Abstract
The effects of supercritical CO2 (SC-CO2) on the microbiological, sensory (taste, odour, and colour), nutritional (vitamin C content), and physical (cloud, total acidity, pH, and °Brix) qualities of orange juice were studied. The CO2 treatment was performed in a 1 litre capacity double-walled reactor equipped with a magnetic stirring system. Freshly extracted orange juice was treated with supercritical CO2, pasteurised at 90°C, or left untreated. There were no significant differences in the sensory attributes and physical qualities between the CO2 treated juice and freshly extracted juice. The CO2 treated juice retained 88% of its vitamin C, while the pasteurised juice was notably different from the fresh juice and preserved only 57% of its vitamin C content. After 8 weeks of storage at 4°C, there was no microbial growth in the CO2 treated juice.
INTRODUCTION
The organoleptic qualities of freshly squeezed orange juice are desired in some consumer markets. The shelf life of such a product is unfortunately limited to a few days due to the presence of an endogenous microflora of 104 to 105 cfu/ml. This microflora is comprised mainly of acidotolerant species, such as Lactobacillus and Leuconostoc;[Citation1] yeast, such as Saccharomyces and Candida; as well as moulds.[Citation2] The presence of other bacteria, such as E. coli 0157, in orange juice has been reported.[Citation3] The proliferation of these microorganisms provokes a rapid degradation of the organoleptic (taste, odour, colour, and aromas) and the nutritional properties.[Citation4] High levels of pectin esterase in orange juice cause the de-esterification of pectin. The resulting pectate forms a complex with calcium ions present in the juice and subsequently causes a loss of turbidity.[Citation5] Pasteurisation at 90°C for 1 min is the method currently used to prevent the degradation of quality caused by the pectin esterase and to reduce microbial populations. Pasteurisation, however, causes a degradation of aromas, flavours, and nutritional values and also induces a change in colour by promoting non-enzymatic browning reactions.[Citation6] Other methods for controlling the action of microorganisms and endogenous enzymes are known. Bae et al.[Citation7] reported that A. acidoterrestris spores were completely inactivated by SC-CO2 to undetectable levels in above 65°C, 100 bar for 40 min and 70°C, 80 bar for 30 min, but the SC-CO2 did not affect the pH and °Brix of apple juice. Jarupan and Mohammad[Citation8] have shown that supercritical CO2/liquid CO2 treatment at ≥6 MPa and 50°C was sufficient to inactive yeast up to 6 log after 15 min treatment time. Shomer et al.[Citation9] reported a 35-day increase in shelf life for orange juice after heating the juice to 60°C for 15 min followed by storage with 6 mM CO2 at 4°C. Wei et al.[Citation10] reported a 99% reduction of the Listeria monocytogenes and Salmonella typhymurium populations in orange juice after a treatment with 6.18 MPa pressurised CO2 at 35°C. According to Balaban et al.,[Citation11] the reduction of pH during treatments with supercritical CO2 affected enzyme activity and reduced the number of microorganisms. The use of Pectin Methylesterase Proteic Inhibitor to stabilise orange juice cloud has been reported by Castaldo et al.[Citation12] However, the addition of other substances affects the quality of the juice and is not acceptable.
In this study, the microbiological properties, sensory attributes (taste, odour, and colour), nutritional (vitamin C concentration), and physical properties (cloud, total acidity and °Brix) of orange juice treated with SC-CO2 (25 MPa, 40°C) were studied.
MATERIALS AND METHODS
Apparatus
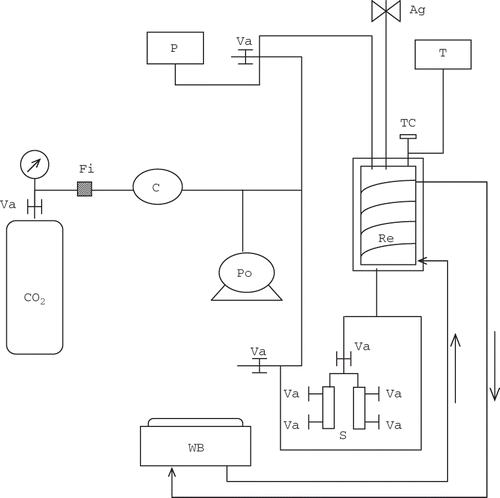
presents a diagram of the apparatus used to treat the orange juice with SC-CO2 (25 MPa, 40°C). The apparatus was equipped with a double-walled stainless steel reactor (model 29501-3, Newport Scientific Inc., Jessup, MD, USA), a compressor (model 46-1387, Newport Scientific Inc.), a pump (model EK16, American LEWA), a number of transmission valves, a magnetic stirrer (model MM-016, series C-8609, PPI, DYNA/MAG Mixers), and a cylinder of CO2 directly attached to the reactor. The temperature of the CO2 in the reaction chamber during the treatments was controlled via a thermocouple (O.F. Ekland, Cape Coral, FL, USA) placed inside the reactor and was maintained by the circulation of water from a water bath (model RMS 20, series H01008, MGW LUDA, Texas City, TX, USA) into the double-walled chamber. The pressure inside the reactor was controlled by a manometer and was regulated by valves. Connection tubing allowed sampling and circulation of CO2 from the cylinder to the reactor chamber. Sampling was done near a flame (to prevent contamination) into metallic 20-ml detachable tubes and the sample was subsequently transferred to sterile 50-ml beakers or flasks. The metallic tubing was rinsed with sterile distilled water between samplings and after each experiment washed in distilled water and sterilised in an autoclave. Cleaning and sterilisation of the reaction chamber and the connection tubing were done by circulating distilled water and ethanol (70%) followed by sterile distilled water.
Orange Juice
A commercial juice extractor (model HJ28-04, Black-Decker, Quebec, QC, Canada) was used to prepare the juice from California grown oranges (5014471, Sunkist Naval) supplied by a local distributor. The initial microbial count of 8.2 × 104 cfu/ml in the freshly extracted juice was evaluated on plate count agar (PCA).
Microorganism
Lactobacillus helveticus is one of the main microorganisms primarily found among the endogenous microflora of freshly extracted orange juice. Pure cultures of L. helveticus (in Man, Rogosa, and Sharpe [MRS]) with initial counts of 106 cfu/ml were used to inoculate the orange juice at 1% (v/v).
Preparation of Juices
Fresh orange juice was inoculated to 1% (v/v) with pure L. helveticus cultures (106 cfu/ml). A portion of the contaminated juice was treated in triplicate with SC-CO2 (25 MPa, 40°C) for 90 min with stirring at 200 rpm. A second portion was heated at 90°C for 60 s, in triplicate. A third portion was left untreated. As a further control, a sample of uncontaminated fresh juice was also left untreated. The juice portions were then stored in 250-ml sterile flasks at 4°C for 8 weeks. Each week, three flasks from each treatment and from the untreated juices were analysed for microbiological quality as well as nutritional (vitamin C) and physico-chemical (cloud, total acidity, pH, °Brix, and colour) qualities. Two other portions of fresh juice, uncontaminated but treated with CO2 and heat, respectively, were stored in the same conditions and were reserved for sensory attribute testing.
Physico-Chemical Analysis
| • | Microbiological analysis was performed by plate counts on MRS-agar. | ||||
| • | The pH was monitored with a pH-meter (model 620, series 1956, Fisher Accumet, St-Laurent, QC, Canada) in 50 ml of orange juice with constant stirring. | ||||
| • | Total acidity content of the orange juices was determined by titration with 0.1 N NaOH in the presence of phenolphthalein (1%) with constant stirring. Acid content in 50 ml of juice is expressed as follows: μmol (citric acid)/ml (juice) = VNaOH × NNaOH × 0.064/50. | ||||
| • | Turbidity was determined as described by Versteeg et al.[Citation12] Samples were centrifuged at 360 rpm for 10 min. The turbidity of the supernatant was determined with a spectrophotometer (model 335201, serial 3702107001, Milton Roy, Ivyland, PA, USA) at 660 nm after calibration with distilled water. | ||||
| • | Colour was evaluated by colorimetry (model CT-310, Minolta, Osaka, Japan) after standard calibration on white. The L, a, and b values representing, respectively, brightness, red colour, and yellow colour of orange juice samples were measured in transparent glass containers well filled so that the cover was in contact with the juice. | ||||
| • | Vitamin C content of treated juices was determined by high performance liquid chromatography (HPLC, model HP 1050; Capitol Heights, MD, USA). The HPLC apparatus was equipped with an interaction column (25 cm × 4 mm × 5μ, model RP-18, series 624076) with a pre-column, an UV detector (model 79853C, 244 mm), an 80.8 bars pressure pump (model 79852A) at 35°C, and an HP autosampler injector (model 1050, series 79855-900001). The solvent (0.15% ammonium tetrabuthyl, pH 2.85) was circulated at 0.750 ml/min. The diluted samples (1/20) were centrifuged at 8000 g for 10 min and 20 ul were then injected for analysis. | ||||
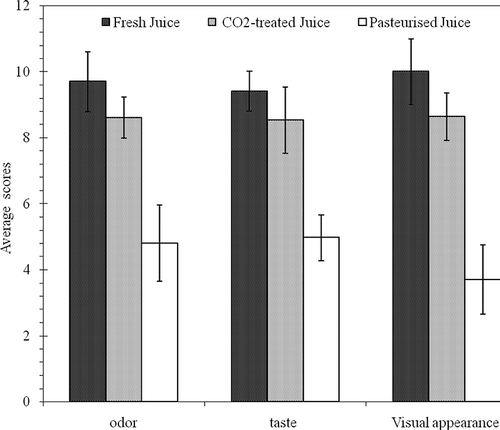
Each week, the taste, the odour, and the visual appearance of the uncontaminated juices treated with either the SC-CO2 or heat (pasteurisation, 90°C for 60 s) were evaluated in comparison to freshly extracted juice by eight tasters. The non-graduated scale method with repetitions was used.[Citation14] This method consists of presenting to the tasters freshly extracted juice as a reference followed by the samples to be evaluated. Each sample had a code, and the codes changed each week. In the first part of the test, the tasters smelled the reference juice and attributed a score. Then they smelled the other sample juices, and gave each of them a score relative to the reference juice. In the second part, the taste of the reference juice was given a score, and then the sample juices were evaluated relative to it. Between each drink of juice, the taster took a gulp of water and waited 5 min. Third, the appearance of the sample juices is judged, once again in relation to the reference juice. Evaluation sessions for the same eight tasters were held once a week for 8 weeks, and at each session the sample codes were changed.
Statistical Analysis
Microbial survival was expressed as the log10 of the ratio between the number of viable cells after treatment and the number of cells initially present (log10[N/No]). The results are statistically examined as mean values by one-way analysis of variance. Reported D-values and lags (L) corresponding to the time during which the number of cells remained constant before inactivation are averages of three experiments. For sensory evaluation, analysis of variance (ANOVA) followed by Tukey's multiple comparison test were performed on the data. ANOVA was used to detect possible differences between treatments or between tasters, with a probability of either 0.01 or 0.05. Tukey's test, with the standard error and the least significant difference, utilises the statistical table of critical values of the Students t-distribution at a probability of 5% (P = 0.05), allowing the comparison of samples to each other.[Citation14]
RESULTS AND DISCUSSION
Microbiological Quality of Orange Juice Treated with CO2
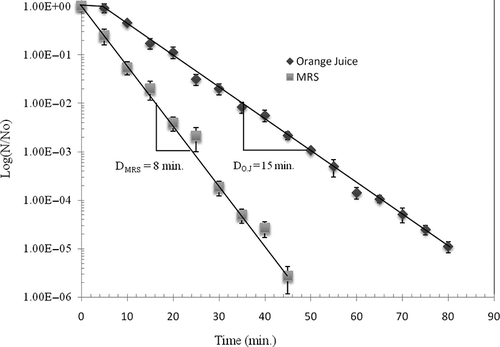
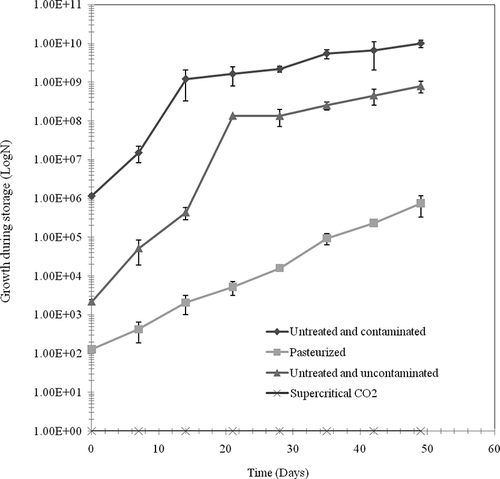
The orange juice was inoculated with a pure culture of L. helveticus, which is one of the bacteria mainly present in the endogenous microbial flora of freshly squeezed orange juice. presents the levels of inactivation of L. helveticus in orange juice and in commercial MRS media by the SC-CO2 treatment (25 MPa, 40°C) at pH 3.7 with stirring at 200 rpm. In the juice, the lag phase was 5 min and the D value was 15 min. These values indicate that the bacteria were more resistant to the SC-CO2 treatment in the orange juice compared to the MRS media where the lag phase and D value were 0 and 8 min, respectively. The efficiency of the SC-CO2 treatment depends not only on the type of microorganisms but also the media and water activity.[Citation15] The relative resistance of L. helveticus to the treatment in the orange juice could be due to the fact that the numerous protein and polysaccharide particles in suspension hindered the contact between the CO2 and the microbial cells. The difference between the orange juice and the culture medium could also be due to differences in viscosity. The transfer of CO2 is slower in more viscous media and the contact between the CO2 and the cells is more difficult to attain. This could, therefore, be responsible for the slower rate of inactivation in the orange juice compared to the MRS media. The lower water content in the orange juice compared to the MRS media could reduce the hydration of the CO2 and subsequently reduce the formation of hydrates. Despite the resistance of L. helveticus in the orange juice, all cells were destroyed after 90 min of treatment. The results of weekly analysis showed that the SC-CO2 treatment allowed a complete purification of orange juice compared to pasteurisation. There was no microbial growth in the flasks containing orange juice treated with SC-CO2 and stored at 4°C. All microbial cells were, therefore, destroyed by the treatment. The pasteurised juice, however, had a microbial count of 126 cfu/ml following the treatment, and after 49 days of storage the number of viable cells increased to 7.21 × 105 cfu/ml indicating a relatively slow rate of growth probably due to the low storage temperature of 4°C (). Growth rate was constant throughout the storage period as indicated by the linear growth rate. The untreated juices (uncontaminated and contaminated) initially contained 2.19 × 103 and 1.17 × 106 cfu/ml, respectively. At the end of the storage period at 4°C, the microbial counts reached 7.85 × 108 and 9.93 × 109 cfu/ml, respectively (). Growth rate was rapid during the first 2 weeks of storage of the contaminated untreated juice and during the first 3 weeks of storage of the uncontaminated untreated juice. Growth rate then slowed for both juices probably due to a depletion of available nutrients. The pasteurised juice, however, had a low initial bacterial count (126 cfu/ml) and had a slow initial rate of growth. In this case, the nutrients were not depleted and growth was linear until the end of the storage period. These results demonstrate that the microbiological quality of orange juice treated with SC-CO2 at the relatively high temperature of 40°C was better than that of pasteurised orange juice. These results concur well with other studies on methods of preserving orange juice with CO2. Shomer et al.[Citation9] studied the effects of CO2 on the microbiological quality of orange juice contaminated with S. cerevisiae and Streptococcus lactis. The authors dissolved 6 mM of CO2 in 350 ml of contaminated orange juice that was then stored at 4°C at atmospheric pressure. The shelf life was prolonged by 25 days with this treatment. Their treatment of a contaminated orange juice with the same quantity of CO2 followed by heat treatment at 60°C for 15 s increased the shelf life of the juice by 35 days. This research demonstrates that the combination of CO2 and temperature preserves orange juice for a longer period of time compared to CO2 treatment at room temperature. Contaminated orange juice treated with CO2 under 13.7 MPa of pressure for 2 days at 35°C reduced the microbial population by 99%.[Citation10] From a strictly microbiological view, the use of SC-CO2 to preserve orange juice is an efficient method that could replace thermal treatments that induce a loss of organoleptic properties.
The Effects of Treatments on Orange Juice Colour
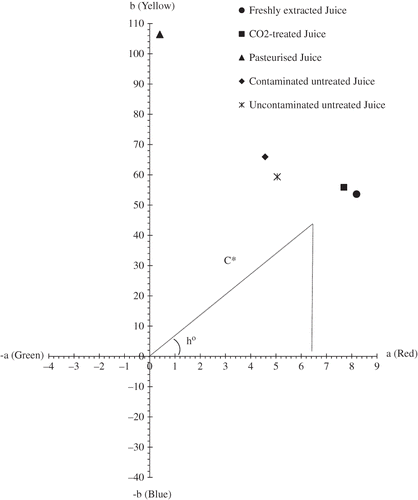
In general, consumers judge and appreciate their food by its colour and taste. Colour, in effect, has a psychological impact on the taste of certain food products for a good number of consumers.[Citation16] In this context, it is therefore necessary and important to preserve the colour of food products during treatment. In this study, the L, a, and b indices were measured during the storage period in order to compare the colour of orange juice following the different treatments (). The L, a, and b indices indicate juice brightness, red colour, and yellow colour, respectively. shows the variation of a and b indices in the juice after 8 weeks of storage at 4°C. To better appreciate the colour modification in the juice after the various treatments, the C* index (colour intensity or chromaticity) and the hue index h° that indicates change in colour, were determined from the a and b values. The C* index is defined as the square root of the sum of squares of a and b.[Citation17] A high C* value indicates a more intense colour:
Table 1 Influence of treatment type and storage time on juice colour
A change in colour provokes an angle variation (h°), defined as:[Citation17]
The L values of untreated juices (contaminated and uncontaminated) did not change significantly during storage at 4°C (P > 0.05). Previous studies by Lee and Nagy[Citation18] also showed that the brightness of orange juice stored at 20°C for 21 weeks did not change. However, the a and b values (positive) varied significantly during storage (P < 0.05). The a value decreased while the b value increased, indicating a reduction in the red colour and an increase in the yellow colour for the untreated juices. At the end of the 8-week storage period, the colour intensity (C*) and the hue angle (h°) of the untreated uncontaminated juice were 59.55 and 85.17, respectively. The initial value of a was 54.09 and that of b was 81.35. In the contaminated untreated juice the C* value was 66.13 and the h° value was 86.08 at the end of the 8-week storage period. These values indicate that there was a modification in the colour of the juice. This colour degradation was probably due to the presence of enzymes, such as polyphenol oxydase (PPO) and non-inactivated microorganisms. However, the variation of the a and b values was slow, probably due to a slowing of reactions at the 4°C storage temperature. Lee and Chen[Citation19] also report that, in untreated juice, the brightness did not change but the red colour decreased slowly and the yellow colour increased rapidly after storage for 19 weeks at 4°C. In the pasteurised juice, the thermal treatment caused an increase in the L (brightness) and b (yellow) values but the a (red) value decreased. The intensity of the colour (C*) and the hue angle (h°) of the pasteurised juice was very high with values of 105.42 and 89.17, respectively. This seems to be due to the significant colour modification occurred during the pasteurisation process. It is recognised that thermal treatments induce changes in the colour of food products.[Citation20,Citation21] The high C* value in pasteurised juice indicates a more intense colour than for other juices. On one hand, non-enzymatic browning reactions must have occurred during pasteurisation,[Citation19] such as the formation of furfural (a browning agent) from vitamin C in the presence of oxygen, a reaction promoted by heat,[Citation22] or the conversion of leucoanthocyanidines to anthocyanidines capable of giving a brown colour after heating in an acid environment.[Citation23] On the other hand, the a index of juice was reduced considerably from 8.19 to 0.36 after pasteurisation, indicating a reduction of red colour and a change towards a more green colouration. This change towards green must have intensified the colour of pasteurised juice.
The L, a, and b values of SC-CO2 treated juice were not significantly different from those of freshly extracted juice (P > 0.05). These values did not vary throughout the storage period. The intensity of colour (C*) and the h° angle were also almost identical to those of freshly extracted juice (), demonstrating that the CO2 treatment did not notably affect the colour of the juice. shows a photo of pasteurised, SC-CO2 treated, and freshly extracted juices. The freshly extracted juice and the SC-CO2 treated juice had the same colour, while pasteurised juice was paler. Research by Cheftel[Citation24] also shows that the treatment with pressure does not affect the colour of food products. In effect, during pressurisation of food products, small molecules such as pigments, vitamins and amino acids are not affected since pressure has little or no effect on covalent bonds. It is worth noting that the L, a, and b indices did not vary throughout the storage period at 4°C, indicating a stability in the colour following the treatments. Inactivation or inhibition of colour-modifying enzymes or microorganisms by the treatments ensures the colour stability of the juices. These results clearly demonstrate that heat pasteurisation of orange juice induces profound modifications in colour quality while the use of pressurised CO2 at moderate temperatures preserves the original colour of the juice.
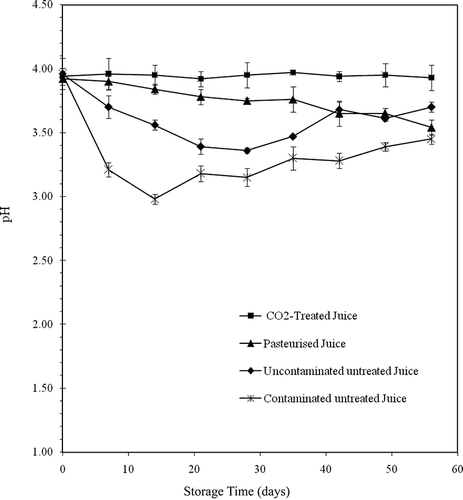
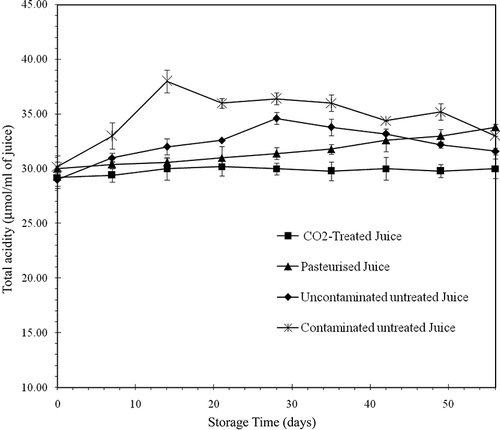
Effect of Treatments on pH and Total Acidity
The pH of fresh orange juice was 3.96 with a total acidity of 30.2 μmol·ml−1. During the CO2 treatment, the pH fell by 0.85 units to 3.11. The pH returned to its initial value approximately 2 h after depressurisation when the CO2 was separated from the juice. The pH and total acidity remained constant during the storage period at 4°C ( and ). Balaban et al.[Citation25] also reported a decrease in pH in orange juice during treatment with pressurised CO2. They found that CO2 under 31 MPa of pressure at 35°C caused a 0.7 unit decrease in pH, and after depressurisation, the pH returned to its initial value. When the pH decreased under experimental conditions, the CO2 was in a supercritical state and was denser, due to the pressure. Under atmospheric conditions, following the release of the pressure, the vapour state CO2 that remained dissolved in the juice was in the aqueous form (CO2 [aq]) because of the acidity of the environment (pH 3.93). The formation of carbonic acid that would reduce the pH was very limited. As well, the carbonic acid would not be dissociated and, therefore, could not cause the increase in acidity. The decrease in pH seems, therefore, to be associated with the state and density of the CO2 in the juice during the treatment. The pH () and total acidity () values of untreated (contaminated and uncontaminated) and pasteurised juices varied throughout the storage period. The pH of the contaminated untreated juice fell to 2.98 while the total acidity increased to 38 μmol·ml−1 during the first 2 weeks of storage, corresponding to the period of rapid microbial growth. The reduction of pH and the increase in total acidity in the uncontaminated untreated and pasteurised juices also corresponded with microbial growth. Goyle and Ojha[Citation26] also observed a reduction of pH and an increase in total acidity in orange juice throughout a refrigerated storage period. The acidification of juices during storage seems to be associated to the presence of microorganisms. The changes in pH and total acidity in the contaminated juices (untreated and pasteurised) are probably due to the acidification (lactic acid) by L. helveticus. The reduction of pH in the stored uncontaminated juice would be due to the production of acids by lactic bacteria (Lactobacilli) naturally present in the juice. However, with the exception of pasteurised juice where the microbial population was not very high, the acidification of untreated juices (contaminated and uncontaminated) did not occur until the end of the storage period. Acidification stopped when microbial growth started to slow, that is, 2 weeks for the contaminated untreated juice and 4 weeks for the uncontaminated untreated juice. The microorganisms had to use certain organic acids as a carbon source for their growth when the nutrients were probably depleted by the large microbial load of the juice. The pH and total acidity of the juice treated with CO2 did not change because the treatment destroyed all microorganisms. The results of this study show that, compared to heat pasteurisation, the use of pressurised CO2 allows a stabilisation of the pH of orange juice.
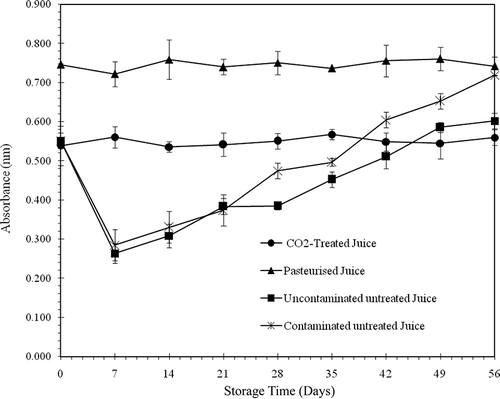
Treatment Effects on Cloud and °Brix
The effects of the treatment on juice turbidity are shown in . The absorbance of freshly extracted juice was 0.552. After pasteurisation, the cloud increased to 0.746 then remained constant throughout the storage period. The CO2 treated juice was less turbid compared to pasteurised juice. Its cloud was almost identical to that of fresh juice, with an absorbance of 0.539. The turbidity remained constant until the end of the storage period for both pasteurised and CO2 treated juices. The cloud stability seems attributable to the inactivation of the pectin-methyl-esterase by the treatments.[Citation11,Citation20,Citation25,Citation27]
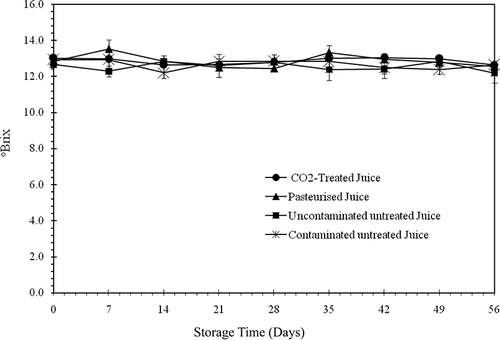
The untreated juices (contaminated and uncontaminated) were much less turbid during their first week of storage as shown in . The cloud decreased over time due to the action of the intact active enzyme. The absorbance of untreated juices (uncontaminated and contaminated) were reduced to 0.264 and 0.285, respectively, after 7 days of storage at 4°C. It is feasible to consider that the decrease in turbidity was rapid in the first hours of storage when the temperature still allowed the enzyme to function. The cloud was then stabilised under the inhibitory effect of the low temperature on the enzyme until the seventh day. The cloud then increased over time to absorbances of 0.602 and 0.719 at the end of the storage period for the untreated juices (uncontaminated and contaminated), respectively. The increase in turbidity seems to be caused by the high biomass of the juice. shows the evolution of °Brix values for the different juices throughout the storage period. Analysis of the variance on °Brix did not reveal any significant differences between juices (P > 0.01). This indicates that heat pasteurisation and pressurised CO2 treatments did not influence the solubility of solid particles, such as sugars. It is somewhat surprising that the solubility of solid particles was not affected by microbial growth in the untreated juices. However, it is conceivable that aging juice during storage became unfavorable to the development of microorganisms because of their nutrient depletion. In this case, the microorganisms had to use part of the solutes and their own metabolites thereby contributing to an increase in °Brix value. The results clearly show that the pressurised CO2 treatment used in this study preserves the original cloud of orange juice, a quality desired by consumers, and does not affect the solubility of solid particles.
Evaluation of Sensory Attributes of Orange Juice
Orange juice is the most consumed fruit juice, not only because of its organoleptic attributes (taste, odour, and aroma), but also because it constitutes an important source of vitamin C.[Citation28] Heat pasteurisation, the primary method of preservation of fruit juices, causes a deterioration of organoleptic and nutritional properties. In this context, it is necessary to develop an alternative preservation protocol that would ideally preserve the quality of the juices while reducing or eliminating the endogenous and exogenous microflora and undesired enzymes. In this part of the study, orange juice treated with pressurised CO2 was compared to pasteurised juice and freshly extracted juice in terms of vitamin C content and, using a taste test, sensory attributes (taste, odour, and visual appearance).
Evaluation of Sensory Attributes by Test Taste
Analysis of the variance of results from the evaluation of odour, taste, and visual appearance revealed no significant differences between the tasters in the study. This indicates that their evaluation of sensory attributes concurred and that the interaction taster-treatment was not significant. There were also no significant differences between repetitions of taste tests over the 8-week testing period indicating that the duration and temperature of storage (4°C) had no effect on juice quality. However, the analysis of the variance of odour, taste, and visual appearance revealed significant differences between treatments (P < 0.01**). Tukey's test, which allows a comparison of treatments for each other, did not reveal significant differences between the CO2 treated juice and freshly extracted juice for all sensory attributes tested. Pasteurised juice was, however, found to be significantly different from freshly extracted and CO2 treated juices for all evaluated sensory attributes (P < 0.05*). shows the results of sensory attribute evaluations. According to these results, it can be concluded that to preserve organoleptic properties of orange juice during preservation treatment, pressurised CO2 at moderate temperatures could be an effective alternative to heat pasteurisation.
Analysis of Vitamin C Content
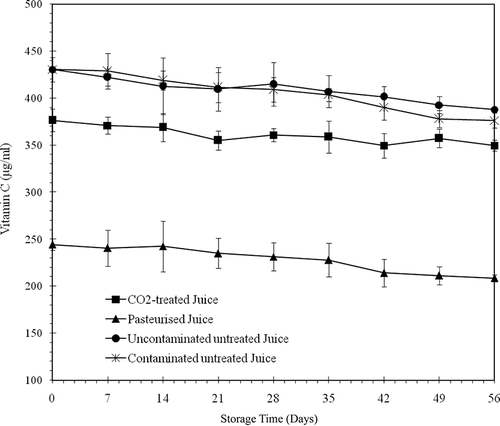
presents the effects of the various treatments on vitamin C content of orange juice before storage at 4°C. The average concentration of vitamin C in freshly extracted juice was 430.64 μg/ml. The vitamin C content remained unchanged in the untreated juices (contaminated and uncontaminated). The vitamin C content was reduced by 12% after the SC-CO2 treatment and by 43% after heat pasteurisation. This large decrease in vitamin C after pasteurisation seems to be attributed to the temperature of pasteurisation.[Citation20]
Table 2 Concentrations of vitamin C in orange juice before and after treatment, prior to storage
High vitamin C content is essential to the quality of orange juice. Furfural, a product of vitamin C degradation, polymerises to produce a browning pigment. This could be one of the causes of the loss in colour and taste of fruit juices.[Citation22,Citation29,Citation30] This non-enzymatic browning reaction is promoted by elevated temperatures. Therefore, this explains the 43% reduction in vitamin C content that occurred after pasteurisation at 90°C. The presence of furfural also explains the intensification of colour in pasteurised juice (C* value of 105, ). The reduction of vitamin C content in pasteurised juice could also be attributed to the presence of residual oxygen.[Citation31] It is known that orange juice is very sensitive to oxygen since oxydases (specifically ascorbic acid oxydase) induce browning and oxydative degradation in acidic environments and at high temperatures. In SC-CO2 treated juice, the replacement of oxygen by the CO2 may explain the preservation of vitamin C. Also, since the vitamin C is not soluble in SC-CO2,[Citation32] it could not be extracted or destroyed. The temperature of CO2 (40°C) could, however, be the cause of the 12% reduction in vitamin C content. There was a reduction in vitamin C content throughout the storage period. In the untreated juices (contaminated and uncontaminated), there were respective losses of 5.88 and 5.05%. In the pasteurised and SC-CO2 treated juices there were supplemental reductions of approximately 6.5 and 4%, respectively, at the end of the storage period. shows the changes in vitamin C content in the stored juices over time. The reduction of vitamin C content during the storage period is probably due to the presence of dissolved oxygen in the juice. A loss of vitamin C in orange juice was also observed by Lee and Chen.[Citation19] These authors report a 2.7% loss of vitamin C in orange juice at 4°C. Goyle and Ojha[Citation26] have reported that orange juice can lose 5 to 8% of its vitamin C during storage. The results clearly show that the treatment of orange juice with 25 MPa of CO2 at 40°C produces a juice with better sensory and nutritional attributes compared to heat pasteurisation traditionally used to conserve fruit juices.
CONCLUSION
Heat pasteurisation is the conventional and most popular method used to prolong the shelf life of fruit juices. While it ensures microbiological stability, the results show that pasteurisation induces a loss of organoleptic qualities (taste, odour, and aroma). The SC-CO2 treatment (25 MPa, 40°C) completely purifies the juice. There was no microbial growth for 56 days after treatment. The taste testers did not find any significant differences in sensory attributes between SC-CO2 treated juice and freshly extracted juice. After CO2 treatment the colour of the juice and 88% of its vitamin C was preserved and its cloud was not affected. The pH initially decreased but eventually returned to its initial value and remained stable. These results show that the use of pressurised CO2 could be an alternative to heat pasteurisation to preserve food products with better sensory and nutritional qualities.
REFERENCES
- Parish , M.E. and Higgins , D. 1988 . Isolation and identification of lactic acid bacteria from samples of citrus molasses and unpasteurized orange juice . Journal of Food Science , 53 : 645 – 650 .
- Parish , M.E. and Higgins , D. 1989 . Yeasts and molds isolated from spoiling citrus products and by-products . Journal of Food Protection , 52 : 261 – 265 .
- Linton , M. , McClements , J.M.J. and Patterson , M.F. 1999 . Survival of Escherichia coli O157:H7 during storage in pressure-treated orange juice . Journal of Food Protection , 62 ( 9 ) : 1038 – 1040 .
- Berry , J.M. , Witter , L.D. and Folinazzo , J.E.F. 1956 . Growth characteristics of spoilage organisms in orange juice and concentrate . Food Technology , 48 ( 11 ) : 553 – 558 .
- Termote , F. , Rombouts , F.M. and Pilnik , W. 1977 . Stabilization of cloud in pectinesterase active orange juice by pectic acid hydrolisates . Journal of Food Biochemistry , 1 : 15 – 21 .
- Pedro , E.M. , Robert , C.S.F. and Olga , M.B. 2006 . Comparative study on shelf life of orange juice processed by high intensity pulsed electric fields or heat treatment . European Food Research and Technology , 222 : 321 – 329 .
- Bae , Y.Y. , Lee , H.J. , Kim , S.A. and Rhee , M.S. 2009 . Inactivation of Alicyclobacillus acidoterrestris spores in apple juice by supercritical carbon dioxide . International Journal of Food Microbiology , 136 : 95 – 100 .
- Jarupan , K. and Mohammad , N.E. 2008 . Application of non-thermal processing for preservation of orange juice . KMITL Science and Technology Journal , 8 ( 2 ) : 64 – 74 .
- Shomer , R. , Cogan , U. and Mannheim , C.H. 1994 . Thermal death parameters of orange juice and effect of minimal heat treatment and carbon dioxide on shelf-life . Journal of Food Processing and Preservation , 18 : 305 – 315 .
- Wei , C.I. , Balaban , M.O. , Fernando , S.Y. and Peplow , A.J. 1991 . Bacterial effect of high pressure CO2 treatment on foods spiked with Listeria or Salmonella . Journal of Food Protection , 54 ( 3 ) : 189 – 193 .
- Balaban , M.O. , Pekyardimci , S. , Chen , J.S. , Arreola , A. , Zemel , G. and Marshall , M.R. 1993 . “ Enzyme inactivation by pressurized carbon dioxide ” . In Science for the Food Industry of the 21st Century, Biotechnology, Supercritical Fluids, Membranes and Other Advanced Technologies for Low Calorie, Healthy Food Alternatives; Yalpani, M , 235 – 252 . ATL Press, Mount Prospect, IL, USA .
- Castaldo , D. , Lovoi , A. , Quagliuolo , L. , Servillo , L. , Balestrieri , C. and Giovane , A. 1991 . Orange juices and concentrates stabilization by a proteic inhibitor of pectin methylesterase . Journal of Food Science , 56 ( 6 ) : 1632 – 1634 .
- Versteeg , G. , Rombouts , F.M. , Spaansen , C.H. and Pilnik , W. 1980 . Thermostability and orange juice cloud destabilizing properties of multiple pectinesterases from orange . Journal of Food Science , 45 ( 4 ) : 969 – 971 . 998
- Poste , L. , Mackie , D. , Butler , G. and Larmond , E. 1991 . Laboratory Methods for Sensory Analysis of Food , 36 – 41 . Canada , , Ottawa : Agriculture Canada Research Branch .
- Haas , G.J. , Prescott , H.E. , Dudley , E. , Dik , R. , Hintlian , C. and Keane , L. 1989 . Inactivation of microorganisms by CO2 under pressure . Journal of Food Safety , 9 : 253 – 265 .
- Sorensen , L.B. , Moller , P. , Flint , A. , Martens , M. and Raben , A. 2003 . Effect of sensory perception of foods on appetite and food intake: a review of studies on humans . International Journal of Obesity and Related Metabolic Disorders , 27 : 1152 – 1166 .
- McGuire , R.G. 1992 . Reporting of objective color measurement . HortScience , 27 ( 12 ) : 1254 – 1254 .
- Lee , H.S. and Nagy , S. 1988 . Measurement of color changes due to browning in stored grapefruit juices . Proceedings of the Florida State Horticultural Society , 101 : 154 – 157 .
- Lee , H.S. and Chen , C.S. 1998 . Rates of vitamin C loss and discoloration in clear orange juice concentrate during storage at temperatures of 4–24°C . Journal of Agricultural and Food Chemistry , 46 : 4723 – 4727 .
- Arreola , A.G. , Balaban , M.O. , Wei , C.I. , Peplow , A. and Marshall , M. 1991 . Effect of supercritical carbon dioxide on some quality attributes of single strength orange juice . Journal of Food Quality , 14 : 275 – 284 .
- Radhika , S. , Devinder , K. , Oberoi , D.P.S. and Sogi , D.S. 2008 . Thermal degradation kinetics of pigments and visual color in watermelon juice . International Journal of Food Properties , 11 : 439 – 449 .
- Finholt , P. , Alsos , I. and Higuchi , T. 1965 . Rate studies on the anaerobic degradation of ascorbic acid . Journal of Pharmaceutical Science , 54 : 181 – 186 .
- Miché , J.C. 1984 . Conservation des Aliments , 186 – 189 . Presses Universitaires de France : Montpellier .
- Cheftel , J.C. 1995 . Hautes pressions, inactivation microbienne et conservation des aliments . Comptes Rendus de l'Académie d'Agriculture de France , 1 : 13 – 38 .
- Balaban , O.M. , Arreola , A.G. , Marshall , M. , Peplow , A. , Wei , C.I. and Cornell , J. 1991 . Inactivation of pectinesterase in orange juice by supercritical carbon dioxide . Journal of Food Science , 56 ( 3 ) : 743 – 746 . 750
- Goyle , A. and Ojha , P. 1998 . Effect of storage on vitamin C, microbial load and sensory attributes of orange juice . Journal of Food Science and Technology (Mysore), India , 35 ( 4 ) : 346 – 348 .
- Chen , J.S. , Balaban , M.O. , Wei , C. , Marshall , R.M. and Wei , Y.H. 1992 . Inactivation of polyphenol oxidase by high pressure carbon dioxide . Journal of Agricultural and Food Chemistry , 40 ( 12 ) : 2345 – 2349 .
- Moshonas , G.M. and Shaw , S.P. 1997 . Flavor and chemical composition of pasteurized and fresh valencia orange juices . Journal of Food Quality , 20 : 32 – 40 .
- Cleeg , K.M. 1966 . Citric acid and browning of solutions containing ascorbic acid . Journal of the Science of Food and Agriculture , 17 : 546 – 549 .
- Nagy , S. and Dinsmore , H.L. 1974 . Relationship of furfural to temperature abuse flavor change in commercially canned single-strength orange juice . Journal of Food Science , 39 : 1116 – 1121 .
- Trammell , D.J. , Dalsis , D.E. and Malone , C.T. 1986 . Effect of oxygen on taste, ascorbic acid loss and browing for HTST-pasteurized, single strentgh orange juice . Journal of Food Science , 51 : 1021 – 1025 .
- Balaban , M.O. and Arreola , A.G. 1991 . Supercritical carbon dioxide applied to citrus processing . Transactions of the Citrus Engineering Conference , 37 : 16 – 37 .