Abstract
Levels of proteolysis of 75 samples belonging to 11 Turkish cheese varieties, including Civil, Canak, Dil, Divle Tulum, Ezine, Hellim, Malatya, Mihalic, Orgu, Urfa, and Van Otlu, were comparatively studied. The cheeses were mainly produced using traditional methods; however, some varieties were industrially produced. Chemical composition and the levels of soluble nitrogen fractions of the cheeses varied depending on the cheese variety. Gel electrophoresis of the cheeses showed that the samples presented different gel patterns with αs1-casein being extensively degraded in many cheeses; whereas the hydrolysis of αs1-casein in Malatya and Hellim was observed to be limited. Peptide profiles by RP-HPLC of the water-soluble fractions were largely different for many of the samples, but some similarities were visualized. Multivariate analysis of the RP-HPLC data grouped the cheeses according to their peptide profiles. The results suggested that each variety of cheese had different levels of proteolysis. The manufacturing technique and ripening conditions employed have played a determinative role on the proteolytic patterns of the cheeses analyzed.
INTRODUCTION
Cheese ripening is a complex and dynamic biochemical process involving the breakdown of protein, hydrolysis of fat, and metabolism of lactose.[Citation1] Proteolysis is catalyzed by proteolytic enzymes, which originate from the coagulant, milk, starter, and/or non-starter bacteria. The primary role of the coagulant, of which 0–15% remains in the cheese, is to hydrolyze intact casein into large (water-insoluble) and intermediate-sized (water-soluble) peptides, which are subsequently degraded by starter or non-starter microflora of cheese during ripening.[Citation2] The complete series of metabolic converting reactions in cheese is still not completely understood, but it is known that the resultant amino acids can serve as precursors of flavor compounds in cheese and can be further decomposed into ammonia, carbonyl compounds, amines, and carbon dioxide.[Citation3,Citation4] The quantity of these products in cheese depends mainly on cheese type, ripening time, and other ripening conditions. Due to the major importance of proteolysis in cheese ripening, indices of ripening are determined using proteolysis data in cheese.[Citation5] The levels of proteolysis vary depending on the cheese type, i.e., each type of cheese has a specific pattern of proteolysis and cheeses are mainly characterized and classified using proteolytic data.[Citation2]
Turkey's annual milk production is about 12.2 million tons and the majority of this is converted into cheese (56.6%), followed by yogurt and ayran (18.6%), liquid milk (12.5%), butter (11.0%), milk powder (0.7%), and ice cream (0.6%).[Citation6] It is estimated that the level of milk reserved for cheese production will increase in the near future as the industrial production of local cheeses is continuously increasing. There are more than 50 varieties of cheese in Turkey, including brine ripened (e.g., white cheese), high temperature (>90°C) scalded cheeses (e.g., Halloumi), pasta-filata type (e.g., Kashar or Kashkaval), as well as herby or spicy cheese varieties (e.g., Van Otlu cheese). Canak cheese is ripened in earthenware pots for at least 6 months underground resulting in the production of strong flavors, which are noted by consumers. Also, Civil cheese is ripened by the spontaneous growth of molds on the surface and it is characterized by a rancid taste despite having a low level of fat. Some varieties of cheese (Dil, Hellim, Malatya, Orgu, and Urfa) are scalded by dipping cheese blocks into hot water or whey (75 to 90°C) and then ripened under brine. Although there is a lack of detailed information on the levels of proteolysis (urea-PAGE patterns, peptide, and free amino acids profiles) in the cheese varieties under investigation, limited levels of proteolysis were expected in some cheeses due to negative effect of scalding on the development of proteolysis. Another cheese variety is Divle Tulum, which is ripened in natural caves (4–5°C and 80% ± 5 relative humidity) for long periods. One of the most interesting cheese varieties in Turkey is Otlu cheese, which uses some special herbs, including Allium, Thymus, Silene, and Ferula species, individually or in appropriate mixtures in the manufacture of this variety.[Citation7–9 Citation Citation9 Indeed, little information is available on the proteolytic patterns in some economically important Turkish cheese varieties. The chemical and microbiological characteristics of the local cheeses have been extensively studied and have previously been published in Hayaloglu et al.[Citation7] and Hayaloglu and Fox.[Citation8] Some of the cheese varieties mentioned above are currently manufactured from pasteurized milk with added starter cultures. However, Civil, Canak, Divle Tulum, and Mihalic (in villages) are artisinally produced without the addition of starter culture, and it is the indigenous microflora of the milk, which contributes to the ripening. The objective of this study was to compare the levels of proteolysis in 11 different cheese varieties of importance to the Turkish dairy industry.
MATERIALS AND METHODS
Materials
A total of 75 samples from 11 different varieties of cheese were collected from different regions of Turkey in 2009. The names of the cheeses were Civil (moldy/mildewy), Canak, Dil, Divle Tulum, Ezine, Hellim, Malatya, Mihalic, Orgu, Urfa, and Van Otlu. The age of the collected cheese samples ranged from 2–8 months old or more. For sample collection, fully matured cheeses or ready-for-sale cheeses were selected as far as possible. The production dates on the label were noted for some samples; however, some samples are still produced by artisanal methods (for example Canak and Divle Tulum cheeses) without packaging and labeling. So, to assess the production dates for these, the manufacturer's recommendations were taken into account. Cheese samples were kept in sterile plastic bags and transported to the laboratory at Food Engineering Department, Inonu University, Turkey. The samples were stored at −20°C until analysis.
Chemical Analysis
Cheeses were analyzed in duplicate for total solids (by the oven drying method at 102°C), fat (by the Van Gulik method), total protein (by the Kjeldahl method), salt (by titration with AgNO3 using Mohr method), and pH as described by Hayaloglu et al.[Citation1]
Nitrogen Fractions and Total Free Amino Acids (FAA)
Water–soluble nitrogen (WSN), 12% trichloroacetic acid soluble nitrogen (TCA–SN), and 5% phosphotungstic acid–soluble (PTA–SN) fractions of the cheese were determined by the methods described by Hayaloglu et al.[Citation1] The WSN, TCA–SN, and PTA–SN were expressed as % of TN. Total free amino acid (FAA) levels in the water–soluble fraction of the cheeses were determined by the Cd–ninhydrin method described in Hayaloglu.[Citation10]
Urea-Polyacrylamide Gel Electrophoresis (Urea-PAGE)
Water-insoluble fractions of the cheeses were freeze-dried and then analyzed by urea-polyacrylamide gel electrophoresis (urea-PAGE) using Protean II XI vertical slab gel unit with dimensions of 20 × 20 cm (Bio-Rad Laboratories Ltd., Watford, UK) according to the method of Andrews.[Citation11] An amount of 8 μL of the sample (sample was prepared by dissolving 10 mg of freeze-dried powder in sample buffer; for details, see Hayaloglu et al.[Citation1]) was loaded onto the gel and electrophoresis was performed in the stacking and separating gels at 280 and 300 V, respectively. The resultant gels were stained directly by the method of Blakesley and Boezi[Citation12] with Coomassie Brilliant Blue G250 overnight. Destaining of the gels was achieved by several washes in distilled water. After destaining, gels were scanned using a scanner HP Scanjet G4010 (Hewlett Packard Co., Palo Alto, CA, USA).
RP-HPLC Analysis
Peptide profiles of the water–soluble fraction of the cheeses were determined by RP–HPLC using a Shimadzu LC 20 AD Prominence HPLC system (Shimadzu Corporation, Kyoto, Japan). The system was comprised of an autosampler (model SIL-20A HT), a solvent delivery system with quartery pumps (model 20AD), and a photo diode array detector (model SPD-M20A prominence) interfaced with a PC with an LC solution software package installed for system control and data acquisition. An Inertsil RP–C8 (250 × 4 mm, 5 μm particle size, 300 Å pore size) analytical column (GL Sciences Inc., Tokyo, Japan) was used. The solvents were: (A) 0.1% (v/v) trifloroacetic acid (TFA, sequencing grade; Sigma-Aldrich Laborchemikalien GmbH, Seelze, Germany) in deionized HPLC–grade water (Milli–Q system; Waters Corp., Molsheim, France) and (B) 0.1% (v/v) TFA in acetonitrile (HPLC grade, Merck KGaA, Darmstadt, Germany) at a flow rate of 0.75 mL/min. Samples of freeze-dried water-soluble fractions were dissolved in solvent A (10 mg/mL), filtered through a 0.45-μm cellulose acetate filter (Sartorius GmbH, Göttingen, Germany); an aliquot (40 μL) of filtrate was injected onto the column. The samples were eluted initially with 100% A for 5 min, then with a gradient from 0 to 50% B (v/v) over 55 min, maintained at 50% B (v/v) for 6 min, followed by a linear gradient from 50 to 60% B (v/v) over 4 min, and finally with 60% B (v/v) for 3 min. The column was washed with 95% B (v/v) for 5 min, followed by equilibration with 100% A for 5 min before the next injection. The eluate was monitored at 214 nm.[Citation1]
Statistical Analyses
The data obtained were analyzed with one-way analysis of variance using the SPSS package program (version 9.0, SPSS Inc., Chicago, IL, USA). RP–HPLC chromatograms of the water-soluble fraction was analyzed by multivariate statistical analyses. Data for multivariate statistical analysis of the RP-HPLC chromatograms were obtained by visually recognizing similar peaks in the chromatograms and using the peak heights as variables in the analysis. The peak heights were obtained by converting the corresponding chromatogram to an ASCII file. PCA was performed using the covariance matrix and varimax rotation and, hierarchical cluster analysis (HCA) was performed by using Euclidean distance and average linkage without standardizing the variables. Statistical analysis was performed using SPSS version 9.0 (SPSS Inc.).
RESULTS AND DISCUSSION
Chemical Composition and pH
The chemical compositions and pH values of the cheeses were dependent on the cheese variety and individual sample due to lack of standard manufacturing protocols. The level of total solids varied between 43.66% (Civil) and 60.13% (Divle Tulum) as shown in . The average total solids contents in cheese samples were above 50% (between 50–55% except for cheeses Civil and Urfa). The values for fat-in-dry matter (FDM) ranged from 4.05% (Civil) to 50.48% (Ezine). The levels of FDM in Ezine cheeses were in accordance with the findings of Karagul-Yuceer et al.[Citation13] The lower levels of fat and FDM in Civil cheese was probably due to the use of low fat milk in its manufacture. Salt concentrations of the cheeses varied within wide ranges (2.46 to 6.91%), with the lowest and highest levels detected in Divle Tulum and Urfa cheeses, respectively. The salt-in-dry matter (SDM) values in Civil, Malatya, Mihalic, Urfa, and Van Otlu cheeses were above the critical levels (10% for SDM) mandated for white-brined cheese. Much higher levels (>15%) of SDM have been reported by Ozer et al.[Citation14] in Urfa cheeses made using bovine and ovine milks. The values for total protein in cheeses were between 18.28% (Urfa) and 34.03% (Civil). The highest levels of protein were detected in Civil cheese and was due to the low level of fat and the proportional effect of fat on other constituents forming total solids. Large variations were noted for pH values among the cheese varieties and within the samples for the same variety as shown in . The pH values were higher in Civil (5.90), Hellim (6.02), Divle (5.49), Mihalic (5.44), Orgu (5.40), and Malatya (5.64) cheeses than in Canak (4.77), Dil (5.24), Ezine (4.95), Urfa (5.07), and Van Otlu (4.66) cheeses. The pH values obtained were in accordance with previously reported values reported for Dil,[Citation15] Urfa,[Citation16] Ezine,[Citation17] and Van Otlu.[Citation18]
Table 1 Chemical composition and pH (Mean ±SD) of cheeses (n = 75)
Nitrogenous Fractions
The nitrogen fractions are important parameters in the determination of the extent of proteolysis. The water‐soluble nitrogen (WSN) or pH 4.6-SN have been used as ripening indices for many cheese varieties; however, the hydrolyzed fractions of the WSN are also water-soluble and remain in the same fraction;[Citation19] hence, further fractionation techniques are required for complete characterization. The levels of WSN, expressed as % of total nitrogen (TN), were in the range of 2.69% (Hellim) and 26.74% (Mihalic) (). The WSN values were above 20% in Civil, Divle Tulum, Ezine, and Mihalic cheeses, while the values for Orgu and Van Otlu cheeses were close to 20%. Therefore, it could be considered that for the majority of the cheeses the level of proteolysis was mid-level. Classification of the cheese in this study based on their ripening indices (levels of WSN) resulted in three groups being identified: Civil and Mihalic cheeses had high levels of proteolysis; Dil, Hellim, and Malatya cheeses had low levels of proteolysis; and, finally, the remainder of the cheeses had mid-levels of proteolysis. Low levels of proteolysis in Dil, Hellim, and Malatya may be due to the negative influence of scalding of the cheese curd.[Citation9] The level of WSN was also lower in Hellim cheese (between 4.6 to 5.5%, as % of TN) as previously reported by Guley and Akbulut.[Citation20] Also, limited levels of WSN have also been previously reported in Malatya[Citation21] and Dil cheeses.[Citation22]
Table 2 Soluble nitrogen fractions and total amino acid concentrations of the cheeses (n = 75)
Levels of secondary proteolysis were determined in the cheese samples using 12% trichloroacetic acid-soluble nitrogen (TCA-SN). A large variation in the concentration of TCA-SN was detected in the cheeses and was probably due to the different ripening times, conditions in the retail outlets, as well as the different microflora employed. The lowest and highest TCA-SN concentrations were determined in Hellim (1.24%) and Divle Tulum (12.29%) cheeses (). Dil, Hellim, Malatya, and Orgu cheeses had similar levels of TCA-SN (2.43, 1.24, 1.45, and 2.47%, respectively). Lower levels of WSN were also noted for these cheeses, which were scalded in hot whey (>90°C). The scalding process inactivates enzymes originated from the coagulant or microorganisms.[Citation20,Citation23] Consequently, the level of proteolysis is very limited in Halloumi cheese produced under these conditions. Canak (9.18%), Divle Tulum (12.29%), Ezine (8.88%), and Van Otlu (10.03%) had higher TCA-SN values in comparison to Hellim and other similar cheeses. The levels of TCA-SN detected in mature Van Otlu cheese are typical for this variety and are in accordance with the findings of Tarakci et al.[Citation24,Citation25] Levels of amino nitrogen, as indicated by the 5% phosphotungstic acids-soluble nitrogen (PTA-SN) were found to be high in the majority of the cheeses, i.e., above 2.5% (as % of TN). The PTA-SN fraction includes mono, di-, and tri-peptides and amino acids with molecular weights smaller than 600 Da.[Citation26] The Civil, Canak, Divle Tulum, Ezine, Mihalic, and Van Otlu cheeses had high levels of free amino acids, which is related to their high concentrations of the PTA-SN (). This was verified by measuring total free amino acid (FAA) by the spectrophotometric method. The concentrations of total FAA was the highest in Divle Tulum (4.19 mg Leu/100 g cheese) cheese followed by the cheeses Civil, Canak, Mihalic, and Van Otlu, which contained substantial concentrations of total FAA. The FAA contents in Dil, Hellim, and Malatya cheeses were lower than 0.5 mg Leu/100 g cheese as were their PTA-SN values. The trends in the levels of total FAA in the cheeses were in agreement with the PTA-SN values. Similar correlations have previously been reported for these variables for Manchego cheese[Citation27] and Turkish white-brined cheese.[Citation1] These two variables, which reflect lower molecular weight peptides and amino acids, give valuable information on the accumulation of amino acids in the cheeses and the effect that manufacturing and ripening conditions (time, temperature, etc.) have on their development.
Urea-PAGE
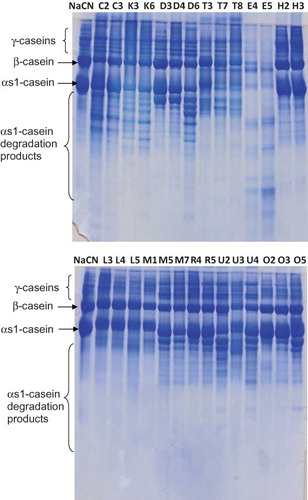
Urea-PAGE was performed on all cheese samples (n = 75); however, representative gels for some sample are shown in . Overall, intervarietal differences were observed between the cheese varieties and also within individual samples of the same variety. Regardless of this variation, urea-PAGE was capable of distinguishing the cheese varieties based on the hydrolysis of the caseins, especially degradation of αs1-casein. Urea-PAGE patterns of the cheeses clearly showed that levels of primary proteolysis were considerably limited in Dil, Hellim, Malatya, Orgu, and Van Otlu cheeses. Although soluble (in water, TCA, or PTA) nitrogenous compounds were high in Van Otlu cheeses, extensive degradation of β-casein was not observed in all samples of Van Otlu cheese. However, degradation of αs1-caseins was dependent on the individual samples. Therefore, these differences may have had an effect on the gel electrophoretograms. Tarakci et al.[Citation18] pointed out that the degradation of αs1-casein was more extensive in ripened Van Otlu cheeses than in unripened cheese collected in retail outlets. Hellim, Malatya, and Orgu (braided) cheeses are scald-type varieties; so, limited casein hydrolysis was detected in these cheeses. Guven et al.[Citation28] reported that the degradation of αs1-casein was slower than that of β-casein in Hellim cheese due to elevated plasmin activity at the higher pH (5.8–6.5). The average pH in Hellim cheeses was 6.0, which favors plasmin activity, as discussed by Sousa and McSweeney.[Citation29] Substantial degradation of the caseins was unexpectedly observed in Dil cheeses despite the high cook temperatures used during its manufacture. However, the levels of the nitrogen fractions measured in this variety () were not high. Extensive degradation of both β- and αs1-caseins were observed in Civil, Canak, Divle Tulum, Ezine, Mihalic, and Urfa cheeses as shown in . These cheeses were at least 3 months or more and were ripened by spontaneous mold growth (Civil), burying underground (Canak), ripening in goat's skin bags and ripening in a natural cave (Divle Tulum), etc. On the other hand, Ezine has a geographical indication status and is ripened for about 6 months before marketing. Mihalic and Urfa cheeses used in this study were artisanally produced samples. Therefore, the casein hydrolysis was more extensive in these cheeses in comparison to the others, and similar differences were detected in nitrogen fractions () and RP-HPLC peptide profiles (see the next section) of these cheeses. Civil cheese has a fibrous structure and the fungi grow on the fibers, which gives a moldy appearance. The fungi affect the biochemistry of the cheese and increase the pH during ripening.[Citation1,Citation29] The hydrolysis of β- and αs1-casein were also monitored in Divle Tulum and Ezine cheeses. A different urea-PAGE pattern of the caseins has been reported by Karagul-Yuceer et al.[Citation17] in Ezine cheese, i.e., the hydrolysis of the caseins was not fast in this variety regardless of whether it was made from cow's or ewe's milk. Considerable differences were noted between samples of Mihalic cheese regarding the level of casein hydrolysis. An irregular pattern of proteolysis is expected in Mihalic cheese due to the different concentrations of salt in the brine used in the manufacture.[Citation9]
Figure 1 Urea-polyacrylamide gel electroforetograms of the cheeses. Lanes were coded with the cheese names (capitals) and samples for the corresponding cheese variety. That is, NaCN is sodium caseinate; C: Civil; K: Canak; D: Dil; T: Divle Tulum; E: Ezine; H: Hellim; L: Malatya; M: Mihalic; R: Orgu; U: Urfa; O: Van Otlu cheeses. Urea-PAGE of all samples was monitored; however, only representative samples were shown in this electrophoretogram. (Color figure available online.)
RP-HPLC Peptide Profile
Some peptides or amino acids released by the hydrolysis of caseins can be determined and characterized using chromatographic techniques. The RP-HPLC peptide profiles of the water-soluble fractions of the 11 different varieties of cheese are shown in and . Only one chromatogram for each cheese variety is shown although data from a much higher number of samples were used for principal component analyses (PCA). The chromatograms of the water-soluble fractions of the cheeses showed that many peptides with early or late retention times were present in Civil, Canak, and Dil cheeses. Major qualitative and quantitative differences were observed between the peptide profiles of each cheese variety. High concentrations of peaks in Civil cheeses were associated with high levels of soluble nitrogen fractions and both early retention time (probably hydrophilic) and late retention time (probably hydrophobic) peptides were screened. Civil cheese samples contained peptides with large peak heights and, hence, this cheese was separated on the PCA plot due to the different concentrations of these peptides (). Similarly, Canak cheese samples were rich in intermediate (20 to 40 min) and hydrophobic (40 to 55 min) peptides. High concentrations of hydrophobic peptides (45–51 min) were detected in Civil and Canak cheeses. Dil is a pasta-filata type cheese and its characteristics are a result of cooking in hot water or 5% NaCl brine at 80–90°C.[Citation15] Even though high cook temperatures are used during its manufacture, relatively high concentrations of peptides (19, 20, 26, 28, 31, and 33 min and such times) were detected from the samples of Dil cheese. High concentrations of peptides were not detected in Ezine cheese even though this cheese is ripened for at least 6 months. The peptides had probably been further degraded into amino acids and volatile compounds, such as acids, aldehydes, and alcohols. Hellim and Malatya cheeses had extremely low concentrations of peptides in comparison to the other cheeses. Only a few peptides were detected with low peak heights, indicating that the high scald temperature negatively influences the production of peptides during the ripening of Hellim and Malatya cheeses. Also, low concentrations of peptides were found in Malatya cheeses that were made from pasteurized milk.[Citation21] Different peptide profiles were obtained in the samples of Van Otlu cheese as shown in . Higher concentrations of peptides eluting with retention times of 13, 19, and 24 min were specific for Van Otlu cheese and the corresponding peaks were not detected in all samples. The majority of peptides eluted in the hydrophilic region (13 to 30 min), showed that the peptides detected are water-soluble and positively contribute to the proteolysis.
Figure 3 RP-HPLC peptide profiles of the water-soluble fractions of Ezine, Hellim, Malatya, Van Otlu, Orgu, and Urfa cheeses. Peptide profiles of all cheeses were analyzed and run by multivariate statistical analysis; however, only representative samples were shown in this figure. (Color figure available online.)
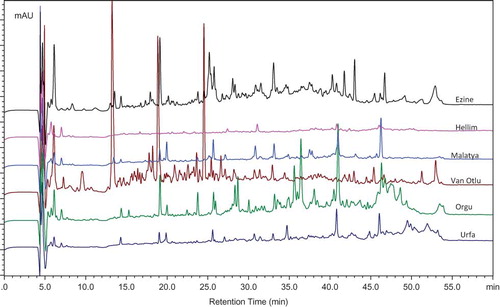
Figure 2 RP-HPLC peptide profiles of the water-soluble fractions of Civil, Canak, Dil, Divle Tulum, and Mihalic cheeses. Peptide profiles of all cheeses were analyzed and run by multivariate statistical analysis; however, only representative samples were shown in this figure. (Color figure available online.)
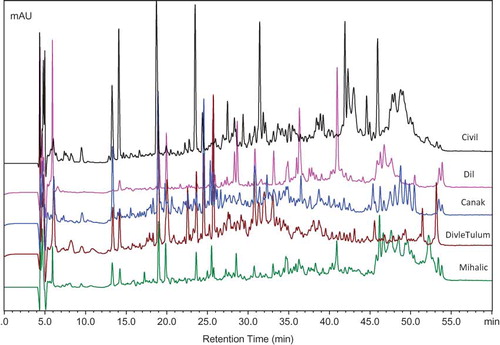
Figure 4 Principal component analysis of the data RP-HPLC profiles of water-soluble fractions of cheeses, including Civil (C), Canak (K), Dil (D), Divle Tulum (T), Ezine (E), Hellim (H), Malatya (MLT), Mihalic (M), Orgu (OR), Urfa (U), and Van Otlu (OT). (Color figure available online.)
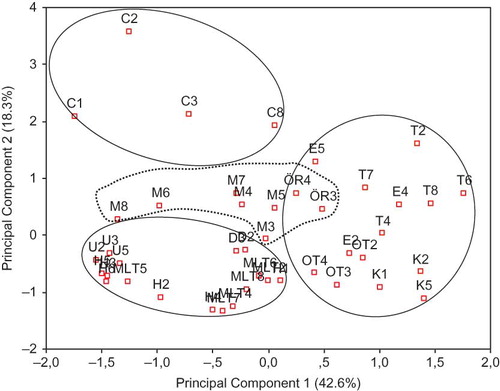
The PCA score plot obtained from the analysis of the peak height data of the RP-HPLC chromatograms is shown in and was used to differentiate the cheeses based on their chromatographic profiles. A cumulative variation of 60.9% was explained by PC1 (42.6%) and PC2 (18.3%) and four main groupings were identified. First, Civil cheeses were clearly separated from the other cheeses forming a distinct group. The second group included the cheeses Dil, Hellim, Malatya, and Urfa, which are scalded in hot whey and were located on the negative side of both PC1 and PC2. The third group contained the cheeses Canak, Divle Tulum, Ezine, and Van Otlu on the positive side of the PC1. The final group included the cheeses Mihalic and Orgu, which were grouped separately in an intermediate region reflecting the similarity in the peptide profiles of these cheeses. However, as highlighted by PCA or HCA (dendrogram not shown) analysis, the distribution of each cheese variety was not regular due to the lack of standardized production methods and ripening conditions for the majority of the cheeses analyzed.
CONCLUSIONS
The levels of proteolysis in the water-soluble–insoluble nitrogen fractions (12% TCA and 5% PTA) were determined in 11 different Turkish cheese varieties. The differences detected were mainly due to their production method and/or ripening conditions. Urea-PAGE patterns of the water-insoluble fractions of the cheeses showed that αs1-casein was degraded in the majority of the cheeses, whereas β-casein was degraded to a lesser extent. Hydrolysis of β-casein was higher in cheeses with a higher pH in comparison to cheeses with a lower pH, indicating higher plasmin activity in the former cheeses. RP-HPLC of water-soluble fractions of the cheeses showed different peptide profiles, but some cheeses were grouped by using PCA analysis based on their chromatographic data. In conclusion, proteolysis in 11 different varieties of cheeses in Turkey exhibited different patterns reflecting the different manufacturing protocols, and ripening conditions for each cheese has an effect on their proteolysis.
ACKNOWLEDGMENTS
This study was funded in part by the Scientific Research Projects Unit of Inonu University, Turkey (Project No. 2007/31). Also, Professor B. Ozer is acknowledged for critical reading of the manuscript.
REFERENCES
- Hayaloglu , A.A. , Guven , M. , Fox , P.F. and McSweeney , P.L.H. 2005 . Influence of starters on chemical, biochemical and sensory changes in Turkish White–brined cheese during ripening . Journal of Dairy Science , 88 : 3460 – 3474 .
- Sousa , M.J. , Ardo , Y. and McSweeney , P.L.H. 2001 . Advances in the study of proteolysis during cheese ripening . International Dairy Journal , 11 : 327 – 345 .
- Broome , M.C. and Limsowtin , G.K.Y. 1998 . Starter peptidase activity in maturing cheese . Australian Journal of Dairy Technology , 53 : 79 – 82 .
- Urbach , G. 1997 . “ The chemical and biochemical basis of cheese and milk aroma ” . In Microbiology and Biochemistry of Cheese and Fermented Milk , 2nd , Edited by: Law , B.A. 253 – 298 . London : Blackie Academic and Professional . Edition
- Fox , P.F. and McSweeney , P.L.H. 1996 . Proteolysis in cheese during ripening . Food Reviews International , 12 : 457 – 509 .
- Anonymous . 2010 . Report for Dairy Industry in the world and Turkey , Ankara , , Turkey : ASUD .
- Hayaloglu , A.A. , Fox , P.F. , Guven , M. and Cakmakci , S. 2007 . Cheeses of Turkey: 1 . Varieties ripened in goat-skin bags. Lait , 87 : 79 – 95 .
- Hayaloglu , A.A. and Fox , P.F. 2008 . Cheeses of Turkey: 3 . Varieties containing herbs or spices. Dairy Science and Technology , 88 : 245 – 256 .
- Hayaloglu , A.A. , Ozer , B.H. and Fox , P.F. 2008 . Cheeses of Turkey: 2 . Varieties ripened under brine. Dairy Science and Technology , 88 : 225 – 244 .
- Hayaloglu , A.A. 2007 . Comparisons of different single-strain starter cultures for their effects on ripening and grading of Beyaz cheese . International Journal of Food Science and Technology , 42 : 930 – 938 .
- Andrews , A.T. 1983 . Proteinases in normal bovine milk and their action on caseins . Journal of Dairy Research , 50 : 45 – 55 .
- Blakesley , R.W. and Boezi , J.A. 1977 . A new staining technique for proteins in polyacrylamide gels using Coomassie Brilliant Blue G250 . Analytical Biochemistry , 82 : 580 – 581 .
- Karagul-Yuceer , Y. , Isleten , M. and Uysal-Pala , C. 2007 . Sensory characteristics of Ezine cheese . Journal of Sensory Studies , 22 : 49 – 65 .
- Ozer , B. , Atasoy , F. and Akin , S. 2002 . Some properties of Urfa cheese (a traditional white-brined Turkish cheese) produced from bovine and ovine milks . International Journal of Dairy Technology , 55 : 94 – 99 .
- Kilic , M. and Isin , T.G. 2004 . Effects of salt level and storage on texture of Dil cheese . Journal of Texture Studies , 35 : 251 – 262 .
- Atasoy , A.F. and Akin , M.S. 2004 . A comparative study on the determination of chemical characteristics and proteolysis levels in Urfa cheeses sold in Sanliurfa province . Harran University Journal of Agricultural Faculty , 8 : 9 – 15 .
- Karagul-Yuceer , Y. , Tuncel , B. , Guneser , O. , Engin , B. , Isleten , M. , Yasar , K. and Mendes , M. 2009 . Characterization of aroma-active compounds, sensory properties, and proteolysis in Ezine cheese . Journal of Dairy Science , 92 : 4146 – 4157 .
- Tarakci , Z. , Coskun , H. and Tuncturk , Y. 2004 . Some properties of fresh and ripened herby cheese, a traditional variety produced in Turkey . Food Technology and Biotechnology , 42 : 47 – 50 .
- Verdini , R.A. , Zorrilla , S.E. and Rubiolo , A.C. 2004 . Characterisation of soft cheese proteolysis by RP-HPLC analysis of its nitrogenous fractions . Effect of ripening time and sampling zone. International Dairy Journal , 14 : 445 – 454 .
- Guley , Z. and Akbulut , N. 24–28 May 2004 . “ Effects of using starter culture on some properties of Halloumi cheese ” . In International Dairy Symposium 222 – 225 . Isparta , , Turkey
- Hayaloglu , A.A. , Deegan , K.C. and McSweeney , P.L.H. 2010 . Effect of milk pasteurization and curd scalding temperature on proteolysis in Malatya, a Halloumi-type cheese . Dairy Science and Technology , 90 : 99 – 110 .
- Kocak , C. , Aydinoglu , G. and Uslu , K. 1997 . A study on proteolysis levels in Dil cheeses sold in Ankara . Gida , 22 : 251 – 255 .
- Papademas , P. and Robinson , R.K. 1998 . Halloumi cheese: The product and its characteristics . International Journal of Dairy Technology , 51 : 98 – 103 .
- Tarakci , Z. , Durmaz , H. , Sagun , E. and Sancak , H. 2005 . Influence of brine concentration on chemical, microbiological and sensory characteristics of herby cheese . Indian Veterinary Journal , 82 : 279 – 282 .
- Tarakci , Z. , Sagun , E. and Durmaz , H. 2006 . The effect of mendi (Chaerophyllum sp.) on ripening of vacuum-packed herby cheese . International Journal of Dairy Technology , 59 : 35 – 39 .
- Jarrett , W.D. , Aston , J.W. and Dulley , J.R. 1982 . A simple method for estimating free amino acids in Cheddar cheese . Australian Journal of Dairy Technology , 37 : 55 – 58 .
- Pavia , M. , Trujillo , A.J. , Guamis , B. and Ferragut , V. 2000 . Proteolysis in Manchego-type cheese salted by brine vacuum impregnation . Journal of Dairy Science , 83 : 1441 – 1447 .
- Guven , M. , Cadun , C. , Karaca , O.B. and Hayaloglu , A.A. 2008 . Influence of rennet concentration on ripening characteristics of Halloumi cheese . Journal of Food Biochemistry , 32 : 615 – 627 .
- Sousa , M.J. and McSweeney , P.L.H. 2001 . Studies of the ripening of Cooleeney, an Irish farmhouse Camembert-type cheese . Irish Journal of Agricultural and Food Research , 40 : 83 – 95 .