Abstract
In this study, the volatile compounds, lipid composition, thermal behavior, and structure of wheat germ oil were analyzed. Forty-four volatile compounds, mainly alcohols, esters, alkenes, and aldehydes, were isolated by headspace solid phase micro extraction. The major constituents were found to be hexanal (15.97%), 2-methyl-2-butene (10.43%), 2,4-heptadienal (8.53%), and limonene (6.83%). The oil was rich in unsaturated fatty acid (83.45%), especially in linoleic acid (64.82%), and there were 75.49% unsaturated fatty acid distributed in Sn-2 position. The main content of unsaponifiable matters was sterol, especially β-sitosterol (64.64%). The thermogravimetric analysis results showed one major regime of weight loss over a temperature range of 280–500°C and an exothermic behavior was observed by the differential scanning calorimetry data.
INTRODUCTION
Wheat germ, which is a main by-product of the wheat milling industry, is considered as a natural source of highly concentrated nutrients. There is a rich deposit of wheat germ in the world every year. However, the applications of wheat germ were limited over the past and most of them were generally used for animal feed.[Citation1] In recent years, with the development of food industry technology, wheat germ can be separated in a fairly pure form from the grain during the milling process and will be a good source to be utilized.
Wheat germ contains about 11% oil, 30% protein, 53% carbohydrate, 12% water, and 4% ash.[Citation2] The oil extracted from wheat germ is reported to have very high nutritional value. It has the highest tocopherol content of all vegetable oils, up to about 2500 mg/kg,[Citation3] in which α-tocopherol represents around 60%. In addition, it is rich in unsaturated fatty acids, mainly linoleic (18:2) and linolenic (18:3).[Citation4] Recently, wheat germ oil has been demonstrated to reduce plasma and liver cholesterol in animals and to delay aging.[Citation5]
With the improvement of people's living standards, the consumption of high quality food is being increased. The beneficial effects of vegetable oils in the human diet have been well known, basically due to their high content in unsaturated fatty acids and their high energy value.[Citation6] The oils, such as olive oil, rice bran oil, corn germ oil, and wheat germ oil, which are healthy, safe, and nutritive, are becoming more and more popular in the world. Therefore, making the best use of a wheat germ source and producing wheat germ oil with good quality are important in developing countries.
It is well known that the quality and stability of oils are the main factors that influence its acceptability and market value. Thus, maintenance of oil quality, acceptable to the consumer is an important function of quality control in the oils and fats industry.[Citation7] The value of the edible oils depends on physical–chemical properties, such as acid value, iodine value, peroxide value, thermal behavior, as well as on triglyceride (TG) composition, bioactivities, and so on. Until now, the extraction and properties of wheat germ oil have been reported in some studies,[Citation8] but a full characterization of wheat germ oil has not been reported. Nowadays, wheat germ oil is becoming more and more popular in developing countries, thus making it desirable to study the oil more comprehensively. This study was aimed at applying some advanced analysis technologies to give a comprehensive characterization of wheat germ oil, which will be a good supplement to the previous report.[Citation2]
MATERIALS AND METHODS
The raw wheat germ was donated by Zhanyuan Corp. (Hefei, China) and was carefully selected and sieved to remove contaminates. The enzymes in the raw wheat germ were inactivated by heating for 30 min at 105°C. Carbon dioxide (purity 99.9%) was purchased from Henglong Gas Corp. (Hefei, China).
Supercritical Carbon Dioxide Extraction
The extraction of wheat germ oil was performed on a HA121-50-01C device (Hua'an Supercritical Fluid Extraction Corp., Nantong, China), and described in detail in our study reported previously.[Citation2,Citation9] Wheat germ oil was obtained under the conditions of wheat germ particle size 60–80 mesh, water content 4.37%, pressure 30 MPa, temperature 40°C, and extraction time 1.7 h.
Analysis of Volatile Compounds
The volatile compounds in wheat germ oil were analyzed by headspace-solid phase microextraction (HS-SPME) according to the method described in detail by Bail et al.,[Citation10] with some modifications. An amount of 6.0 g of wheat germ oil loaded in a SPME compatible vial was extracted at room temperature (25°C) for 6 h. A pre-conditioned Supelco 57348 (Supelco Corporation, PA, USA; 2 cm, 50/30 μm DVB/Carboxen/PDMS Stable-Flex fiber) was used. Once the extraction was finished, the fiber was placed immediately into the gas chromatograph-mass spectrometer (GC-MS) instrument (GC-MS-QP2010, Shimadzu, Kyoto, Japan) with a capillary column (DB-5, 30 m × 0.25 mm, 0.25 μm film thickness, Agilent, CA, USA). The injector and detector temperatures were set at 250°C. The column temperature program was set as follows:[Citation10] the initial temperature of 38°C was held for 1 min and then increased at a rate of 2.5°C min−1 to 175°C. From this point, the temperature was increased at a rate of 50°C min−1 to 220°C, which was held for 2 min. The split ratio was set to 1.0. The transfer line temperature and ion source temperature were set at 250°C. An ionization voltage of 70 eV was acquired in the m/z range 30–450 amu. Compounds were identified by comparison of their retention indices and mass spectra with the mass spectra library.
Pancreatic Lipase-Catalyzed Sn-2 Position Fatty Acid Analysis
The Sn-2 position fatty acid distribution in wheat germ oil was analyzed according to the method of Kosugi et al.[Citation11] and Mansour and Sinclair[Citation12] with some modifications. First, 0.1 g of oil diluted in 2 mL of tris buffer (pH 8.0) was heated at 40°C for 5 min. Then, 0.2 mL of CaCl2 (220 mg/mL), 0.5 mL of sodium cholate (2 mg/mL), and 10 mg of pancreatic lipase were added. The mixture was incubated at 40°C for 5 min and then the reaction was stopped with 1 mL of 6M HCl. Next, 2 mL of diethyl ether was added and the mixture was centrifuged at 5000 rpm for 5 min. The upper layer of the diethyl ether fraction was collected. Then, 2 μL of the diethyl ether fraction was examined on thin layer chromatography (TLC) using a developing solvent system of hexane/diethyl ether/methanoic acid (v/v/v = 70/30/1). The Sn-2 monoacylglycerol (Sn-2-MAG) spot was scratched and extracted by hexane. The fatty acid composition of the Sn-2–MAG and TG[Citation13] in original oil were analyzed by GC-MS according to the method by Wei et al.[Citation9]
Analysis of Unsaponifiable Matter
An amount of 1.0 g of wheat germ oil was saponificated with 10 mL 0.5 mol/L KOH-C2H5OH solution and the unsaponifiable matter was recovered and then was diluted with 2 mL of hexane and injected into the GC-MS instrument (GC-MS-QP2010, Shimadzu) with a capillary column (HP-5, 30 m × 0.25 mm, 0.25 μm film thickness, Agilent). The injector and detector temperatures were set at 320 and 285°C, respectively. The column temperature program was set as follows: the initial temperature of 200°C and then increased at a rate of 30°C min−1 to 285°C and held for 25 min. The split ratio was set to 20.0. The transfer line temperature and ion source temperature were set at 250°C. An ionization voltage of 70 eV was acquired in the m/z range of 30–450 amu. Compounds were identified by comparison of their retention indices and mass spectra with the mass spectra library.
Fourier Transform Infrared Spectrum (FT-IR) Analysis
A Nicolet 6700 spectrometer (Nicolet Instrument Corp., Madison, USA) with a DTGS detector was used for FT-IR analysis. A film of the oil sample was deposited between two disks of KBr. A total of 32 scans were collected for each sample at a resolution of 4 cm−1 in the range between 400 and 4000 cm−1.
Thermal Analysis
Simultaneous differential scanning calorimetry-thermal gravimetric analysis (DSC-TGA) (SDT) Q600 (TA Instruments, New Castle, DE, USA) was used for DSC and TGA of oil. The sample of oil was hermetically sealed in an aluminum pan with an empty pan serving as a reference.[Citation14]
Oxidative Stability
The oxidative stability of wheat germ oil was measured by Rancimat 743 apparatus (Metrohm Corp., Herisau, Switzerland). A 5-mL aliquot of oil was warmed to 120°C at an air flow of 20 mL/min. The oxidative stability was expressed as the oxidation induction time (h).
RESULTS AND DISCUSSION
Volatile Compounds Analysis
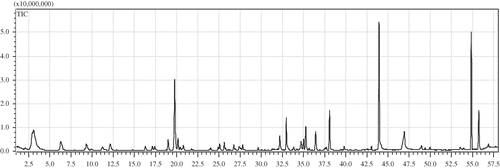
The volatile compounds, which are responsible for the flavor of oil, play an important role in consumer choice. showed the chromatographic localization of the volatile compounds presented in wheat germ oil. The information of the identified 44 volatile compounds was listed in . In wheat germ oil, the isolated and identified compounds were mainly alcohols, esters, alkenes, and aldehydes. The major constituents of the volatile compounds, such as hexanal (15.97%), 2-methyl-2-butene (10.43%), 2,4-heptadienal (8.53%), and limonene (6.83%), which were also identified in other oils, such as grape seed oil and olive oil,[Citation10,Citation15,Citation16] were responsible for fatty and sweaty impression for consumer acceptance.
Table 1 Volatile compounds extracted by HS-SPME in wheat germ oil
Analysis of Unsaponifiable Matter
Wheat germ oil, as a special product with very high nutritional value, has many bioactive compounds. It contained about 4.16% of the unsaponifiable matter[Citation2] and the tocopherol content reached 319 mg/100 g. The main content of unsaponifiable matter was sterol. Sterol, which is one of the important bioactivity components in oils, has a significant effect on lowering blood cholesterol and reducing the incidence of cardiovascular disease. According to the mass spectra analysis, five different unsaponifiable matters were observed and identified in : squalene, cholesterol, campesterol, β-sitosterol, and fucosterol. The main sterol was β-sitosterol (64.64%). A similar result was obtained by Eisenmenger and Dunford.[Citation19]
Table 2 Main unsaponifiable matter identified in wheat germ oil
Sn-2 Position Fatty Acid Composition
Recently, many reports showed that the distribution of fatty acid in TG, which was one of the important factors for nutrition, decided the absorption and metabolism of oils. The distribution of fatty acids in TG is not random, according to certain laws. However, many previous reports only focused on the fatty acid composition of wheat germ oil (WGO) and ignored the Sn-2 position fatty acid composition. As shown in , wheat germ oil was rich in unsaturated fatty acid (83.45%), especially in linoleic acid (64.82%) and oleic acid (13.19%). Pancreatic lipolysis was used to determine the Sn-2 position fatty acid. The compositions of the Sn-2-MAG were: linoleic acid 87.29%, oleic acid 12.11%, and palmitic acid 0.60%. That is to say, there was 75.49% unsaturated fatty acid of wheat germ oil distributed in Sn-2 position, which can be easily absorbed during digestion.
Table 3 Fatty acid composition (%) of TG and Sn-2-MAG in wheat germ oil
Evaluation of FT-IR Spectrum
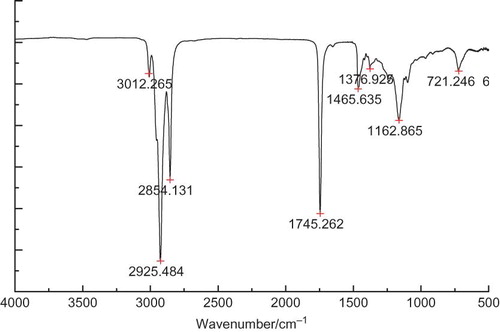
Recent developments in FT-IR technology have expanded its application in food research. It is well known that every oil/fat differs in composition, length, and unsaturated degree of the fatty acids as well as their positions in the chain.[Citation20] FT-IR technology has been an effective tool in the study of edible fats and oils.[Citation21] The FT-IR spectrum of wheat germ oil was presented in . Data showed that TG was the main component in wheat germ oil. The strong absorption band of ester carbonyl functional group of TG (–C=O) was around 1745 cm−1. The stretch vibration of –C–O eater groups appeared at 1162 cm−1.[Citation21] The stretching vibrations of –CH3 and –CH2 appeared at 2980–2950 and 2950–2850 cm−1, whereas the bending vibrations (–CH2) of these groups appeared at 1475–1350, 1350–1150, and 722 cm−1, respectively.[Citation22–24 Citation Citation24 Different composition of oils leads to different absorbance in the FT-IR spectrum. According to previous reports, olive oil, which contains higher content of oleic acid, has a strong absorbance at 3006 cm−1, while sunflower oil and soybean oil, which contain higher linoleic acid, show strong absorbance at 3009 cm−1.[Citation20] Wheat germ oil has higher content of linoleic acid than sunflower oil and soybean oil, so its strong absorbance appeared at 3012 cm−1. The FT-IR spectrum represented a fingerprint for wheat germ oil and can be an effective analytical tool to determine wheat germ oil adulteration with lower priced vegetable oils (such as sunflower oil and soybean oil).[Citation20]
Thermal Analysis
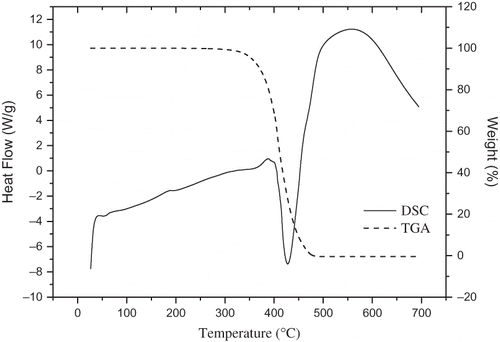
Vegetable oils are mostly used for cooking and frying of foods, so it is important to understand its thermo-physical properties during the cooking and frying processes. It is well known that DSC technology, which can show thermal behavior of oils for their identification, has been widely applied in oil and fat research for the characterization of oils from different vegetables sources.[Citation25] DSC techniques have the potential to be used as a nonchemical method to determine oil quality parameters.[Citation7] The DSC-TGA profiles of wheat germ oil were shown in . An exothermic behavior can be observed. The onset temperature of melting for wheat germ oil was −29.03°C, the peak temperature detected was −14.61°C, and the △H was 21.54 J/g. The essential weight loss occurred mainly in the 280–500°C range. At 516.02°C, the weight lost completely.
Oxidative Stability
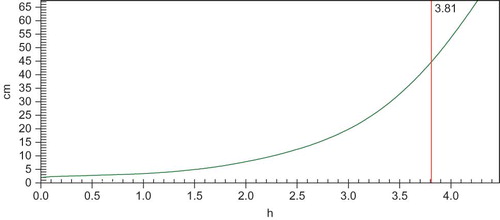
Oxidative stability is one of the most important factors for maintaining the quality of oils. The oil, which remains suitable to consumers for a longer time, is an important objective of quality control.[Citation26] The oxidative stability curve of wheat germ oil was shown in . The oxidative induction time was 3.81 h. As low melting oils contain more unsaturated fatty acid, they are very prone to oxidation and become rancid easily. Wheat germ oil was a low melting oil; the high amount of unsaturated fatty acid made it oxidative more easily. However, compared with other oil with high amount of unsaturated fatty acid, such as corn bran oil,[Citation26] a large amount of tocopherol in wheat germ oil makes it more stable. In order for consumption of wheat germ oil in a long shelf time, supplementation of the diet with wheat germ oil capsules seems to be the easiest way to elevate its intake.[Citation27]
CONCLUSION
Wheat germ could be successfully utilized as a source of edible oil with good quality for human consumption. According to our analysis of volatile compounds in wheat germ oil, 44 volatile compounds were isolated and the identified compounds were mainly alcohols, esters, alkenes, and aldehydes, which were responsible for fatty and sweaty impression. The oil contained high unsaturated fatty acid (83.45%), most of which distributed at Sn-2 position. The main content of unsaponifiable matter in it was sterol, especially β-sitosterol (64.64%). Sterol and tocopherol, which are bioactivity components, have been found in wheat germ oil, and have demonstrated to reduce plasma and liver cholesterol in animals and to delay aging. With the increased demand for natural nutritive edible oils, wheat germ oil with high amounts of high unsaturated fatty acid and bioactivity components should be greatly developed. However, wheat germ oil was a low melting oil and prone to oxidation. Therefore, how to keep its quality and acceptability is important. The results of the present work will be a good supplement to the properties of wheat germ oil reported in previous reports.
ACKNOWLEDGMENTS
This work was financially supported by the National Key Technology Research and Development Program in the 11th Five Year Plan of China (2010BAD01B07), and the High-Tech Research and Development Program of China (2010AA101503).
REFERENCES
- Ge , Y. , Sun , A. , Ni , Y. and Cai , T. 2001 . Study and development of a defatted wheat germ nutritive noodle . European Food Research and Technology , 212 ( 3 ) : 344 – 348 .
- Jiang , S.T. and Niu , L.Y. 2011 . Optimization and evaluation of wheat germ oil extracted by supercritical CO2 . Grasas Y Aceites , 62 ( 2 ) : 181 – 189 .
- Schuler , P. 1990 . “ Natural antioxidants exploited commercially ” . In Food Antioxidants; Hudson, B.J.F , 99 – 170 . New York : Elsevier Applied Science .
- Wang , T. and Johnson , L. 2001 . Refining high-free fatty acid wheat germ oil . Journal of the American Oil Chemists’ Society , 78 ( 1 ) : 71 – 76 .
- Leenhardt , F. , Fardet , A. , Lyan , B. , Gueux , E. , Rock , E. , Mazur , A. , Chanliaud , E. , Demigne , C. and Remesy , C. 2008 . Wheat germ supplementation of a low vitamin E diet in rats affords effective antioxidant protection in tissues . Journal of the American College of Nutrition , 27 ( 2 ) : 222
- Molero Gómez , A. and Martínez de la Ossa , E. 2000 . Quality of wheat germ oil extracted by liquid and supercritical carbon dioxide . Journal of the American Oil Chemists’ Society , 77 ( 9 ) : 969 – 974 .
- Tan , C. , Che Man , Y. , Selamat , J. and Yusoff , M. 2002 . Comparative studies of oxidative stability of edible oils by differential scanning calorimetry and oxidative stability index methods . Food Chemistry , 76 ( 3 ) : 385 – 389 .
- Radlove , S. 1945 . A note on the composition of wheat-germ oil . Journal of the American Oil Chemists’ Society , 22 ( 7 ) : 183 – 184 .
- Wei , Z. , Liao , A. , Zhang , H. , Liu , J. and Jiang , S. 2009 . Optimization of supercritical carbon dioxide extraction of silkworm pupal oil applying the response surface methodology . Bioresource Technology , 100 ( 18 ) : 4214 – 4219 .
- Bail , S. , Stuebiger , G. , Krist , S. , Unterweger , H. and Buchbauer , G. 2008 . Characterisation of various grape seed oils by volatile compounds, triacylglycerol composition, total phenols and antioxidant capacity . Food Chemistry , 108 ( 3 ) : 1122 – 1132 .
- Kosugi , Y. , Oshima , A. , Koike , S. , Fukatsu , M. , Minami , K. , Miyake , Y. and Masui , K. 2002 . Determination of fatty acid composition at sn-2 acyl position in triacylglycerol by capillary gas chromatography with lipase from rhizopus delemar . Journal of Oleo Science , 51 ( 9 ) : 599 – 605 .
- Mansour , M. and Sinclair , A. 1993 . The trans fatty acid and positional (sn-2) fatty acid composition of some Australian margarines, dairy blends and animal fats . Asia Pacific Journal of Clinical Nutrition , 2 : 155 – 163 .
- Obayashi , H. , Nakano , K. , Shigeta , H. , Yamaguchi , M. , Yoshimori , K. , Fukui , M. , Fujii , M. , Kitagawa , Y. , Nakamura , N. and Nakamura , K. 1996 . Formation of crossline as a fluorescent advanced glycation end product in vitro and in vivo . Biochemical and Biophysical Research Communications , 226 ( 1 ) : 37 – 41 .
- Fasina , O.O. and Colley , Z. 2008 . Viscosity and specific hat of vegetable oils as a function of temperature: 35°C to 180°C . International Journal of Food Properties , 11 ( 4 ) : 738 – 746 .
- Luna , G. , Morales , M.T. and Aparicio , R. 2006 . Characterisation of 39 varietal virgin olive oils by their volatile compositions . Food Chemistry , 98 ( 2 ) : 243 – 252 .
- Krist , S. , Stuebiger , G. , Unterweger , H. , Bandion , F. and Buchbauer , G. 2005 . Analysis of volatile compounds and triglycerides of seed oils extracted from different poppy varieties (Papaver somniferum L.) . Journal of Agriculture and Food Chemistry , 53 ( 21 ) : 8310 – 8316 .
- Cavalli , J.F. , Fernandez , X. , Lizzani-Cuvelier , L. and Loiseau , A.M. 2004 . Characterization of volatile compounds of French and Spanish virgin olive oils by HS-SPME: Identification of quality-freshness markers . Food Chemistry , 88 ( 1 ) : 151 – 157 .
- Baccouri , B. , Temime , S. , Campeol , E. , Cioni , P. , Daoud , D. and Zarrouk , M. 2007 . Application of solid-phase microextraction to the analysis of volatile compounds in virgin olive oils from five new cultivars . Food Chemistry , 102 ( 3 ) : 850 – 856 .
- Eisenmenger , M. and Dunford , N. 2008 . Bioactive components of commercial and supercritical carbon dioxide processed wheat germ oil . Journal of the American Oil Chemists’ Society , 85 ( 1 ) : 55 – 61 .
- Yang , H. , Irudayaraj , J. and Paradkar , M. 2005 . Discriminant analysis of edible oils and fats by FTIR, FT-NIR and FT-Raman spectroscopy . Food Chemistry , 93 ( 1 ) : 25 – 32 .
- Vlachos , N. , Skopelitis , Y. , Psaroudaki , M. , Konstantinidou , V. , Chatzilazarou , A. and Tegou , E. 2006 . Applications of Fourier transform-infrared spectroscopy to edible oils . Analytica Chimica Acta , 573 : 459 – 465 .
- Guillen , M.D. and Cabo , N. 1997 . Infrared spectroscopy in the study of edible oils and fats . Journal of the Science of Food and Agriculture , 75 ( 1 ) : 1 – 11 .
- Tariq , M. , Ali , S. , Ahmad , F. , Ahmad , M. , Zafar , M. , Khalid , N. and Khan , M.A. 2011, . Identification, FT-IR, NMR (1H and 13C) and GC/MS studies of fatty acid methyl esters in biodiesel from rocket seed oil. Fuel Processing Technology 92 (3), 336–341
- Argyropoulou , C. , Daferera , D. , Tarantilis , P. , Fasseas , C. and Polissiou , M. 2007 . Chemical composition of the essential oil from leaves of Lippia citriodora HBK (Verbenaceae) at two developmental stages . Biochemical Systematics and Ecology , 35 ( 12 ) : 831 – 837 .
- Chiavaro , E. , Rodriguez-Estrada , M.T. , Barnaba , C. , Vittadini , E. , Cerretani , L. and Bendini , A. 2008 . Differential scanning calorimetry: A potential tool for discrimination of olive oil commercial categories . Analytica Chimica Acta , 625 ( 2 ) : 215 – 226 .
- Arain , S. , Sherazi , S.T.H. , Bhanger , M.I. , Talpur , F.N. and Mahesar , S.A. 2009 . Oxidative stability assessment of Bauhinia purpurea seed oil in comparison to two conventional vegetable oils by differential scanning calorimetry and Rancimat methods . Thermochimica Acta , 484 ( 1–2 ) : 1 – 3 .
- Kolanowski , W. 2010 . omega-3 lc pufa contents and oxidative stability of encapsulated fish oil dietary supplements . International Journal of Food Properties , 13 ( 3 ) : 498 – 511 .