,17] and Salvia macrosiphon (Marve)Citation Citation Citation[18–20] have been traditionally used as herb seeds and pharmaceutical sources for hundreds of years, due to their valuable medical effects. However, these seeds are known to contain mucilage in large amounts that could be suitable for applications as stabilizing and thickening agents.Citation Citation[3,10] In this sense, our research works have been focused on the study of physical properties of seeds, extraction optimization, chemical analysis, functional properties, and food applications of hydrocolloid extract of these domestic seeds since four years ago.
Wild sage seed (Salvia macrosiphon) is a small rounded seed, which readily swells in water to give mucilage.Citation[19] This mucilage can be extracted quantitatively with water by a centrifugal filtering system. Bostan et al. optimized the extraction process conditions of sage seed gum (SSG) using response surface methodology.Citation[18] At optimum conditions, the yield, apparent viscosity (1%, 122 s−1 and 25°C), and emulsion stability of SSG were determined as 10.1%, 312 mPa.s, and 403 min, respectively. The SSG powder obtained at optimum conditions contained 6.72% moisture, 0.85% lipid, 8.17% ash, 2.84% protein, 1.67% crude fiber, and 79.75% carbohydrate. There is no more information about the chemical structure of SSG because of the novelty of the subject. Razavi et al.Citation[20] studied the steady-shear flow behavior of SSG dispersions as a function of SSG concentration (0.5–2%) and temperature (20–50°C). The results demonstrated that SSG dispersion has high zero-shear-rate-viscosity and very shear-thinning behavior at all conditions tested. In addition, it was even more pronounced than those reported for commercial hydrocolloids like xanthan, guar gum, and locust bean gum. The Heschel-Bulkley model was found to be the most suitable model to describe steady-shear flow behavior of SSG dispersions. They suggested application of this new gum as a stabilizer, thickener, binder, and gelling agent in food, cosmetics, and pharmaceutical systems. Up to now, many papers have been published on the viscoelastic characterization of different seed gums,Citation Citation Citation Citation Citation Citation Citation Citation Citation[21–29] while no information on the sage seed extract is available. Therefore, the purpose of this study was to investigate the linear viscoelastic properties of sage seed gum, as well as their structural breakdown/recovery behavior under steady shear, as a function of gum concentration.
MATERIALS AND METHODS
Gum Extraction
Sage seed gum was extracted at optimum conditions (temperature 25°C, water to seed ratio 51:1, and pH 5.5) using the same procedure as described by Bostan et al.Citation[18] The dispersions were dried overnight in a forced convection oven (Model 4567, Kimya Pars Com., Mashad, Iran) at 70°C, then milled and sieved using a mesh 18 sifter.
Sample Preparation
Seven concentration levels of sage seed gum in aqueous solutions (0.5, 0.75, 1, 1.25, 1.5, 1.75, and 2% w/w) were prepared by dispersing the sage gum powder in deionized distilled water at ambient temperature (25°C). Sodium azide (0.02%) was added to all dispersions as an anti-microbial preservative. The samples were stirred at 300 rpm for 24 h, and kept for 24 h at room temperature (25°C) to complete the hydration prior to the experiments. The samples were then loaded onto the rheometer to evaluate the rheological properties.
Rheological Tests
All rheological measurements were carried out using a controlled-stress/strain rheometer (Gemini 150, Bohlin Instruments Ltd., Malvern, UK) equipped with cone-plate geometry (40 mm of diameter, 4° cone angle, and 1 mm gap). The temperature was fixed, using a Peltier system, at 20°C and then each sample was equilibrated, at least for 5 min, before the rheological test. All rheological tests were performed, at least, in triplicate.
Small amplitude oscillatory shear (SAOS) measurements
First of all, stress sweep tests in oscillatory shear were conducted in the range of 0.01 to 150 Pa at 20°C, and a constant frequency of 1 Hz. These experiments were carried out in order to determine the maximum deformation attainable by all samples in the linear viscoelastic range (LVR). Frequency sweep tests were then conducted, within the LVR (maximum strain of 1%), in the range of 0.01 to 10 Hz, at a constant temperature of 20°C. The mechanical spectra obtained were characterized by the storage modulus (G′), loss modulus (G″), complex modulus (G*), complex dynamic viscosity (η*), and the loss tangent (tan δ) as a function of frequency (f, Hz).
Creep/recovery measurements
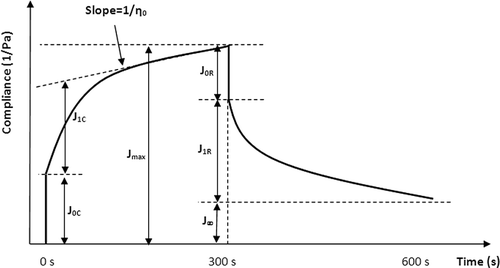
Creep/recovery tests were performed at constant shear stress (1 Pa) and temperature (20°C). The stress was applied instantly and kept for a period of 300 s. After removing stress, recovery compliance was measured also during 300 s. The creep and recovery compliances (JC and JR ) were the resulting strain (γ) divided by the applied stress (τ) during creep and recovery periods, respectively. In this study, based on the creep/recovery behavior observed (), the four-component Burger model, comprising the association in series of the Maxwell model and the Kelvin–Voigt model, was used to describe the transient data. In this model, the creep compliance, JC , as a function of time, corresponds to the following equation:Citation[30]
Full mechanical characterization of a system can be established by calculating the contribution of the compliances of EquationEqs. (1) and Equation(2), at the maximum deformation to which the system is subjected. The percentage deformation of each element of the system can be calculated by:Citation[31]
In addition, the final percentage recovery (R, %) of the entire system can be calculated by the following equation:
In-shear structural recovery measurements
In-shear structural recovery of the samples was determined according to the procedure of Mezger.Citation[32] The sample was loaded onto the rheometer at 20°C, then a three-stepped shear flow test was performed as follows: (1) a constant shear rate of 1 s−1 was applied for 120 s (with pre-shear at 1 s−1 for 30 s), and subsequently, (2) a constant shear rate of 300 s−1 was applied for 60 s, and then (3) a constant shear rate of 1 s−1 was applied for 180 s. The in-shear recovery value was calculated as the ratio of average apparent viscosity (η a3) obtained during the first 120 s of the third step to the average η a1 value determined in the first step.
RESULTS AND DISCUSSION
SAOS Rheological Functions
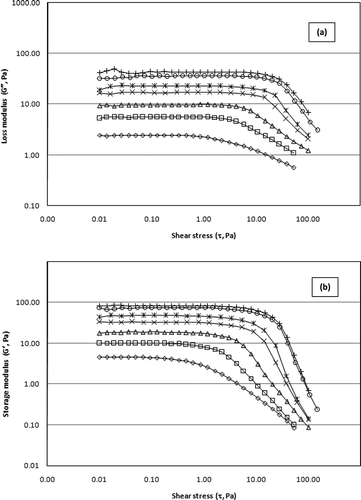
Figure 2 shows the values of the linear viscoelasticity moduli, G′ and G″, as a function of shear stress obtained from stress sweep performed at a fixed frequency (1 Hz) and temperature (20°C), in a shear stress range from 0.01 to 150 Pa. As can be observed, all SSG samples show an initial linear viscoelastic region where both linear viscoelastic moduli are independent of the stress. also illustrates the effect of gum concentration on the extension of the linear viscoelastic regime and on the stress dependence in the nonlinear region. The SSG samples exhibited higher dependency to stress amplitude at lower concentrations; therefore, lower stress values must be attained to ensure linear viscoelastic properties. Generally, there was no abrupt change in G′ up to a stress amplitude of 0.33 Pa for 0.5% concentration. Thus, the frequency sweep tests conducted at 1% strain were well within the LVR, and the gel network was not damaged by the strain imposed during the measurements. In all cases, the storage modulus was approximately two orders of magnitude greater than the loss modulus over the entire range of stresses, indicating the presence of a gel structure.
Figure 2 Stress dependence (1 Hz, 20°C) of (a) loss modulus and (b) storage modulus for sage seed gum at different concentrations (◊, 0.5%; □, 0.75%; △, 1%; ×, 1.25%; *, 1.5%; ◯, 1.75%; +, 2%).
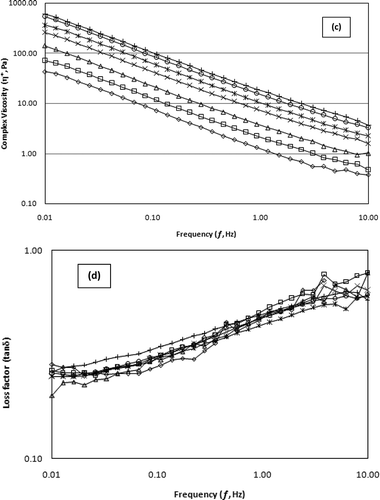
Figures 3a–3d shows the mechanical spectra of SSG samples as a function of frequency at 20°C and strain amplitude of 1.0%. It can be found that all the samples were characterized by a gel behavior with a slight dependency of G′ and G″ on the frequency, over the range of 10−2 to 10 Hz, with G′ greater than G″, linear reduction in log η* with increasing log f, and Tan δ < 1. The G′ and G″ curves of all concentrations were almost parallel and a crossover between the two moduli was not observed throughout the frequency domain. The mechanical spectra of SSG at different concentrations indicate a weak gel behavior.Citation[33] This type of behavior has been observed for some gums, such as psyllium gels,Citation[34] waxy maize starch/xanthan mixtures,Citation[35] Corchorus olitorius leaves’ hydrocolloid,Citation[36] konjac gluco-mannan/xanthan solutions,Citation[37] gellan gels,Citation[38] and k-carrageenan/LBG gels.Citation[39] These “gel like” properties may be attributed to tenuous association of ordered chains and it has been reported for some conventional gums like xanthan above ∼0.3 w/v.Citation[40] On the other hand, some researchers observed a liquid-like behavior at a lower frequency (G″ > G′) and solid-like behavior at a higher frequency (G′ > G″), which is a typical behavior of concentrated polymer solutions.Citation Citation Citation Citation Citation Citation Citation[21,23,24,26–29] The SSG gels exhibited very similar/higher values of both G″ and G′, compared with the other gums like guar and tragacanth,Citation[41] fenugreek gum, guar gum, tara gum and locust bean gum,Citation[24] Mucuna flagellipes seed gum,Citation[23] fenugreek seed gum,Citation[26] and lesquerella fendleri seed gumCitation[29] reflecting pronounced elastic behavior and strong network development in sage seed gum as a new hydrocolloid.
In the present study, SSG gels exhibited lower Tan δ at low frequencies, but it increased slightly at higher frequencies, showing a higher contribution of the viscous component at higher frequencies. Tan δ is a measure of the energy loss per cycle relative to energy stored per cycle, which characterizes the sol–gel transition. The low values of tan δ (<1) show a tendency toward more solid-like behavior and it observed the loss factor values around 0.2–0.3 for amorphous polymers, and near 0.01 for glassy crystalline polymers and gels.Citation Citation[34,42] In this article, the loss factor of SSG gels was mean 0.25 (±0.02) at 0.01 Hz and increased to average value of 0.67 (±0.08) at 10 Hz in the concentration range of 0.5 to 2.0%.
The frequency dependencies of G′ and G″ for physical gels were approximated by a power-law model as follows:Citation[42]
The magnitudes of slopes (n′ and n″), intercepts (k′ and k″), and R 2 (determination coefficient) were summarized in . It was found that sage seed gum displayed gel-like behavior because the slopes (n′ = 0.20–0.24 and n″ = 0.37–0.41) were positive, which were much lower than those reported for a Maxwellian fluid (G′ ∞ ω2 and G″ ∞ ω). There was no difference between n′ or n″ values at different concentrations, indicating no effect of concentration on the slopes’ values. The magnitudes of k′ (7.28–107.15) were much higher than those of k″ (3.42–51.66), showing the G″/G′ ratio was mean 0.47, and confirming a gel-like behavior of SSG in the concentration range of 0.5–2.0%.Citation[43] As shown in , increase in the concentration of SSG also increased considerably the values of k′ and k″, suggesting an increase in the stiffness of continuous phase due to the thickening effect of SSG. In this article, the values of intercepts increased about twofold with an increase in SSG concentration by 0.25%.
Table 1 Frequency dependency of elastic and viscous moduli of SSG at different concentrations and constant temperature of 20°C
Figure 4 shows how the dynamic rheological parameters of SSG gels vary with respect to gum concentration. In general, both storage and loss moduli increased dramatically with increasing the SSG concentration, reflecting stronger gel structure at higher concentrations, although the loss factor remained relatively unchanged. The concentration dependencies of the storage and loss moduli can be represented by the following power law relationships:
The power-law exponent value of approximately 2 is in good agreement with that reported for one-component thermo-reversible gels.Citation Citation Citation[44–46] Square power-law relationship is valid only when the concentration range investigated is far greater than the critical gelling concentration.Citation[47]
Creep/Recovery Compliance
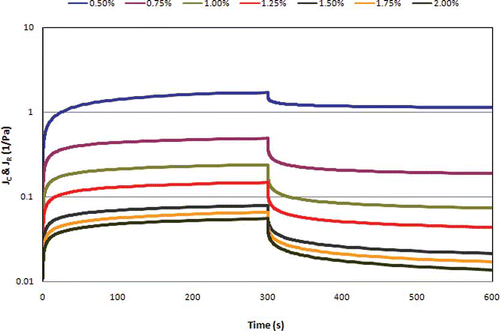
The examination of viscoelastic properties of SSG gels was followed by shear creep/recovery experiments. The creep/recovery curves of sage seed gum as a function of concentration is shown in . It is observed that SSG samples showed viscoelastic behavior, and the creep/recovery compliances decreased with concentration because of solidity. There was no full recovery even after 600 s for all cases studied. In viscoelastic materials, according to Burger's model, recovery of the applied stress is partial, controlled by the more elastic or viscous character of the sample, situated at an intermediate position between solid and liquid. The greater the compliance, the easier it is to deform the sample. In this study, the strains generated in the sample containing 0.5% SSG was high; however, for the other concentrations studied, the curve of compliance versus time had the form characteristic of a predominantly elastic network, indicating a viscoelastic solid behavior of sage seed gum (). It means that when the stress was applied, there was a sharp and instantaneous increase in strain, followed by a slight increase over time, which can be attributed to some re-arrangement of network structure. On removal of the stress, there was also a corresponding sharp reduction in compliance, with little residual deformation at the end of the recovery period. At concentrations higher than 1.25%, the SSG gels were almost recovered to their original states, and permanent deformation was small after removal of stress, indicating an elastic gel structure (). The higher the recovery phase, the higher is the viscoelastic solid character of the sample. In addition, it is obvious that the systems very quickly approached the equilibrium compliance conditions.
Figure 5 Compliance versus time in “creep and recovery test” for sage seed gum at different concentrations (applied shear stress: 1 Pa; temperature: 20°C). (Color figure available online.)
The fitting of JC = f(t), in the interval 0 ≤ t ≤ 300 s, for all the concentrations studied and based on the Burger model (EquationEq. 1), yielded values of r ≥ 0.983 in all cases, indicating that the Burger model of four elements was adequate to describe SSG creep behavior. shows an example of the experimental creep compliance data and the values predicted by the Burger model for SSG gel at 2% concentration. The values of Burger model parameters, including J 0C , J 1C , η0, and λ ret and the correlation coefficient obtained, are listed in .
Table 2 Creep parameters of the Burger model, obtained from the fits by EquationEq. (1), for sage seed gum at different concentrations (20°C).a
The instantaneous elastic compliance (J 0C ) represents the value of instantaneous shear creep compliance at initial time, and it may be related to the undisturbed hydrocolloid network structure.Citation[37] A higher value of J 0C reflects a higher degree of non-retarded Hookean-type (elastic) deformation, representing that the network is relatively free to rearrange between cross-links.Citation[22] In this case, J 0C decreased with concentration, indicating that the SSG becomes more rigid and has greater hardness at higher concentration.
The retarded compliance (J 1C ) represents the principal component of the viscoelastic behavior of SSG. It was observed that J 1C decreased with concentration (). The decrease of this parameter is associated with a more solid (elasticity) and less viscoelastic behavior. In all cases studied, the contribution of J 1C to the total deformation was larger than the contribution of J 0C , reinforcing the concept of a behavior closer to a viscoelastic material than to an elastic one. The sum of J 0C and J 1C is called the steady state compliance.Citation[30] Thus, creep process of SSG gels consisted mainly of steady state compliance, which is important in view of gelation.
The Newtonian viscosity of the free dashpot (η0) increased considerably with SSG concentration (). η0 measures the mechanical behavior of the fluid part of the system. In fact, this parameter is associated with the breakdown of the gel network structure. It also characterizes the linear region of the viscous compliance.Citation[30] In this study, the slope of the linear region (1/η0) approached to zero when concentration increased from 0.5 to 2.0%. It means that the time period of creep phase was sufficient to access steady flow conditions.
The retardation time (λ ret ) was not affected by concentration, except for increasing from 0.5 to 0.75%, which it was decreased greatly by 50% (). The retardation time is unique for each material. Generally, the higher the retardation time of a system network, the longer it takes to reach full deformation on application of shear stress.Citation[30] This also implies that retardation times are inversely related to network viscoelasticity.
The fits of the values of recovery compliance (JR ) as a function of time, have been made based on EquationEq. (2). The results obtained, together with the parameters B and C, and the correlation coefficients are presented in . For all cases analyzed, correlation coefficients were 0.999, confirming the excellent fitting quality of EquationEq. (2) in describing the recovery phase. J 0R and J 1R represent instantaneous elastic compliance and retarded compliance at the recovery phase, respectively. It can be seen that there was a similar trend for J 0R and J 1R with concentration, as observed for J 0C and J 1C in the creep phase (). The residual compliance at the end of the recovery period (J ∞) decreased greatly as the SSG concentration increased, showing more recovery and elasticity at higher concentrations. Furthermore, it was found that the B parameter decreased generally with an increase in SSG concentration, whereas the C parameter increased slightly when the concentration increased, indicating the speed of structural recovery of sage seed gum network increased by increasing the concentration of SSG ().
Table 3 Recovery parameters, obtained from the fits by EquationEqs. (2) and Equation(3), for sage seed gum at different concentrations (20°C).a
Table 4 shows J max, J 0C *, J 1C *, J 2C *, J 0R *, J 1R *, J ∞*, and R for SSG samples. It can be seen that J max decreased greatly with concentration, however, J 0C *, J 1C *, and J 2C * parameters were affected slightly by the concentration of SSG. It seems that when concentration is increased, the increase in interaction of molecules allows smaller deformation of the SSG network. In all cases, the retarded elastic deformation percentage, J 1C *, was greater than the instantaneous elastic deformation percentage, J 0C *, and the viscosity flow deformation percentage, J 2C *. It means that the retarded elastic deformation played the most important role in the total creep deformation, followed by the instantaneous elastic deformation and the viscosity flow deformation. In the case of recovery, we see that the percentage deformation of the elements of the recovery phase (J 0R *, J 1R *, J ∞*) are in the same proportion of the creep phase. As it can be found from , the contribution of Kelvin–Voigt element (J 1R *) was higher than the other two elements, Maxwell spring (J 0R *) and Maxwell dashpot (J ∞*), except for 0.5% concentration, in which the contribution of Maxwell dashpot (J ∞*) was considerably greater than the others. Moreover, the contribution of residual compliance at the end of the recovery period (J ∞*) decreased by about 43% as the concentration of SSG increased from 0.5 to 2%.
Table 4 Maximum compliance and creep/recovery normalized parameters for sage seed gum at different concentrations (20°C).a
In this study, the final percentage recovery values (R, %) ranged from 34.03 to 77.71%, indicating a viscoelastic behavior of sage seed gum (). As the concentration of SSG samples increased, the value of R increased and SSG network can recover up to approximately 78% of its maximum deformation. This parameter gives clear evidence of SSG elasticity behavior with concentration. The results obtained by creep/recovery analysis were in agreement with the dynamic assay results ( and ).
Table 5 In-shear recovery properties of sage seed gum as a function of concentration at 20°C.a,b,c
In-Shear Structural Recovery
The in-shear structural recovery test was carried out in order to investigate the capability of the SSG gels to recover their original structure under low shear conditions after decomposition under high-shear conditions.Citation Citation[32,35] The results of in-shear recovery experiments are presented in . It can be found that increasing SSG concentration had no distinct effect on the in-shear structural recovery. In addition, the structural recovery of SSG samples were obtained relatively high (>50%) and it was mean at 57.63%, indicating rapid rearrangement of structure after the high shear step. This result is useful for practical applications, in which the resistance to high shear conditions (like pumping and mixing) and fast recovery of the initial viscosity (anti-thixotropy) are very important factors for selection of a suitable stabilizer/thickener. As expected, increase in SSG concentration increased considerably the apparent viscosity both at low and high shear rates (). For example, increasing the concentration from 0.5 to 1.0, 1.5, and 2.0% resulted in an increase of apparent viscosity of step 1 (η a1) by 162, 298, and 639%, respectively. This result was in agreement with the steady shear results reported for SSG.Citation[20] In addition, increase in concentration of samples by 0.25% increased the apparent viscosity (η a ) by about 44 ± 0.01%, independent of shear rate used. It means that the viscosity decrease caused by thixotropic behavior from steps 1–3 was little.
CONCLUSION
Mechanical spectra obtained by frequency sweep tests showed that the sage seed gum at all concentrations ranging from 0.5 to 2% (w/w) exhibited weak gel behavior like some commercial gums, because G′ was larger than G″ throughout the frequency range and the loss factor was larger than 0.1. Creep tests at different concentrations allowed gaining a better understanding of viscoelastic properties of sage seed gum. The decreasing of J 0 and J 1, and increasing of η0 with concentration may be explained by more stabilizing of the SSG network. A semi-empirical approach was used to obtain the contribution of each compliance parameter to the total deformation of the system. The contribution of J 1 to the total deformation was larger than the contribution of J 0, reinforcing the concept of a behavior closer to a viscoelastic material than to an elastic one. The in-shear recovery properties of SSG gels showed that there is no dependency to concentration and the structure can be relatively highly recovered after applying high shear conditions. The results of this article will contribute to the research of new potential of hydrocolloids, as a substitute to the conventional ones as thickening/gelling agents, and to the development of novel food formulations, addressing the demands of the modern consumer.
ACKNOWLEDGMENTS
The authors would like to thank Cristina Fuentes and Juan Andres Sandoval for their technical assistance.
REFERENCES
- Will, R.; Loechner, U.; Yokose, K. Hydrocolloids. CEH report. (accessed April 12, 2010), 2010 http://www.sriconsulting.com/CEH/Public/Reports/582.7000 (http://www.sriconsulting.com/CEH/Public/Reports/582.7000)
- Williams , P.A. and Phillips , G.O. 2000 . “ Introduction to good hydrocolloids ” . In Handbook of Hydrocolloids , Edited by: Phillips , G.O. and Williams , P.A. 1 – 19 . Cambridge , , UK : Woodhead Publishing .
- Razavi , S.M.A. , Farhoosh , R. and Bostan , A. 2007 . Functional properties of hydrocolloid extract of some Iranian seeds. Research project No. 1475 , Iran : Unpublished report. Ferdowsi University of Mashhad .
- Koocheki , A. , Mortazavi , S.A. , Shahidi , F. , Razavi , S.M.A. , Kadkhodaee , R. and Milani , J.M. 2010 . Optimization of mucilage extraction from Qodume Shirazi seed (Alyssum homolocarpum) using response surface methodology . Journal of Food Process Engineering , 33 ( 5 ) : 861 – 882 .
- Koocheki , A. , Mortazavi , S.A. , Shahidi , F. , Razavi , S.M.A. and Taherian , A.R. 2009 . Rheological properties of mucilage extracted from Alyssum homalocarpum seed as a new source of thickening agent . Journal of Food Engineering , 91 : 490 – 496 .
- Koocheki , A. and Razavi , S.M.A. 2009 . Effect of concentration and temperature on flow properties of Alyssum homolocarpum seed gum solutions: Assessment of time dependency and thixotropy . Food Biophysics , 4 : 353 – 364 .
- Razavi , S.M.A. , Emadzadeh , B. and Zahedi , Y. June 15–19 2008 . “ Comparisons of different methods for measuring yield stress in selected hydrocolloids ” . In Proceedings of 9th International Hydrocolloids Conference (IHC) June 15–19 , Singapore
- Razavi , S.M.A. and Kardzhiyan , H. 2009 . Flow properties and thixotropy of selected hydrocolloids: Experimental and modeling studies . Food Hydrocolloids , 23 ( 3 ) : 908 – 912 .
- Razavi , S.M.A. , Mohammadi Moghaddam , T. and Amini , A.M. 2009 . Physico-mechanic and chemical properties of Balangu seeds (Lallemantia royleana) . International Journal of Food Engineering , 4 ( 5 ) : 1 – 12 .
- Koocheki , A. , Taherian , A.R. , Razavi , S.M.A. and Bostan , A. 2009 . Response surface methodology for optimization of extraction yield, viscosity, hue and emulsion stability of mucilage extracted from Leidium perfoliatum seeds . Food Hydrocolloids , 23 : 2369 – 2379 .
- Karazhiyan , H. , Razavi , S.M.A. and Phillips , G.O. 2011 . Extraction optimization of a hydrocolloid extract from cress seed (Lepidium sativum) using response surface methodology . Food Hydrocolloids , 25 : 915 – 920 .
- Karazhiyan , H. , Razavi , S.M.A. , Phillips , G.O. , Fang , Y. , Al-Assaf , S. , Nishinari , K. and Farhoosh , R. 2009 . Rheological properties of Lepidium sativum hydrocolloid extract as a function of concentration, temperature and time . Food Hydrocolloids , 23 : 2062 – 2068 .
- Razavi , S.M.A. , Mortazavi , S.A. , Matia-Merino , L. , Hosseini-Parvar , S.H. , Motamedzadegan , A. and Khanipour , E. 2009 . Optimization study of gum extraction from Basil seeds (Ocimum basilicum L.) . using response surface methodology. International Journal of Food Science and Technology , 44 : 1755 – 1762 .
- Razavi , S.M.A. , Bostan , A. and Rezaie , M. 2010 . Image processing and physico-mechanical properties of Basil seed (Ocimum Basilicum) . Journal of Food Process Engineering , 33 ( 1 ) : 51 – 64 .
- Hosseini-Parvar , S.H. , Matia-Merino , L. , Goh , K.K.T. , Razavi , S.M.A. and Mortazavi , S.A. 2010 . Steady shear flow behavior of gum extracted from Ocimum basilicum L. seed: Effect of concentration and temperature . Journal of Food Engineering , 101 : 236 – 241 .
- Razavi , S.M.A. , Mahdavian Mehr , H. and Zahedi , Y. 2010 . Some engineering properties of Plantago major L. (Barhang) seed . Iranian Food Science and Technology Research Journal , 5 ( 2 ) : 10 – 17 .
- Razavi , S.M.A. , Mahdavian Mehr , H. and Zahedi , Y. 2010 . Optimization of gum extraction conditions from Plantago major L. using the response surface method . Journal of Herbs, Spices and Medicinal Plants , Submitted manuscript
- Bostan , A. , Razavi , S.M.A. and Farhoosh , R. 2010 . Optimization of hydrocolloid extraction from wild sage seed (Salvia macrosiphon) using response surface methodology . International Journal of Food Properties , 13 ( 6 ) : 1380 – 1392 .
- Razavi , S.M.A. , Mahdavian Mehr , H. and Zahedi , Y. 2013 . Optimization of gum extraction conditions from Plantago major L. using the response surface method . Electronic Journal of Environmental, Agricultural and Food Chemistry , (Accepted paper)
- Razavi , S.M.A. , Taheri , H. and Quinchia , L.A. 2011 . Steady shear flow properties of wild sage (Salvia macrosiphon) seed gum as a function of concentration and temperature . Food Hydrocolloids , 25 ( 3 ) : 451 – 458 .
- Bourbon , A.L. , Pinherio , A.C. , Ribeiro , C. , Miranda , C. , Maia , J.M. , Teixeria , J.A. and Vicente , A.A. 2010 . Characterization of galactomannans extracted from seeds of Gleditsia triacanthos and Sophora japonica through shear and extensional rheology: Comparison with guar gum and locust bean gum . Food Hydrocolloids , 24 : 184 – 192 .
- Koop , H.S. , Praes , C.E.O. , Reicher , F. , Petkowicz , C.L.O. and Silveria , J.L.M. 2009 . Rheological behavior of gel xanthan with seed galactomanan: Effect of hydroalcoholic-ascorbic acid . Materials Science and Engineering C , 29 : 559 – 563 .
- Nwokocha , L.M. and Williams , P.A. 2009 . Isolation and rheological characterization of Mucuna flagellipes seed gum . Food Hydrocolloids , 23 : 1394 – 1397 .
- Wu , Y. , Cui , W. , Eskin , N.A.M. and Goff , H.D. 2009 . An investigation of four commercial galactomannans on their emulsion and rheological properties . Food Research International , 42 : 1141 – 1146 .
- Michon , C. , Chapius , C. , Langendroff , V. , Boulenguer , P. and Cuvelier , G. 2004 . Strain hardening properties of physical weak gels of biopolymers . Food Hydrocolloids , 18 : 999 – 1005 .
- Brummer , Y. , Cui , W. and Wang , Q. 2003 . Extraction, purification and physicochemical characterization of fenugreek gum . Food Hydrocolloids , 17 : 229 – 236 .
- Martin , S. , Freitas , R.A. , Obayashi , E. and Sierakowski , M.R. 2003 . Physico–chemical aspects of galactoxyloglucan from the seeds of Hymenaea courbaril and its tetraborate complex . Carbohydrate Polymers , 54 : 287 – 295 .
- Oblonsek , M. , Sostar-Turk , S. and Lapasin , R. 2003 . Rheological studies of concentrated guar gum . Rheologica Acta , 42 : 491 – 499 .
- Holser , R. , Carriere , C.J. and Abbott , T.P. 2000 . Rheological properties of lesquerella gum fractions recovered by aqueous extraction . Industrial Crops and Products , 12 : 63 – 69 .
- Steffe , J.F. 1996 . Rheological Methods in Food Process Engineering , Second , East Lansing, MI : Freeman Press . Edition
- Dolz , M. , Hernandez , M.J. and Delegido , J. 2008 . Creep and recovery experimental investigation of low oil content food emulsions . Food Hydrocolloids , 22 : 421 – 427 .
- Mezger , T.G. 2002 . “ Rotational tests ” . In The Rheology Handbook: For Users of Rotational and Oscillatory Rheometers; Zorll, E , 55 – 68 . Hannover : Vincentz Verlag .
- Clark , A.K. and Ross-Murphy , S.B. 1987 . Structural and mechanical properties of biopolymer gels . Advances in Polymer Sciences , 83 : 57 – 192 .
- Farahnaky , A. , Askari , H. , Majzoobi , M. and Gh , Mesbahi . 2010 . The impact of concentration, temperature and pH on dynamic rheology of psyllium gels . Journal of Food Engineering , 100 : 294 – 301 .
- Wang , B. , Wang , L. , Li , D. , Ozkan , N. , Li , S. and Mao , Z. 2009 . Rheological properties of waxy maize starch and xanthan gum mixtures in the presence of sucrose . Carbohydrate Polymer , 77 : 472 – 481 .
- Yamazaki , E. , Kurita , O. and Matsumura , Y. 2009 . High viscosity of hydrocolloid from leaves of Corchorus olitorius L . Food Hydrocolloids , 23 ( 3 ) : 655 – 660 .
- Fitzsimons , S.M. , Tobin , J.T. and Morris , E.R. 2008 . Synergistic binding of konjac gluco-mannan to xanthan on mixing at room temperature . Food Hydrocolloids , 22 : 485 – 491 .
- Rodriguez-Hernandez , A.L. , Durand , S. , Garnier , C. , Tecante , A. and Doublier , J.L. 2003 . Rheology-structure properties of gellan systems: Evidence of network formation at low gellan concentration . Food Hydrocolloids , 17 : 621 – 628 .
- Fernandez , P.B. , Goncalves , M.P. and Doublier , J.L. 1991 . A rheological characterization of kappa-carrageenan/galactomanan gels: A comparison of locust bean gum samples . Carbohydrate Polymers , 16 : 253 – 274 .
- Ross-Murphy , S.B. , Morris , V.J. and Morris , E.R. 1983 . Molecular viscoelasticity of xanthan polysaccharide . Faraday Symposia the Chemistry Society , 18 : 115 – 129 .
- Chenlo , F. , Moreira , R. and Silva , C. 2010 . Rheological behavior of aqueous systems of tragacanth and guar gums with storage time . Journal of Food Engineering , 96 : 107 – 113 .
- Rao , M.A. 1999 . “ Rheological behavior of processed fluid and semisolid foods ” . In Rheology of Fluid and Semisolid Foods: Principles and Applications; Rao, M , 153 – 218 . USA : Aspen Publishers, Inc .
- Ross-Murphy , S.B. 1984 . “ Rheological methods ” . In Biophysical Methods in Food Research; Chan, H.W.S , 138 – 199 . London : Blackwell Scientific Publication .
- Ferry , J.D. 1948 . Mechanical properties of substances of high molecular weight IV. Rigidities of gelatin gels: Dependence on concentration, temperature and molecular weight . Journal of American Chemistry Society , 70 : 2244 – 2249 .
- Koltisko , B. , Keller , A. , Litt , M. , Baer , E. and Hiltner , A. 1986 . Mechanical properties of thermoreversible atactic polystyrene gels . Macromolecules , 19 : 1207 – 1212 .
- Mao , C.F. and Rwei , S.P. 2006 . Cascade analysis of mixed gels of xanthan and locust bean gum . Polymer , 47 : 7980 – 7987 .
- Ross-Murphy , S.B. and McEvoy , H. 1986 . Fundamentals of hydrogels and gelation . Britain Polymer Journal , 18 ( 1 ) : 2 – 7 .