Abstract
Ohmic heating is an interesting method for heating meat ball; however, its effectiveness relies on the electrical and thermo-physical properties of the product. Therefore, this research aimed to determine the electrical conductivities and thermo-physical properties of pork-meat balls. The results showed that electrical conductivities of samples could be well-fitted into the mathematical models as a function of temperature and the ratio of each ingredient due to their high R2 and low RMSE. The addition of salt and sodium tripolyphosphate led to a significant increase in electrical conductivity. The specific heats and thermal conductivities of meat balls were measured, whereas the convective heat transfer coefficients were determined by a lump-heat capacity technique. An equation was developed to estimate the convective heat transfer coefficients as a function of temperature difference between particle and surrounding fluid.
INTRODUCTION
Ohmic heating is a promising technique for heating solid and solid-liquid mixture foods, due to its high heating rate and uniformity of temperature within the product when compared to the conventional methods that rely on the conduction, convection, and radiation heat transfers. For the conventional heating, it always has the coldest point inside the solid foods, especially for the large-size solids due to their low thermal conductivities. As a result, they need long processing time to raise the temperature at the coldest point to the specified level.[Citation1] Subsequently, the product quality, particularly at the surface, is lessened because of over cooking. Although ohmic heating is considered as a solution for this problem, the success in applying the ohmic heating technique is strongly dependent on the electrical conductivity of the product.[Citation2] If the electrical conductivity is high and uniform, the heat will be generated quickly and evenly during ohmic heating, which can lead to better nutrient and flavor retention and product integrity.
During the last decade, there have been a number of researchers who studied the electrical conductivities of meat products.[Citation3] Shirsat et al.[Citation4] illustrated the effect of salt, fat, and temperature on the conductivities of meat emulsion batters. It appeared that an increase in salt content resulted in the enhancement of electrical conductivity and ohmic heating rate of emulsion samples. On the other hand, an increase in fat content caused the reverse effect. In addition, Shirsat et al.[Citation5] studied the electrical conductivities of lean and fat components of meat individually and in combination. They found that lean pork is highly conductive compared with fat. The addition of fat to lean pork resulted in the reduction of electrical conductivities of products. Similarly, Sarang et al.[Citation6] stated that lean chicken, pork, and beef are much more electrically conductive than fat. Moreover, the relationships between the electrical conductivities of all samples and temperatures are linear. Tulsiyan et al.[Citation7] measured the electrical conductivities of chicken breast at the temperatures beyond 100°C. Zell et al.[Citation8] pointed out that whole beef muscles must be injected with salt solution at multiple positions and then tumbled to ensure an even salt distribution within the whole beef muscles so that the inside temperatures would be rapidly and uniformly increased when they were heated by the ohmic method. Bozkurt and Icier[Citation9] studied the effect of voltage gradients applied during ohmic cooking on some quality properties of ground beef samples that had different initial fat contents. Even though there have been some studies on the electrical conductivities of some meat products, a limited attention has been given to the electrical conductivities of meat balls that varied their compositions and temperatures.
Apart from the electrical conductivity, there are some additional properties that affect the temperature of food during ohmic heating, such as specific heat, thermal conductivity, and convective heat transfer coefficient, between solid and liquid. Marra et al.[Citation10] investigated the specific heat and thermal conductivity of mashed potato in order to apply these properties for simulating the temperatures of mashed potato during ohmic heating. The correlations between these thermo-physical properties and temperatures were developed. Balasubramaniam and Sastry[Citation11] and Baptista et al.[Citation12] developed the mathematical models for predicting the Nusselt number and the subsequent fluid to particle heat transfer coefficients in the continuous tube flow. In their studies, the convective heat transfer coefficients were calculated applying the method of a lumped-heat capacity system. In addition, Barigou et al.[Citation13] summarized the fluid to particle heat transfer coefficients that were investigated from various studies in order to apply these data for the calculation of heat transfer in solid-liquid food flows especially for the continuous aseptic processing.
Due to the deficiency of published data on the electrical conductivity and thermo-physical properties of meat ball, the main objectives of the present study were (1) to investigate the electrical conductivities of pork-meat balls under various ratios of ingredients and (2) to determine the important thermo-physical properties that are vital for the application of ohmic heating on meat ball, including specific heat, thermal conductivity, and convective heat transfer coefficient. These data are valuable especially for both industry and academia that desire to estimate the meat ball temperature during ohmic heating by mathematical models.
MATERIALS AND METHODS
Sample Preparation
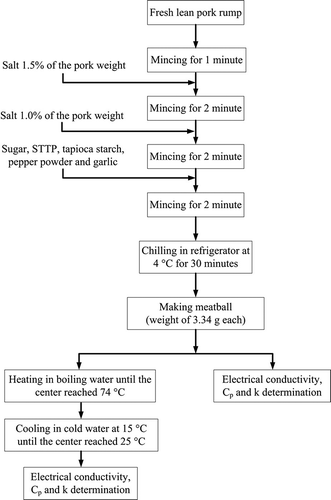
The meat ball samples were prepared from “Betagro” lean pork rump (produced from Betagro group, Bangkok, Thailand) purchased from a supermarket. It was minced by a “Robot Coupe” food processor model R201 Ultra (manufactured by Robot-Coupe Australia Pty Ltd., NSW, Australia) for 1 min and then added with salt (NaCl) at 1.5% of the pork weight. After that the mixture was minced again for 2 min before pouring additional salt for 1% and mincing for another 2 min. Later, specified amounts of sugar, sodium tripolyphosphate (STPP), tapioca starch, pepper powder, and garlic were filled into the mixture prior to 2 min of mincing. The well-minced sample was kept in a refrigerator setting temperature at 4°C for 30 min before making meat balls (weight of 14.6 g each). It is noted that for the sample preparation the salt was added in two stages in order to make it uniformly distributed. The homogeneous distribution of salt inside the minced sample is vital because it affects on the amount of protein extracted from the meat structure that would act as the emulsifier for the meat ball. A portion of fresh meat balls were measured for their electrical conductivities (σ), specific heats (Cp ), and thermal conductivities (k), whereas the rest were heated in boiling water until the center temperature reached 74°C and then cooled down to 25°C by cold water at approximately 15°C. The heated meat balls were also measured for their properties. This preparation procedure was chosen in this study, since after a preliminary test it appeared that this process provided the good quality of meat balls. illustrates the procedure throughout the control sample preparation.
Electrical Conductivity Determination
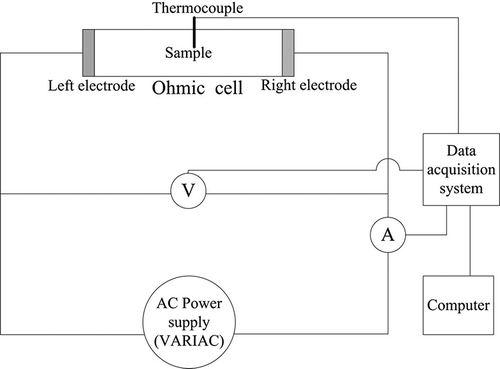
The control samples and the meat balls that varied the ratios of their ingredients, as presented in , were measured for their electrical conductivities applying a lab-scale ohmic heating apparatus that was assembled at the department of Food Science and Technology, Kasetsart University. This electrical conductivity measuring system resembles that of many researchers.[Citation4–7 Citation Citation Citation7,Citation10,Citation14] A schematic diagram of the ohmic heating circuit is shown in . The cylindrical ohmic cell was made from acrylic pipe with an inside diameter of 0.01468 m, while the electrodes were stainless steel grade 316L. The distance between electrodes is 0.01663 m. The electrical field strength applied in this measurement was 20.5 V cm−1. A type-K thermocouple located at the center of the ohmic cell was used for measuring the sample temperature. The temperature data were recorded at a 1-s time interval by a “Yokogawa” data logger model DX 1012 (produced by Yokogawa Electric Corporation, Tokyo, Japan). The electrical voltage and current were measured by a Fluke digital multimeter model 8808A (made by Fluke Corporation, Everett, WA, USA) that was connected to a computer with a RS-232 cable in order to record the data. Prior to the electrical conductivity measurement of meat ball samples, the measurement system accuracy was verified using a standard solution of 0.1 M NaCl at a temperature between 30 and 90°C. The experiments were performed in triplicate for both accuracy verification and measurement of electrical conductivities of the meat balls. Electrical conductivity of the sample was calculated by applying EquationEq. (1):
Table 1 The amount of each ingredient in the meat ball samples
Mathematical Models for Electrical Conductivity Estimation
The measured values of electrical conductivities of meat balls were fitted into various equation patterns as a function of temperature and the ratio of each ingredient. In fitting the models, the experimental data were separated between the samples before and after heating in boiling water. The best-fitted models were evaluated by their coefficients of determination (R 2) and root mean square errors (RMSE). These statistical values were calculated by EquationEqs. (2) and Equation(3):
Specific Heat, Thermal Conductivity, and Moisture Content Measurements
There were four cases of meat balls that were measured for their specific heat, thermal conductivity, and moisture content values comprising meat balls (1) before heating, (2) after heating in boiling water, (3) after ohmic heating using the identical heating rate as those heated in boiling water (approximately 4.9°C/min), and (4) after ohmic heating using the average heating rate of 24.5°C/min. For these measurements, the meat balls were prepared applying the same recipe as the control sample in the previous section. In the ohmic heating, a meat ball sample was put into the ohmic heating system that is illustrated in and then filling up the distilled water until the ohmic cell was full before applying the electrical current. The inside diameter of the cylindrical ohmic cell that was applied for heating meat balls prior to specific heat, thermal conductivity, and moisture content measurements was 0.04324 m, whereas the distance between electrodes was 0.03645 m. The heating rates of samples in cases 3 and 4 were controlled by adjusting the “SILICON” variable voltage transformer (VARIAC 0-250V) model TDGC2-3K (manufactured by Yueqing Zhongming Electric, Yueqing Zhejiang, China) during the heating process. The specific heats and thermal conductivities of samples were measured at 25°C for three replications using “DECAGON DEVICES” thermal properties analyzer model KD2 Pro (Decagon Devices Inc., Pullman, WA, USA). The moisture content was determined by the oven method using 2 g of sample and 105°C drying temperature for 24 h. Thereafter, the sample was cooled in a desiccator, weighed, and re-dried for 2 h. The process was repeated until a change in weight between the successive dryings at 2 h intervals was not over 2 mg.[Citation15] The weight loss after drying in the oven was used to calculate the moisture content of the meat ball sample and was expressed on a wet basis (wb). The moisture content determination was done in three replications.
Determination of Convective Heat Transfer Coefficient
The convective heat transfer coefficients between a meat ball and the surrounding water inside the cylindrical ohmic cell were determined applying the method of lumped-heat capacity system assuming that the internal resistance to heat transfer is negligible if comparing with the convective heat transfer resistance at the particle surface .[Citation16] A spherical stainless-steel particle (Cp = 477 J/kg °K; ρ = 7900 kg/m3)[Citation17] that has the same diameter as the meat ball (≈27.9 mm) was made and welded with a joint position of a thermocouple type T. The convective heat transfer coefficient between the spherical metal and surrounding water was considered to be close to that of the meat ball and surrounding water because the value of convective heat transfer coefficient for the case of natural convection is strongly related to the shape and size of the solid, Grashof number, Prandtl number, and thermal conductivity of the surrounding liquid.[Citation16] These parameters are obviously not associated to the type of solid material. Prior to the experiment, the cylindrical ohmic cell with an inside diameter of 0.04324 m was placed into a “STUART SCIENTIFIC” water bath model SBS 30 (Bibby Scientific Ltd., Staffordshire, UK) setting the temperature at a specified level. After the water temperature inside the ohmic cell equilibrated to those inside the water bath, the thermocouple with a spherical stainless steel was connected to the data logger and then immersed in water inside the ohmic cell. The temperature history of the spherical stainless steel was recorded until it reached the equilibrium point with the surrounding water. The experiments were conducted at seven water temperature levels comprising 30, 35, 40, 45, 60, 70, and 80°C with three replications. The initial temperature of spherical stainless steel was approximately 25°C. After collecting the experimental data, the fluid-to-particle convective heat transfer coefficients were determined using EquationEq. (4):[Citation11,Citation12]
The experimental data from each water temperature level were plotted in a graph by specifying t as the x-axis and as the y-axis. The slope of each graph was determined by linear regression. The hfp
could be calculated after knowing the slope and values of Ap
, mp
, and Cpp
.
Model Fitting and Statistical Analysis
All of the fitting of experimental data into the mathematical models in this study were performed by a least square method using the SPSS version 12.0 software package (SPSS Inc., Chicago, IL, USA). Moreover, this software was used for the one-way analysis of variance (ANOVA) and a Duncan's multiple range test in the statistical analysis using the measured data of specific heat, thermal conductivity, and moisture content values from three replications.
Table 2 The relationship between electrical conductivity of 0.1 M NaCl solution and temperature
RESULTS AND DISCUSSION
Accuracy of the Electrical Conductivity Measuring System
The measured electrical conductivities of 0.1 M NaCl solution were fitted into a linear equation as a function of temperature. The equation developed in this work and those from Lobo[Citation18] and Jittanit[Citation19] are presented in . The graph comparing the electrical conductivities of 0.1 M NaCl solution estimated by these three equations is depicted in . The plot shows that the values of electrical conductivities at low temperatures measured in this work were close to those from the references. However, the electrical conductivities from the present study were lower than those of Lobo[Citation18] with the increasing deviation when the temperature was raised. On the other hand, they became closer to those of Jittanit[Citation19] at the higher temperatures. The average differences between the electrical conductivities of 0.1 M NaCl solution in this study and those of Lobo[Citation18] and Jittanit[Citation19] were 12.2 and 1.7%, respectively. Kanjanapongkul et al.[Citation14] indicated that the electrical conductivities of 0.1 M NaCl solution measured by their ohmic heating system were lower than those of Lobo,[Citation18] especially at the high temperatures. The deviation was 6.3% at the temperature of 70°C. Sarang et al.[Citation6] claimed that the maximum difference between their measured and reference electrical conductivity values of standard salt solutions was 9%. The differences among these researches might be caused by the different internal resistances in the circuit wiring and connectors located between the digital multimeter and the ohmic cell.[Citation14] These internal resistances resulted in the voltage drop across them and subsequently reduced the voltage drop across the cell. If deeming EquationEq. (1), the variable “V” is inversely related to the calculated electrical conductivity; however, the “V” that was used for calculating the electrical conductivity was the voltage measured by the digital multimeter instead of the voltage drop across the cell excluding that across the circuit wiring, connectors, and each electrode. Thus, if the voltage drop across the circuit wiring, connectors, and each electrode occurred, the “V” would be higher than the real value and afterward the calculated electrical conductivity would be undervalued. During ohmic heating, the voltage drop across the circuit wiring, connectors, and each electrode would also generate heat resulting in the increase in the internal resistances due to the temperature dependent of the metal resistance.[Citation20] Therefore, the calculated electrical conductivities would be further undervalued along the ohmic heating process. According to the results, it appeared that the ohmic heating system in this work could be applied for the measurement of electrical conductivities of meat balls; nonetheless, the accuracy of the system should be concerned especially when the sample temperature was raised. Although some deviations could happen during the measurement, this ohmic heating apparatus is convenient to use comparing with the general electrical conductivity instrument that uses a probe to generate magnetic field, and then the subsequent eddy current is utilized as the measurement signal that can be converted to the electrical conductivity of the sample. It is because for this ohmic heating apparatus that the meat ball sample could be uniformly heated and measured its temperature and electrical conductivity simultaneously; on the other hand, the general instruments that apply the eddy current method cannot heat the sample during the measurement. Furthermore, the electrical conductivity measurement of general instruments is the most accurate at a specified temperature, such as 20°C. Outside this temperature range, the temperature compensation of the conductivity measurement is required.
Figure 3 Estimated electrical conductivities of 0.1 M NaCl solution at temperature range between 30–90°C (□, Lobo[Citation18]; ◊, Jittanit[Citation19]; ♦, present study).
![Figure 3 Estimated electrical conductivities of 0.1 M NaCl solution at temperature range between 30–90°C (□, Lobo[Citation18]; ◊, Jittanit[Citation19]; ♦, present study).](/cms/asset/a1552209-63e7-43a7-85b7-0460a766c83b/ljfp_a_604891_o_f0003g.gif)
Electrical Conductivities of Meat Balls
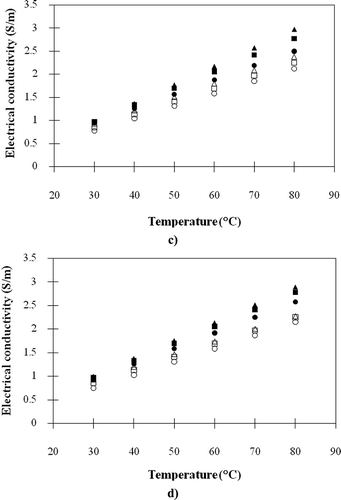
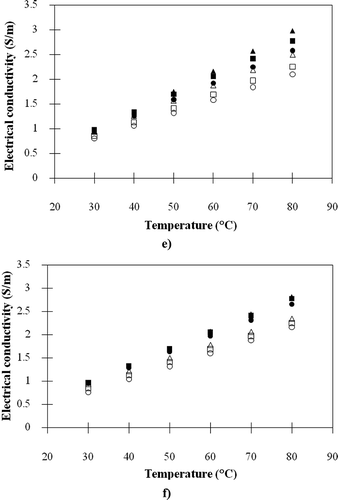
The electrical conductivities of the fresh and heated meat ball samples varying amount of each ingredient are presented in . It appeared that the electrical conductivities of meat ball samples ranged between 0.677–3.156 S/m indicating that they can be effectively heated by an ohmic heating method. Lyng and McKenna[Citation21] pointed out that the food with electrical conductivities between 0.01–10 S/m are considered appropriate for the ohmic heating technique. Apparently, – illustrated that the electrical conductivities of fresh meat balls were higher than those of samples heated in boiling water prior to electrical conductivity measurement. The explanation is that a number of ions in samples diffused to the surrounding water during heating; as a result, the electrical conductivities of meat balls decreased while those of surrounding water increased. Furthermore, the volume expansion of meat ball during boiling was another reason since after expansion the ion content per volume of meat ball would descend. Sarang et al.[Citation6] pointed out that heating can result in the change of electrical conductivities of meat products because of structural changes in the tissue, such as cell wall protopectin breakdown, removal of non-conductive gas bubbles, softening, and lowering in aqueous phase viscosity. shows that the addition of salt led to the obvious increase in the electrical conductivities of meat balls. The consequence of the STTP addition on the electrical conductivities of meat balls was similar to those of the salt; however, the degree of effect was lower because the weight of added STTP was much less than salt as shown in . Referring to Shirsat et al.[Citation4] and Zell et al.,[Citation8] either the addition of salt in meat emulsion batters or the injection of salt solution into the meat pieces resulted in the significant increase of their electrical conductivities. They explained that the electrical conductivity of food was directly related to its ion movement; thus, if the salt is added in a damp sample, the ions inside the sample will be raised resulting in the enhancement of its electrical conductivity. Conversely to salt and STTP, the escalating of proportions of tapioca starch, sugar, pepper powder, and garlic in meat balls led to the fall of electrical conductivity as illustrated in –. It is because the majority of tapioca starch and sugars are carbohydrates that are non-electrolytic. The pepper and garlic also have low electrical conductivities because their major components are carbohydrate and moisture, respectively.
Figure 4 Electrical conductivities of the meat ball samples: (a) control, −10% salt and +10% salt; (b) control, −10% STPP and +10% STPP; (c) control, −10% starch and +10% starch; (d) control, −10% sugar and +10% sugar; (e) control, −10% pepper and +10% pepper; (f) control, −10% garlic and +10% garlic (■, control [fresh]; ▲, −10% [fresh]; ●, +10% [fresh]; □, control [heated]; ∆, −10% [heated]; ο, +10% [heated]).
![Figure 4 Electrical conductivities of the meat ball samples: (a) control, −10% salt and +10% salt; (b) control, −10% STPP and +10% STPP; (c) control, −10% starch and +10% starch; (d) control, −10% sugar and +10% sugar; (e) control, −10% pepper and +10% pepper; (f) control, −10% garlic and +10% garlic (■, control [fresh]; ▲, −10% [fresh]; ●, +10% [fresh]; □, control [heated]; ∆, −10% [heated]; ο, +10% [heated]).](/cms/asset/6c4a1aaf-4634-4a74-9f00-bdceb72b60ea/ljfp_a_604891_o_f0004g.gif)
Mathematical Models for Electrical Conductivity Estimation
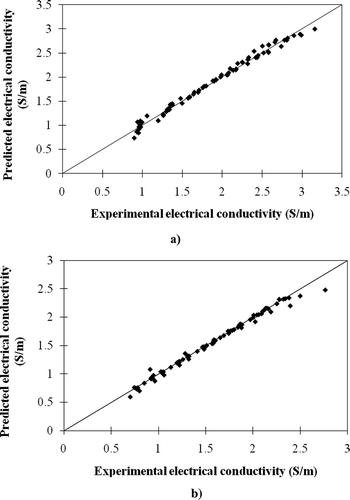
The results in the previous section show that there are some relationships between the electrical conductivities of meat balls and both temperature and the ratio of each ingredient. After fitting the experimental data into various equations using a least square method, two good fitting models were created for the samples before and after heating in boiling water. These models with their R 2 and RMSE are presented in . It was found that the effect of adding STTP on the electrical conductivity of the fresh meat ball was insignificant but it was significant in the case of boiled meat ball. The comparisons between the experimental data and the model prediction are depicted in . It appeared that these empirical models are simple to use but provide a reasonably precise estimation. In practice, the equation developed for the fresh sample is useful if applying the ohmic heating process to cook the meat ball, whereas that developed for the heated samples can be applied if ohmic heating process is used to heat the cooked meat balls that are contained in a food as a component.
Table 3 The empirical equations for estimating the electrical conductivities of meat balls
Specific Heats, Thermal Conductivities, and Moisture Contents of Meat Balls
The measured specific heat, thermal conductivity, and moisture content values of four cases of samples are shown in . The results showed that the specific heats of heated meat balls (sample nos. 2 to 4) were insignificantly different but they were significantly higher than those of the fresh meat balls. The variation in the specific heats of food products can be caused by the different compositions of foods. Subramanian and Viswanathan[Citation22] and Legrand et al.[Citation23] stated that the specific heat of food products will increase if their moisture contents were raised. The moisture content determination in this study reveals that the moisture content of fresh meatball was significantly lower than those of the heated meat ball. It was 71.2%wb for fresh meatball but between 72.4–73.1%wb for heated meatballs as shown in . Therefore, the increase in the specific heats of samples after heating occurring in this work is unsurprising. After deeming the thermal conductivities of samples, it is obvious that the thermal conductivities of samples are insignificantly different between all samples. Although the thermal conductivity of food related to its composition especially moisture like the specific heat,[Citation22,Citation23] the effect of moisture content on the thermal conductivity is less than that on the specific heat. This statement can be confirmed by the finding of Legrand et al.,[Citation23] who developed the equations for predicting the specific heat and thermal conductivity of cooked red bean as a function of moisture and temperature. In their study, they indicated that the multiplier of moisture variable in the specific heat equation was 30.378, whereas that in the thermal conductivity equation was merely 7.8 × 10−3. The specific heat and thermal conductivity of meat ball are needed if the researcher or industry desires to estimate the meat ball temperature during ohmic heating by mathematical models.
Table 4 The specific heats, thermal conductivities, and moisture contents of meat balls prepared by various processes
Table 5 The liquid-to-particle convective heat transfer coefficients determined at various ΔT
Fluid-to-Particle Convective Heat Transfer Coefficients
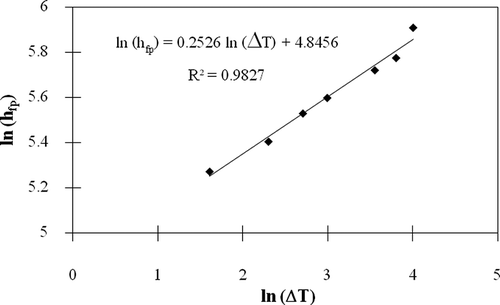
One of the heat components that are influential to the temperature of meat balls in liquid during ohmic heating is the convective transferred heat between meat balls and liquid. The amount of convective transferred heat is straightforwardly associated with the convective heat transfer coefficient.[Citation17] In this study, the heat convection was considered as natural convection since the motion of liquid was initiated by the liquid temperature difference not due to the externally mechanical force. The convective heat transfer coefficients were determined by the lump-heat capacity method as formerly described. The results were presented in . The convective heat transfer coefficients were found to be in the range between 195–369 W/m2°C, which are in the comparable range to those investigated by Astrijm and Bark[Citation24] and Jittanit.[Citation19] Moreover, it is clear that the convective heat transfer coefficients were boosted along the increasing of the differences between the particle and surrounding fluid temperatures. The explanation is that this coefficient directly depends on the fluid velocity around the particle.[Citation16] For the natural convection, the most important factor that affects on the velocity of fluid along the particle surface is the temperature difference between the particle and surrounding fluid. If plotting a graph by specifying as the y-axis and
as the x-axis, a linear correlation between these two values was observed as illustrated in . This correlation was converted to be a more user-friendly pattern as shown in EquationEq. (5). This equation can be utilized for estimating the value of convective heat transfer coefficient between the meat ball or any particulate food with a diameter of more or less 27.9 mm and the surrounding water under the case of natural convection:
CONCLUSION
In this study, it was found that meat balls can be effectively heated by an ohmic heating method due to their high electrical conductivities. The addition of some ingredients that contain ionic substances, such as salt and STTP, could raise the electrical conductivity of meat ball. Two good-fitting empirical equations were developed for estimating the electrical conductivities of meat balls. They presented the electrical conductivity as a function of temperature and the ratio of each ingredient. The specific heats of fresh meat balls were slightly lower than those of heated meat balls. However, the thermal conductivities of meat balls are insignificantly different between each treatment. The fluid-to-particle convective heat transfer coefficients were found to be in the range between 195–369 W/m2°C. A simple model was developed to estimate the convective heat transfer coefficients as a function of temperature difference between the particle and surrounding fluid. The electrical and thermo-physical properties investigated in this work are useful if wanting to calculate the meat ball temperature during ohmic heating by mathematical models. In the future, the application of ohmic heating for meat balls in continuous mode should be studied so that the pasteurization or sterilization of meat balls could be developed to be an in-line process.
NOMENCLATURE
| A | = |
Cross sectional area of electrode (m2) |
| Ap | = |
Surface area of the particle (m2) |
| Cp | = |
Specific heat (J/kg°C) |
| Cpp | = |
Specific heat of the particle (J/kg°C) |
| dp | = |
Diameter of the particle (m) |
| g | = |
Acceleration due to gravity (m/s2) |
| hfp | = |
Fluid-to-particle convective heat transfer coefficient (W/m2°C) |
| I | = |
Electrical current (Ampere) |
| kf | = |
Thermal conductivity of the fluid (W/m°C) |
| kp | = |
Thermal conductivity of the solid particle (W/m°C) |
| L | = |
Distance between the electrodes (m) |
| MC | = |
Moisture content (%wb) |
| mp | = |
Mass of the particle (kg) |
| t | = |
Time (s) |
| T | = |
Temperature (°C) |
| Tf | = |
Temperature of the fluid (°C) |
| Ti | = |
Initial temperature of the particle (°C) |
| Tp | = |
Temperature of the particle (°C) |
| ΔT | = |
Tf – Tp = Temperature difference between fluid and particle (°C) |
| V | = |
Applied voltage (Volt) |
| R 2 | = |
Coefficients of determination |
| RMSE | = |
Root mean square errors |
| X | = |
Weight ratio between the ingredient (as specified by subscript) and the meat |
Greek Letters
| β | = |
Volumetric thermal expansion coefficient (K−1) |
| σ | = |
Electrical conductivity (S m−1) |
| μ | = |
Fluid viscosity (Pa·s) |
| ρ f | = |
Density of the fluid (kg/m3) |
| ρ p | = |
Density of the particle (kg/m3) |
Dimensionless Numbers
| Bi | = |
Biot number |
| Gr | = |
Grashof number = |
| Nu | = |
Nusselt number = |
| Pr | = |
Prandtl number |
ACKNOWLEDGMENTS
This research was financially supported by the Kasetsart University Research and Development Institute, Bangkok, Thailand.
REFERENCES
- Ozkan , N. , Ho , I. and Farid , M. 2004 . Combined ohmic and plate heating of hamburger patties: Quality of cooked patties . Journal of Food Engineering , 63 : 141 – 145 .
- Goullieux , A. and Pain , J.P. 2005 . “ Ohmic heating ” . In Emerging Technologies for Food Processing; Sun, W , 469 – 500 . California : Elsevier Academic Press .
- Saif , S.M.H. , Lan , Y. , Wang , S. and Garcia , S. 2004 . Electrical resistivity of goat meat . International Journal of Food Properties , 7 ( 3 ) : 463 – 471 .
- Shirsat , N. , Lyng , J.G. , Brunton , N.P. and McKenna , B. 2003 . Conductivities and ohmic heating of meat emulsion batters . Journal of Muscle Foods , 15 : 121 – 137 .
- Shirsat , N. , Lyng , J.G. , Brunton , N.P. and McKenna , B. 2004 . Ohmic processing: Electrical conductivities of pork cuts . Meat Science , 67 : 507 – 514 .
- Sarang , S. , Sastry , S.K. and Knipe , L. 2008 . Electrical conductivity of fruits and meats during ohmic heating . Journal of Food Engineering , 87 : 351 – 356 .
- Tulsiyan , P. , Sarang , S. and Sastry , S.K. 2008 . Electrical conductivity of multicomponent systems during ohmic heating . International Journal of Food Properties , 11 : 1 – 9 .
- Zell , M. , Lyng , J.G. , Cronin , D.A. and Morgan , D.J. 2009 . Ohmic cooking of whole beef muscle—Optimisation of meat preparation . Meat Science , 81 : 693 – 698 .
- Bozkurt , H. and Icier , F. 2010 . Ohmic cooking of ground beef: Effects on quality . Journal of Food Engineering , 96 : 481 – 490 .
- Marra , F. , Zell , M. , Lyng , J.G. , Morgan , D.J. and Cronin , D.A. 2009 . Analysis of heat transfer during ohmic processing of a solid food . Journal of Food Engineering , 91 : 56 – 63 .
- Balasubramaniam , V.M. and Sastry , S.K. 1994 . Liquid-to-particle convective heat transfer in non-Newtonian carrier medium during continuous tube flow . Journal of Food Engineering , 23 : 169 – 187 .
- Baptista , P.N. , Oliveira , F.A.R. , Oliveira , J.C. and Sastry , S.K. 1997 . The effect of translational and rotational relative velocity components on fluid-to-particle heat transfer coefficients in continuous tube flow . Food Research International , 30 ( 1 ) : 21 – 27 .
- Barigou , M. , Mankad , S. and Fryer , P.J. 1998 . Heat transfer in two-phase solid-liquid food flows: A review . Trans IChemE , 76 : 3 – 29 .
- Kanjanapongkul , K. , Yoovidhya , T. , Tia , S. and Wongsa-Ngasri , P. 2008 . Protein removal from fish mince washwater using ohmic heating . Songklanakarin Journal of Science and Technology , 30 ( 3 ) : 413 – 419 .
- Jittanit , W. , Niti-Att , S. and Techanuntachaikul , O. 2010 . Study of spray drying of pineapple juice using maltodextrin as an adjunct . Chiang Mai Journal of Science , 37 ( 3 ) : 498 – 506 .
- Holman , J.P. 2002 . Heat transfer , 9th , 334 – 350 . Boston , MA : McGraw-Hill . 140–217
- Cengel , Y.A. 1998 . Heat Transfer: A Practical Approach , 949 – 959 . Boston , MA : McGraw-Hill .
- Lobo , V.M.M. 1984 . Electrolyte Solution: Literature Data on Thermodynamics and Transport Properties , 340 Coimbra , , Portugal : Coimbra Editora .
- Jittanit , W. 2001 . Influence of heat transfer between liquid and particle in ohmic heating , 172 Thonburi , , Thailand : Master's Thesis, Food Engineering Department, King Mongkut's University of Technology .
- Mott , N.F. and Jones , H. 1958 . The Theory of the Property of Metals and Alloys , 243 – 245 . New York , , USA : Dover Publication Inc .
- Lyng , J.G. and McKenna , B.M. 2007 . “ Ohmic pasteurization of meat and meat products ” . In Handbook of Farm, Dairy, and Food Machinery; Kutz, M , 553 – 577 . New York : William Andrew Inc .
- Subramanian , S. and Viswanathan , R. 2003 . Thermal properties of minor millet grains and flours . Biosystems Engineering , 84 : 289 – 296 .
- Legrand , A. , Leuliet , J.C. , Duquesne , S. , Kesteloot , R. , Winterton , P. and Fillaudeau , L. 2007 . Physical, mechanical, thermal and electrical properties of cooked red bean (Phaseolus vulgaris L.) for continuous ohmic heating process . Journal of Food Engineering , 81 : 447 – 458 .
- Astrijm , A. and Bark , G. 1994 . Heat transfer between fluid and particles in aseptic processing . Journal of Food Engineering , 21 : 97 – 125 .