Abstract
A high performance liquid chromatography procedure for the quantitative determination of three marker benzene derivatives, 2,4,6-trihydroxy-3-prenyl acetophenone (tHPA) (1), 2,4,6-trihydroxy-3-geranyl acetophenone (tHGA) (2), and p-O-geranyl coumaric acid (GCA) (3), in the Melicope ptelefolia ethanolic leaf extracts, a medicinal herb obtained from a few locations of the Peninsula Malaysia, was described. The quantitative analysis was performed using high performance liquid chromatography-photodiode array detection on Xterra octadecylsiyl silica (ODS; 3.0 × 150 mm, 3.5 μm) column kept at 32°C, using gradient elution with acetonitrile and water containing 0.1% formic acid at a flow-rate of 1 ml/min with UV detection wavelength at 280 nm. All calibration curves showed good linearity (R2 of 0.999 to 1.0000) within the concentrations range of 2.5 × 10−3 to 0.1 mg/mL. The method was shown to be simple, sensitive, and reliable for qualitative and quantitative analysis of the marker compounds in M. ptelefolia leaf preparations.
INTRODUCTION
Quality control and stability evaluation of medicinal plants in preparation for therapeutic purposes is important for safe utilization. The United States Food and Drug Administration (USFDA), European Medicines Agency (EMEA), State Food and Drug Administration (SFDA) of China, and the World Health Organization (WHO) agreed on the basis that appropriate fingerprint chromatograms should be used as a strategy for identification and quality evaluation of traditional medicine preparation.[Citation1]
M. ptelefolia (Champ. Ex Benth.) Hartley belongs to the Rutaceae family. It is a common plant in Malaysia, vernacularly known as “tenggek burung.”[Citation2,Citation3] The young leaves are eaten raw as a traditional salad or “ulam,” a popular appetizer among the Malay ethnic group.[Citation4] The whole plant is used in traditional medicine for treating itchiness and wounds, to improve the digestive system, and as an emmenagogue.[Citation5,Citation6] The species have been reported to exhibit antibacterial,[Citation7,Citation8] fungicidal,[Citation9] antioxidant, and nitric oxide inhibitory activities.[Citation10,Citation11]
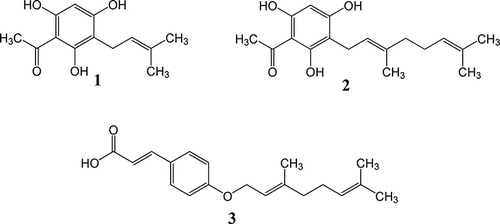
Previously, we had isolated three benzene derivatives, identified as 2,4,6-trihydroxy-3-prenyl acetophenone (tHPA) (1), 2,4,6-trihydroxy-3-geranyl acetophenone (tHGA) (2), and p-O-geranyl coumaric acid (GCA) (3) (), from the ethanolic extract of M. ptelefolia leaves.[Citation12] In another study, the results of which are being reported elsewhere, tHGA has also been found to exhibit anti-asthmatic activity. The aim of the study is to develop a high-performance liquid chromatography-photodiode array detection (HPLC-DAD) method that could simultaneously quantify the three marker compounds (1–3) in extract preparations of the plant. HPLC was chosen for the quantitative analysis as it has been shown to be a reliable instrumental method.[Citation13,Citation14] The current study arose from the need to quantify the content of this bioactive compound in standardized extracts, in preparation for further pharmacological and preclinical studies on the medicinal plant. Development of a validated method of quantitation will also be valuable in the quality control of herbal, phytomedicinal, or nutraceutical products derived from this medicinal plant.
EXPERIMENTAL
Plant Material
Plant materials were collected from Pahang (Sample no. 1), Terengganu (Sample no. 2), and Selangor (Samples no. 3 and 4). Samples 1 and 2 were obtained from a natural forest while samples 3 and 4 were from trees growing in a rubber estate. The plant material was identified and authenticated by a resident botanist through comparison with herbarium specimens of M. ptelefolia (SK153/02) kept at the Mini Herbarium, Institute of Bioscience, Universiti Putra Malaysia (UPM).
Reagents
The solvents used were of HPLC grade (Merck, Darmstadt, Germany). Deionized water was prepared using a Millipore water purification system (Billerica, Boston, MA, USA). Acetonitrile was purchased from (Merck) and degassed in an ultrasonic bath for 30 min before use.
High-Performance Liquid Chromatography System
The experimental equipment consisted of a JASCO Series 4 Liquid Chromatograph (LC) (Benelux, The Netherlands) system with the built-in modules of gradient pump (JASCO PU 2086), vacuum degasser, and autosampler (AS-2055) equipped with a 100 μL sample-loop, column oven and variable wavelength photodiode array detector (JASCO MD-2010). Chromatographic conditions were as follows: the column used was an Xterra® RP-18 (3.5 μm particle size, 3.0 × 150 mm i.d.; Waters, Dublin, Ireland) kept at column oven temperature of 32°C, injection volume of 20 μL, and detector wavelength at 280 nm. Solvents of acetonitrile (A) and water (B) containing 0.1% formic acid for the mobile phase were filtered through 0.45 μm membrane filter (HA-filter; Millipore, Bedford, USA).
Preparation of Sample Solution
The leaf samples of M. ptelefolia were air-dried under shade for 48 h. The dried leaves were then ground to a fine mesh and extracted via sonication (30-min session repeated five times) with 95% ethanol as a solvent to sample ratio of 5:1 (v/v). The extracts obtained after each 30-min session were pooled, filtered through Whatman filter paper (110 mm diameter; Whatman International Ltd., Maidstone, England), and processed to complete dryness via rotary evaporation and lyophilization to yield a greenish black colored M. ptelefolia ethanolic extract. A 1 mg/mL sample solution was prepared in HPLC grade methanol and filtered through a 0.22 μm filter membrane for HPLC analysis.
Preparation of Calibration Curves
Standard stock solutions of tHPA, tHGA, and GCA were prepared by dissolving 10 mg of each in methanol (10 mL) to give solutions of 1 mg/mL (1000 ppm). A series of seven dilutions were made by transferring volumes of 0.25, 0.5, 1.0, 2.0, 4.0, 8.0, and 10 mL of the standard stock solutions into 100 mL volumetric flasks, after which methanol was added to each to make up to the mark. The prepared solutions were filtered through a 0.22 μm filter membrane and the resultant filtrates were used for HPLC analysis. For each concentration, 20 μL of the solution was injected in six replicates. Peak area ratios corresponding to the dilution concentrations of each marker compound were extrapolated to give the calibration curve, whereby linear regression analysis was performed for each marker compound.
Recovery of tHPA, tHGA, and GCA
The recoveries of the three marker compounds were determined by the following equation: Recovery (%) = (A – B)/C × 100%, wherein A is the amount of sample solution with spiked standards, B is the amount of sample solution; and C is the added amount of the standards. To the prepared sample solution of 1 mg/mL, different amounts of the standard marker compounds (0.5, 1.0, 2.0 mg) were added. After filtering through a 0.22 μm Millipore filter membrane, the mixture was subjected to HPLC analysis to calculate the recovery. The recovery results of the analytes imply accuracy of instrumental data assessment, which is affected by the effectiveness of the sample preparation.
RESULTS AND DISCUSSION
Optimization of HPLC Conditions
The HPLC conditions for the ethanolic M. ptelefolia leaf extract were optimized to give the best resolution of the three marker components: tHPA, tHGA, and GCA (). Under the optimized chromatographic conditions, sufficient separation of the marker components was achieved, with the retention times of tHPA, tHGA, and GCA determined as RT: 6.25, 17.35, and 20.84 min. Separation was achieved using a gradient system consisting of acetonitrile, water, and 0.1% formic acid (F.A.) eluted at 1 mL/min on an Xterra® octadecylsiyl silica column (ODS; 3.0 × 150 mm, 3.5 μm) maintained at 32°C. Good resolution was obtained between tHPA and tHGA, and tHGA and GCA with values of 27 and 7, respectively. System suitability of the HPLC method was achieved as the calculated capacity factors values of the tHPA, tHGA, and GCA standards were 10, 3, and 12 and the theoretical plates were found to be 1841, 35038, and 26816, respectively. The efficiency of the method is depicted in in which tHPA, tHGA, and GCA from 20 μL injection of the 1 mg/mL extract, were eluted in ca. 25 min at detection wavelength of 280 nm. Compared to other wavelengths, tHPA, tHGA, and GCA showed better absorptivity and sensitivity at 280 nm. The chromatographic conditions have been optimized for best selectivity.
Figure 2 HPLC chromatogram of marker compounds in 1 mg/mL ethanolic leaf extract of M. ptelefolia (tHPA [RT: 6.25 min], tHGA [RT: 17.35 min], and GCA [RT: 20.84 min]). RT = retention time. (Description: Under the optimized HPLC conditions for the ethanolic M. ptelefolia leaf extract, the best resolution of the three marker components: tHPA, tHGA, and GCA in 1 mg/mL of crude extract was obtained.)
![Figure 2 HPLC chromatogram of marker compounds in 1 mg/mL ethanolic leaf extract of M. ptelefolia (tHPA [RT: 6.25 min], tHGA [RT: 17.35 min], and GCA [RT: 20.84 min]). RT = retention time. (Description: Under the optimized HPLC conditions for the ethanolic M. ptelefolia leaf extract, the best resolution of the three marker components: tHPA, tHGA, and GCA in 1 mg/mL of crude extract was obtained.)](/cms/asset/0f60c96f-86ba-492a-847f-80b844e5f1fa/ljfp_a_609952_o_f0002g.gif)
Calibration and Method Validation
Validation of a developed method is an integral part of any good analytical practice, which can be observed in the quality, reliability, and consistency of the analytical results. The developed HPLC method was thus validated for its linearity, specificity, accuracy and precision, limits of detection (LOD) and quantification (LOQ), and stability according to the ICH guidelines.[Citation15]
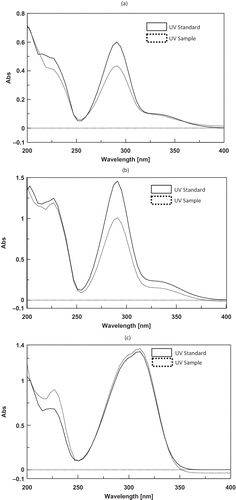
Specificity of the method was evaluated by comparing the retention times and ultraviolet (UV) absorption spectra of the eluted peaks with those of the pure standards (). The retention times and UV spectra showed very good agreement for every HPLC run. Linearity was determined by a series of six injections of the standard solutions of the three benzene derivatives. The elicited response areas were directly proportional to the concentration of the analytes between 2.5 × 10−3 to 0.1 mg/mL, as analyzed by the optimized HPLC method. The evaluation is made by visually inspecting a plot of peak area as a function of analyte concentration in which regression equations and coefficients (R 2) corresponding to the marker compounds were determined as follows:
Figure 3 Overlay of UV spectra of the maker compounds in the sample and standards at the same retention time: (a) tHPA, (b) tHGA, and (c) GCA. (Description: Specificity of the method was evaluated by comparing ultraviolet (UV) absorption spectra at the same retention times of the eluted sample peaks with those of the pure standards.)
These results showed that the area responses obeyed the equation y = a + mx, where the intercept a was zero within 5% confidence limits and the squared correlation coefficient (R 2) was from 0.9999 to 1.0000.
The crude sample and standard solutions of the three marker compounds of M. ptelefolia were analyzed in six replications by the described HPLC method. The retention times of tHPA, tHGA, and GCA in the test samples corresponded well to those of the standards with precision values of 2.86, 1.71, and 1.0 relative standard deviation (RSD), respectively. The areas, which correspond to the concentrations of the marker compounds in the sample preparations, could be directly calculated from extrapolation of the calibration curve. The intra- and interday precisions expressed as RSD, and accuracy expressed as recovery for the three analytes were determined from samples that were spiked with the standard solutions of the three marker compounds. Five determinations of two different concentrations were performed and precision of areas in intra- and interday studies were both <2% RSD (). The recovery results for six replicates of tHPA, tHGA, and GCA were excellent, which ranged from 101 to 96% with ≤1% RSD. For all three marker compounds, the criteria for accuracy, precision of retention times, and areas were fulfilled.
Table 1 Intra- and inter-day precision of HPLC analysis of tHPA, tHGA, and GCA
The LOD and the LOQ were calculated based on the standard deviation of the response and the slope obtained from the linearity plot of each marker compound. The limit of detection was established as a signal six times higher than noise. The LOD of tHPA, tHGA, and GCA were determined to be 1, 0.1, and 0.1 ng/mL, respectively. Meanwhile, the LOQ of tHPA, tHGA, and GCA were determined to be 10, 1, and 1 ng/mL, respectively. Based on the LOQ values, the method is shown to be adequately sensitive in detecting the three benzene derivatives, thus providing a reliable method of quantifying their concentrations in the ethanolic leaf extract.
Quantitative Analysis of the Marker Compounds in Leaf Extracts of M. ptelefolia
The method was applied in the quantitative analysis of the three marker components in M. ptelefolia leaf extract samples obtained from three different locations of the Peninsular Malaysia. The presence of the three marker compounds in the samples were verified by comparing the retention times and UV spectra to those of authentic standards. From the concentration values obtained for each marker constituent from a 1 mg/mL solution of the crude extract (), the amounts of the marker constituents in the crude extract was calculated. The percentage contents of the three marker compounds (w/w) in the crude leaf extracts varied between 2–3% for tHPA, 5–10% for tHGA, and 14–21% for GCA (). There appears to be less variation in the percentage content of tHPA between the four samples compared to those of tHGA and GCA. Furthermore, the percentage contents of the three compounds in samples 3 and 4, which came from the same location, were quite consistent. As expected, the ecological factors of the growth site greatly influence the production of these metabolites.
Table 2 Concentrations of tHPA, tHGA, and GCA in 1 mg/mL of ethanolic crude M. ptelefolia sample solution (mean ± SD; n = 3)
Table 3 Content of the three marker compounds (w/w) in the leaf crude extract
CONCLUSION
An HPLC method has been developed for the quantitative and qualitative measurements of the three marker compounds in the ethanolic leaf extract of M. ptelefolia, vis a vis 2,4,6-trihydroxy-3-prenyl acetophenone (tHPA), 2,4,6-trihydroxy-3-geranyl acetophenone (tHGA), and p-O-geranyl coumaric acid (GCA). A validation test performed in this study indicated that the developed method was simple, precise, accurate, and specific. The method can be used, with reasonable confidence, for the identification and quantification of the three marker compounds as demonstrated by the successful application on the leaf extracts prepared at different times between the months of May to June 2010. Development of the validated HPLC-DAD method will complement standardization, stability assessments, and quality control of medicinal preparations containing M. ptelefolia leaf extract.
ACKNOWLEDGMENTS
The research work is a part of a project under the R&D Initiatives, supported by the Institute of Pharmaceutical and Nutraceutical Malaysia-Ministry of Science, Technology and Innovation (IPHARM-MOSTI).
REFERENCES
- Ying , X. , Zhi-Hong , J. , Hua , Z. , Xiong , C. , Yuen-Fan , W. , Zhong-Qiu , L. , Zhao-Xiang , B. , Hong-Xi , X. and Liang , L. 2007 . Combinative method using HPLC quantitative and qualitative analyses for quality consistency assessment of an herbal medicinal preparation . Journal of Pharmaceutical and Biomedical Analysis , 43 : 204 – 212 .
- Hartley , T.G. 2001 . On the taxonomy and biogeography of Euodia and Melicope (Rutaceae) . Allertonia , 8 : 1 – 319 .
- Burkill , I.H. 1993 . A Dictionary of the Economic Products of the Malay Peninsula , 3rd , Kuala Lumpur : Ministry of Agriculture, Malaysia .
- Shuib , N.H. , Shaari , K. , Khatib , A. , Maulidiani , Kneer , R. , Zareen , S. , Salahudin , M.R. , Lajis , N.H. and Neto , V. 2010 . Discrimination of young and mature leaves of Melicope ptelefolia using 1H NMR and multivariate analysis . Food Chemistry , 126 : 640 – 645 .
- Loi , D.T. 1977 . Nhung Chay Thoue va vi Thoue Vietnam (Glossary and Vietnamese Medicinal Plants) , 145 Hanoi : Science and Technics Publication .
- Pham-Hoang , H. 1993 . An Illustrated Flora of Viet Nam Vol. II , 515 Mekong Printing: Montreal
- Manandhar , M.D. , Hussaini , F.A. , Kapil , R.S. and Shoeb , A. 1985 . Bacteriostatic heterocycles from Euodia lunu-ankenda . Phytochemistry , 24 : 199 – 200 .
- Rasadah , M.A. and Zakaria , M. 1988 . “ Antibacterial activity of the extracts from Melicope and Eodia species ” . In Proceedings of the Seminar on Malaysian Traditional Medicine 173 – 178 . Institute of Advanced Studies, University of Malaya, Malaysian Institute of Chemistry: Kuala Lumpur , , Malaysia
- Kumar , V. , Karunaratne , V. , Sanath , M.R. , Meegalle , K. and Macleod , J.K. 1990 . Two fungicidal phenylethanones from Euodia lunu-ankenda root bark . Phytochemistry , 29 : 243 – 245 .
- Abas , F. , Lajis , N.H. , Israf , D.A. , Shaari , K. and Umi Kalsom , Y. 2006 . Antioxidant and nitric oxide inhibition activities of selected Malay traditional vegetables . Food Chemistry , 95 : 566 – 573 .
- Abas , F. , Shaari , K. , Israf , D.A. , Syafri , S. , Zainal , Z. and Lajis , N.H. 2010 . LC-DAD-ESIMS, analysis of nitric oxide inhibitory fractions of tenggek burung (Melicope ptelefolia Champ Ex Benth.) . Journal of Food Composition and Analysis , 23 : 107 – 112 .
- Shaari , K. , Safri , S. , Abas , F. , Lajis , N.H. and Israf , D.A. 2006 . A geranylacetophenone from the leaves of Melicope ptelefolia . Natural Product Research , 20 : 415 – 419 .
- Giuseppe , S. , Alfonso , D.-G. , Giuseppina , T. , Caterina , L.-P. , Annarita , P. , Barbara , N. , Rocco , D.-P. and Carmela , S. 2007 . Antioxidative activity and lycopene and β-carotene contents in different cultivars of tomato (Lycopersicon Esculentum) . International Journal of Food Properties , 10 : 321 – 329 .
- Sibel , K. , Fahrettin , G. and Sami , E. 2005 . Hydroxymethyl furfural content of concentrated food products . International Journal of Food Properties , 8 : 367 – 375 .
- Dann , M.M. 1996 . ICH Validation of Analytical Procedures: Methodology , Canada : ICH Topic Q2 B; Ministry of Health .