Abstract
The aim of the current study was to investigate the properties of Chinese honeys. A total of 33 honeys were collected from various botanical and geographical sources in China. Nine unifloral honeys were identified as Acacia, Vetch, Linden, Coptidis, Red date, Astragalus, Codonopsis, Eriobotrya, and Eucalyptus according to their pollen granule characteristics. The chemical properties of free amino acids were further assessed; the total amino acid contents ranged from 240.7 mg/kg in light honey to 812.6 mg/kg in dark honey. Remarkable variations in proline and total amino acid contents were demonstrated in honeys from different botanical or geographical sources.
INTRODUCTION
Honey is the natural sweet product produced by honey bees (Apis mellifera) from plant nectars, secretions (honeydew) of living parts of plants, or excretions of plant-sucking insects.[Citation1] As a natural resource of sugars, honey has been used for infants, seniors, and invalids for a long time; it is a more easily digestible and palatable food carbohydrate than sucrose. Honey is widely appreciated as a food preservative[Citation2,Citation3] in the concentrated form of sugar.[Citation4] Several researches have supported antimicrobial activity of honeys.[Citation5–7 Citation Citation7
Honey is also considered to be one of the important traditional medicines since ancient times.[Citation8] Its roles have been reported in the treatment of burns, gastrointestinal disorders, asthma, infections and skin ulcers,[Citation9,Citation10] cataracts, and other eye ailments.[Citation6,Citation9,Citation11,Citation12] Antioxidants in honey have been applied to treat different diseases caused by oxidative stress.[Citation13,Citation14] Honey has been used for effectively reducing the risk of heart diseases, cancers, immune system declination, cataracts, and different inflammatory processes.[Citation15]
Amino acids account for around 1% (w/w) in honey. Proline, an indicator of honey quality,[Citation16,Citation17] is one of the predominant amino acid compositions that account for total amino acid contents. Proline content varies considerably in different honeys. Besides proline, there are 26 amino acids in honey; their relative proportions rely on the origin of honey. Proline and other amino acids in honey come from pollens, nectar, or are produced by the bees themselves.[Citation18,Citation19] Pollen from nectariferous plants is the major amino acid source of honey; the amino acid profile of a honey could reflect the characteristics of their botanical or geographical origin.[Citation20]
Several honey samples from Burkina Faso were analyzed to determine their proline contents as well as their radical scavenging activities.[Citation21] The correlation between radical scavenging activity and proline content was higher than that for total phenolic compounds in honeys. This suggested that the amino acid content of honey should be considered more frequently when determining its antioxidant activity. There is a long history of apiculture in China where it is rich in spices and has a wide distribution of honeybees. Some investigations have been done in Chinese honeybees and honey products, while profound studies on their chemical and biological properties are still limited. It is expected that the properties of various honeys are different because of different botanical and geographical sources. The major purpose of the current study was to evaluate nutritional and medicinal potentials of Chinese unifloral honeys, and to establish parameters valuable for further research. Therefore, the present work was to investigate the floral origins, and the food quality aspects in terms of physical properties (color, consistency, aroma, and taste) and chemical properties (amino acids).
MATERIALS AND METHODS
Honey Samples
A total of 33 honey samples were collected from various botanical and geographical sources in China (the original production places are listed in ). All of the samples were harvested from 2009 to 2010 directly from beekeepers (without further processing) and then stored in dark conditions at a temperature between 0 and 4°C. Part of the unprocessed unifloral honeys were locally obtained from Wang's Bee Products (Changchun, China) with a guarantee of authenticity and known history.
Table 1 Pollen identification and sensory characteristics of Chinese unifloral honeys.a
Chemicals and Reagents
The 17 amino acid standards, including alanine (Ala), arginine (Arg), aspartic acid (Asp), cysteine (Cys), glutamic acid (Glu), glycine (Gly), histidine (His), isoleucine (Ileu), leucine (Leu), lysine (Lys), methionine (Met), phenylalanine (Phe), proline (Pro), serine (Ser), threonine (Thr), tyrosine (Tyr), and valine (Val), as well as three internal standards, including norleucine (Nle), phenylisothiocyanate (PITC), and triethylamine (TEA), were obtained from Agela Technologies Inc. (Newark, DE, USA). Sodium acetate, acetonitrile, and methanol (high performance liquid chromatography [HPLC] grade) were obtained from Fisher Chemical Co. (Hampton, NH, USA). All other chemicals were of analytical grade.
Sensory Test of Chinese Unifloral Honeys
A series of organoleptic tests were performed to certify the validity of the floral origins of the collected honey samples. The floral origins of the samples were specified regarding hive locations, seasons, and available floral sources, and further confirmed by means of sensory analyses, such as aroma, taste, consistency, and color characteristics, according to previous reports.[Citation10,Citation16,Citation22]
Pollen Analysis
All of the samples were subjected to pollen analysis according to the method of Lutier and Vassière.[Citation23] For identification of the floral source, 5 g of diluted honey sample was centrifuged at 10,000 rpm for 15 min, to separate the pollens. The separated pollen granules were spread with the help of a brush on a slide containing a drop of lactophenol. The slide was examined microscopically at 400×, using an optical SZ61 bright-field microscope (Olympus, Tokyo, Japan). The slides of pollen granules from flowers of nectariferous plants (Acacia, Vetch, Linden, Coptodis, Red date, Astragalus, Codonopsis, Eriobotrya, and Eucalyptus) of Chinese honeys were also made for comparison. The aim of the analysis was to confirm that the analyzed samples could be declared as unifloral honeys.
Amino Acid Analysis of Honeys by HPLC
The total amino acid extracts were isolated from 33 honey samples according to the method described by Bouseta et al.,[Citation24] starting from 12.5 g of honey treated with 375 μl of norleucine (an internal standard) solution (1 mg/ml in 0.1 N HCl). After extraction, the final volume was 10 ml, the pH was adjusted to 3.2, and the sample was refrigerated prior to derivatization. The extracts (300 μl) were dried under reduced pressure, at t ≤ 38°C, and allowed to react for 10 min with 18 μl of solution of water, triethylamine, and absolute ethanol (1:1:7 (v/v/v)), and 2 μl of phenylisothiocyanate. After filtration through a nylon membrane of 0.45 μm, direct injections of the derivatizated samples were subjected to a High Performance Liquid Chromatograph (Shimadzu, Tokyo, Japan), equipped with a Venusil-AA amino acid analysis column (5 μm, 250 × 4.6 mm, Angela Technologies Inc.). The CTO-10Avp thermostatted column compartment was set at 40°C during the HPLC analyses. Detection was done at 254 nm with an SPD-10Avp detector.
Chromatographic condition for separation was carried out according to Agela Chemical Co. with a gradient of two mobile phases. Phase A: sodium acetate buffer (25 mM, pH 6.5)–acetonitrile; Phase B: acetonitrile–water (v/v = 4:1). Linear gradient elution was programmed as 0.0 min, 0% B; 4 min, 3% B; 16 min, 11% B; 17 min, 21% B; 32 min, 34% B; 34 min, 100% B; 38 min, 0% B. The flow rate was set at 1.0 ml/min. Amino acid identification was done by means of comparing the retention times of HPLC peaks obtained from standard compounds and honey samples. Quantification was achieved using calibration curves obtained from amino acids and ammonium solutions of known concentrations containing the same amount of internal standard.
Statistical Analysis
All analyses were carried out in triplicate and the data were expressed as means ± standard deviations. Statistical analyses between experimental results were based on the Student's t-test. Significant differences were statistically considered at the level of p < 0.05.
RESULTS AND DISCUSSION
Sensory Properties
A sensory test of a honey sample was carefully conducted according to the international rules of the International Honey Committee. The harmonized methods of previous literature[Citation10,Citation16,Citation22] and the European Honey Commission[Citation25] were utilized. The honey samples were characterized according to several selected sensory characteristics, such as aroma, taste, color, and consistency. The main sensory characteristics are characterized in . The colors of Chinese honeys defined by visual observation were noticeably different and varied from almost colorless to dark brown. The brightest were Acacia, Eriobotrya, and Linden honeys, almost colorless to pale yellow and white to cream or ivory, with a yellow or dark brown tinge, respectively. Current results showed that honeys from different botanical and geographical sources possessed diverse sensory properties in aroma, color, and consistency, which verified sensory diversity of Mexico honeys.[Citation26]
Identification of Floral Source
Pollen in natural honey comes from respective nectariferous plants; therefore, the pollen composition can be extensively applied to identify the botanical and geographical origin of honey, to discriminate honey types, and to examine honey quality as well. The microscopic observation on pollen morphologic characteristics and composition is one of the prevailing ways of honey examination. Generally, a honey is considered mainly from one plant (unifloral) if the pollen frequency of that plant is higher than 45%. Pollen granules from taxa are under- or over-presented in relation to their flowers yield of respective nectariferous plant. For unifloral honeys with under-represented pollen, the minimum percentage of the taxon that gives the honey name is 10–20% or 20–30%; for unifloral honeys with over-represented pollen, the minimum percentage of the taxon that gives the honey is 70–90%.[Citation27] Pollen analysis was used to specifically identify the floral origins of 33 honeys and the results are shown in . The micro-structures of Acacia pollen, Vetch pollen, Linden pollen, Coptidis pollen, and Red date pollen were clearly presented on the microscopic slides.
The morphological characteristics of Acacia pollen were near triangle and three apertures, with a side length of 0.079 μm and height of 0.059 μm. While the morphological characteristics of pollen from Acacia honey were also triangle and three apertures, similar to the appearance and structure of Acacia pollen (). The morphological characteristics of Vetch pollen were oval and one aperture, with a long diameter of 0.13 μm, and a short diameter of 0.086 μm. The morphological characteristics of pollen from Vetch honey were also oval and one aperture, similar to the appearance and structure of Vetch pollen (). The morphological characteristics of Linden pollen were near triangle and three apertures, with a side length of 0.092 μm and height of 0.066 μm, while the morphological characteristics of pollen from Linden honey were also triangle and three apertures, similar to the appearance and structure of Linden pollen (). The morphological characteristics of Ziziphus jujuba pollen were near triangle and three apertures, with a side length of 0.076 μm and height of 0.042 μm. The morphological characteristics of pollen from Red date honey were also triangle and three apertures, similar to the appearance and structure of Ziziphus jujuba pollen (). The morphological characteristics of Coptidis pollen were near triangle and three apertures, with a long diameter of 0.064 μm, and a short diameter of 0.056 μm. The morphological characteristics of pollen from Coptidis honey were oval and three apertures, similar to the appearance and structure of Coptidis pollen ().
Figure 1 Micro-structure of pollen granules from several unifloral honeys. (a) Acacia honey; (b) Vetch honey; (c) Linden honey; (d) Red date honey; (e) Coptidis honey. (Color figure available online.)
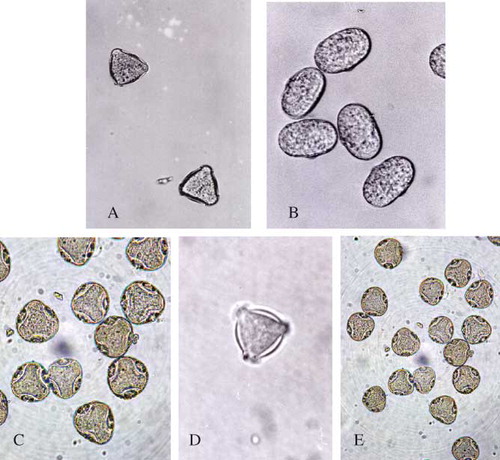
As shown in , the samples of honey H1, H2, and H3 were classified as Acacia (Robinia pseudoacacia L.). The samples of honey H4, H5, H6, and H7 were classified as Vetch (Vicia cracca L.). The samples of honey H8, H9, H10, H11, and H12 were classified as Linden (Tilia spp.). The samples of honey H13, H14, H15, and H16 were classified as Coptidis (Coptis chinensis). The samples of honey H17, H18, H19, and H20 were classified as Red date (Ziziphus jujuba Mill.). The samples of honey H21, H22, and H23 were classified as Astragalus (Astragalus microcephalus Willd.). The samples of honey H24, H25, and H26 were classified as Codonopsis (Codonopsis pilosula (Franch.) Nannf.). The samples of honey H27, H28, and H29 were classified as Eriobotrya (Eriobotrya japonica (Thunb.) Lindl.). The samples of honey H30, H31, H32, and H33 were classified as Eucalyptus (Eucalyptus robusta Smith.).
Free Amino Acid Contents in Chinese Unifloral Honeys
The quantitative determination of free amino acids in honeys were performed by following a RP-HPLC method in which the extraction and derivatization of analyses with phenylisothiocyanate (PITC) were developed and validated by Bouseta et al.[Citation24] HPLC was able to separate and quantify 17 amino acids in honeys. The chromatogram of 17 amino acid standards is shown in and a typical chromatogram of free amino acids from a Linden honey sample is shown in . The proline contents varied from 139.2 to 631.5 mg/kg (). The highest proline content was observed in a Eucalyptus honey (sample H30, a dark-colored honey) and the lowest proline content was observed in a Vetch honey (sample H7, a light-colored honey). There were no significantly (p < 0.05) different proline contents within several species (Vetch, Red date, Astragalus, and Eriobotrya) of unifloral honeys from different geographical sources. Part of the samples from unifloral honeys (Acacia, Linden, Coptidus, Codonopsis, and Eucalyptus) with different geographical sources exhibited significant (p < 0.05) differences in proline contents, while part of samples from these sources did not exhibit significant (p < 0.05) differences in proline content within the same species of unifloral honey. Among all unifloral honey species, the Eucalyptus honeys exhibited the highest proline content (average 574.7 mg/kg), followed by Red date honeys (average 529.1 mg/kg), Coptidis honeys (average 423.1 mg/kg), Linden honeys (average 421.7 mg/kg), Codonopsis honeys (average 346.8 mg/kg), Eriobotrya honeys (average 285.4 mg/kg), Astragalus honeys (average 272.4 mg/kg), Acacia honeys (average 247.9 mg/kg), and Vetch honeys (average 144.9 mg/kg) in a descending order.
Figure 2 Chromatogram of amino acids. (a) Chromatogram of 17 amino acid standards; (b) typical chromatogram of a sample (Linden honey). 1. Asp; 2. Glu; 3. Ser; 4. Gly; 5. His; 6. Arg; 7. Thr; 8. Ala; 9. Pro; 10. Tyr; 11. Val; 12. Met; 13. Cys; 14. Ile; 15. Leu; 16. Nle (internal standard); 17. Phe; 18. Lys. (Color figure available online.)
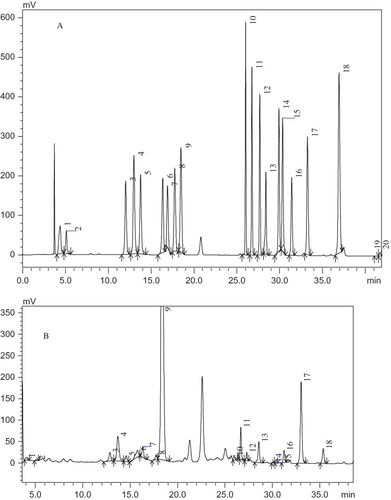
Table 2 Amino acid contents of 33 Chinese unifloral honeys.a
The total amino acid contents (TAACs) varied from 240.7 to 812.6 mg/kg (). The highest TAAC was also observed in Eucalyptus honey (sample H30) and the lowest TAAC was observed in a Vetch honey (sample H7). Significant (p < 0.05) different TAACs were found between unifloral honeys and between samples within the same species of unifloral honey except for Astragalus and Eriobotrya honeys. Among all unifloral honey species, the Eucalyptus honeys exhibited the highest TAAC (average 761.6 mg/kg), followed by Linden honeys (average 744.2 mg/kg), Red date honeys (average 742.4 mg/kg), Coptidis honeys (average 686.8 mg/kg), Codonopsis honeys (average 553.8 mg/kg), Eriobotrya honeys (average 406.9 mg/kg), Astragalus honeys (average 389.9 mg/kg), Acacia honeys (average 354.5 mg/kg), and Vetch honeys (average 262.9 mg/kg) in a descending order. A previous study revealed that various honeys contained 11–21 free amino acids with proline predominating.[Citation28] Proline was the most abundant amino acid in honey, and was used as a standard to quantify amino acid content in honey.
CONCLUSIONS
A total of 33 honey samples from nine floral sources were collected from various geographical areas in China. Honey contains an extensive number of amino acids. Different honey has different compositions and contents in amino acids because of the diversity of geographical areas. The concentration and type of amino acid depends on the floral origin of honey and individual geographical areas in China. Amino acids contents found in Linden honeys were all higher than those in the light colored pure honeys (Acacia and Vetch honey), closer to those in dark colored honeys (Eucalyptus, Coptidis, and Red Date honey). The Linden honey samples appeared to deserve further investigation into its individual biological active components, which may be an attractive source of nutraceuticals and medicinal ingredients.
ACKNOWLEDGMENTS
This project was sponsored by Wang's Bee Garden. The authors would like to extend thanks to Wang's Bee Garden for kindly providing honey samples.
REFERENCES
- Mendes , E. , Brojo-Proença , E. , Ferreira , I.M. and Ferreira , M.A. 1998 . Quality evaluation of Portuguese honey . Carbohydrate Polymers , 37 : 219 – 223 .
- Cherbuliez , T. 2001 . The medicine from the bees , Apimondia : CD-ROM .
- Ferreira , I. , Aires , E. , Barreira , J.C.M. and Estevinho , L.M. 2009 . Antioxidant activity of Portuguese honey samples: Different contributions of the entire honey and phenolic extract . Food Chemistry , 114 : 1438 – 1443 .
- FA , O. 1996 . Value-Added Products from Beekeeping , Rome , , Italy : FAO Agricultural Services Bulletin. FAO .
- Allen , K.L. , Molan , P.C. and Reid , G.M. 1991 . The variability of the antibacterial activity of honey . Apiacta , 26 : 114 – 121 .
- Molan , P.C. 1992 . The antibacterial activity of honey. 1—The nature of antibacterial activity . Bee World , 73 : 5 – 28 .
- Alnaqdy , A. , Al-Jabri , A. , Al-Mahrooqi , Z. , Nzeako , B. and Nsanze , H. 2005 . Inhibition effect of honey on the adherence of Salmonella to intestinal epithelial cells in vitro . International Journal of Food Microbiology , 103 : 347 – 351 .
- White , J.W. 1979 . “ Composition of honey ” . In Honey: A Comprehensive Survey; Crane, E , 157 – 158 . London : Heinemann .
- Orhan , F. , Sekerel , B.E. , Kocabas , C.N. , Sackesen , C. , Adalioglu , G. and Tuncer , A. 2003 . Complementary and alternative medicine in children with asthma . Annals of Allergy, Asthma & Immunology , 90 : 611 – 615 .
- Silva , L.R. , Videira , R. , Monteiro , A.P. , Valentao , P. and Andrade , P.B. 2009 . Honey from Luso region (Portugal): Physicochemical characteristics and mineral contents . Microchemical Journal , 93 : 73 – 77 .
- Castaldo , S. and Capasso , F. 2002 . Propolis, an old remedy used in modern medicine . Fitoterapia , 73 : S1 – S6 .
- Al-Mamary , M. , Al-Meeri , A. and Al-Habori , M. 2002 . Antioxidant activities and total phenolics of different types of honey . Nutrition Research , 22 : 1041 – 1047 .
- Aljadi , A.M. and Kamaruddin , M.Y. 2004 . Evaluation of the phenolic contents and antioxidant capacities of two Malaysian floral honeys . Food Chemistry , 85 : 513 – 518 .
- Beretta , G. , Granata , P. , Ferrero , M. , Orioli , M. and Maffei-Facino , R. 2005 . Standardization of antioxidant properties of honey by a combination of spectrophotometric/fluorimetric assays and chemometrics . Analytica Chimica Acta , 533 : 185 – 191 .
- The National Honey Board. Honey-health and therapeutic qualities. Lashley Street Longmont, 2002. http://www.nhb.org (http://www.nhb.org)
- Bogdanov , S. , Ruoff , K. and Persano-Oddo , L. 2004 . Physico-chemical methods for the characterisation of unifloral honeys: A review . Apidologie , 35 : S4 – S17 .
- Bogdanov , S. 1999 . Harmonised methods of the international honey commission , Bern , , Switzerland : Swiss Bee Research Center, FAM, Liebefeld .
- Bergner , K.G. and Koromi , J. 1968 . Zum Aminosaurengehalt von Honigen . Z. Bienenforsch , 9 ( 5 ) : 182 – 184 .
- White , J.W. , Reithof , M. and L; Kushnir , I. 1961 . Composition of honey. VI. The effect of storage on carbohydrates, acidity and diatase content . Journal of Food Science , 26 : 63 – 71 .
- Cotte , J.F. , Casabianca , H. , Giroud , B. and Albert , M. 2004 . Characterization of honey amino acid profiles using high-pressure liquid chromatography to control authenticity . Analytical and Bioanalytical Chemistry , 378 : 1342 – 1350 .
- Meda , A. , Lamien , C.E. , Romito , M. , Millogo , J. and Nacoulma , O.G. 2005 . Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity . Food Chemistry , 91 : 571 – 577 .
- Mossel , B. , Bhandari , B.R. , D'Arcy , B. and Caffin , N. 2003 . Determination of the viscosity of some Australian honeys based on composition . International Journal of Food Properties , 6 ( 1 ) : 87 – 97 .
- Lutier , P. and Vassière , B. 1993 . An improved method for pollen analysis of honey . Review of Palaeobotany and Palynology , 78 : 129 – 144 .
- Bouseta , A. , Scheirman , V. and Collin , S. 1996 . Flavor and free amino acid composition of lavender and Eucalyptus honeys . Journal of Food Science , 61 ( 4 ) : 683 – 694 .
- Bogdanov , S. , Martin , P. and Lüllmann , C. 1997 . Harmonised methods of the European honey commission . Apidologie , 28 ( Special Issue ) : 59
- Ruiz-Navajas , Y. , Viuda-Martos , M. , Fernandez-Lopez , J. , Zaldivar-Cruz , J.M. , Kuri , V. and Perez-Alvarez , J.A. 2011 . Antioxidant activity of artisanal honey from Tabasco, Mexico . International Journal of Food Properties , 14 ( 2 ) : 459 – 470 .
- Terrab , A. , Diez , M.J. and Heredia , F.J. 2002 . Characterization of Moroccan unifloral honeys by their physicochemical characteristics . Food Chemistry , 79 : 373 – 379 .
- White , J.W. and Doner , L.W. 1980 . Honey composition and properties . Beekeeping in the United States Agriculture Handbook; Agriculture Research Service, USDA: Washington, DC, , : 335