Abstract
The effects of detoxification process on conformational and functional properties of Jatropha curcas proteins were investigated. The molecular fractions and structure of proteins were affected more easily by the detoxification process. Compared with the proteins in Jatropha curcas meal, the detoxification process decreased the thermal stability and led to an increase in the surface hydrophobicity with a decrease in the contents of sulfhydryl and disulfide bond of proteins in detoxified Jatropha curcas meal. These conformational sand compositional changes in proteins influenced by the detoxification process, contributed to the relatively good changes in functional properties of detoxified Jatropha curcas meal, such as solubility, water and fat holding capacities, emulsifying properties, and foaming properties. Therefore, the detoxification treatment was not only a promising way to detoxify, but also an effective means to improve the functional properties of detoxified Jatropha curcas meal.
INTRODUCTION
With the development of the world economy and rapid growth of the population, there is an increasing demand for protein consumption. Due to high-volume, cheapness, and good-quality, the plant-based proteins industry has been receiving more and more attention, such as the soy protein industry, which serves as an excellent model for the use of other crops.[Citation1,]
Jatropha curcas, a small tree or shrub distributed in the subtropical and tropical regions of the world, is fueled by the availability of its oil, which can be easily converted into biodiesel that meets American and European standards. After extraction of the oil, the Jatropha curcas meal is rich in protein, between 50 and 64%. Except for lysine, all other essential amino acids in the meal have been reported to be higher concentrations than those of the FAO reference pattern suggested for pre-school children.[Citation2–4] Therefore, high protein content in Jatropha curcas meal and the excellent profile of essential amino acids in Jatropha curcas meal protein (JMP) would make it a strong candidate for food applications. However, compared to the soybean protein industry, the JMP industry is relatively young; commercial use of JMP has been limited by its low value as food and nonfood additives due to the presence of high toxic and antinutritional components, such as phorbolesters, curcin, trypsin inhibitor, glucosinolates, and amylase inhibitors.[Citation5,Citation6]
In order to make JMP a potentially important ingredient for food and nonfood applications, recently, various methods, such as germination, dehulling, cooking, roasting, autoclaving, fermentation, and extrusion cooking, have been employed to detoxify Jatropha curcas meal.[Citation5,Citation7–9] In the authors’ previous study, a method of hydrolysis of enzymes (cellulase plus pectinase) followed by washing with ethanol (65%) had been successfully applied to detoxify Jatropha curcas meal.[Citation10] The toxin and anti-nutritional composition in Jatropha curcas meal can be decreased to a tolerable level; at the same time, the crude protein in detoxified meal reached 74.68%.
Functional properties of protein are important in food processing and food product formulation. Despite having a relatively high effect on the content of toxic and antinutritional components, the detoxification process may also influence the functional properties of JMP, which have an effect on the application of the JMP in food and nonfood applications. Until now, information, such as functional properties, surface/interfacial activity, surface hydrophobicity, and thermal stability of protein in detoxified Jatropha curcas meal, is still rare in the literature. In order to get some insight into the technical applications for detoxified Jatropha curcas meal in the food and nonfood industries, the effect of the authors’ detoxification treatment of hydrolysis of enzymes (cellulase plus pectinase) followed by washing with ethanol (65%) on functional properties, structure, and thermal properties of protein in detoxified Jatropha curcas meal were determined.
MATERIALS AND METHODS
Materials
Jatropha curcas seeds were procured from Si Chuan Province, China. 5,5′-Dithio-bis 2-nitrobenzoic acid (DTNB) and 1,8-anilinonaphthalenesulfonate (ANS) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Cellulase and pectinase (the enzyme activities of the cellulase and pectinase were 126 FPU and 1.8 U/mg, respectively) were obtained from Novozymes A/S (Wuxi, China). All other chemical reagents used were of analytical grade and were purchased from Sinopharm chemical reagent factory (Co., Ltd., Beijing, P.R. China).
Preparation of Jatropha curcas Meal (JM) and Detoxified Jatropha curcas Meal (DJM)
The Jatropha curcas were deshelled and ground. The ground kernel was defatted using a screw extruding-expanding pretreatment technique at 90°C, followed by petroleum ether extraction (boiling point = 40–60°C). The defatted ground kernel of Jatropha curcas meal was served as the JM. The DJM was prepared according to the detoxification method described by Xiao et al.[Citation10] The JM was treated with enzymes (cellulase plus pectinase) for 1 h. The procedure was carried out under this condition: cellulase (5 mg/g) and pectinase (10 mg/g); 50°C; pH 4.5–5.0; time 1 h. Hydrolysis was stopped by heating at 105°C for 15 min. Hydrolysates was clarified by centrifugation (4500× g, 15 min) and then the precipitation was washed using 65% ethanol (v/v = 5:1) at 50°C with constant stirring for 1 h. Then the solvent was removed by centrifugation (4500× g, 15 min) and recovered. The residue was freeze dried and designated as DJM.
Preparation of Detoxified Jatropha curcas Meal Protein (DJMP) and Jatropha curcas Meal Protein (JMP)
DJMP and JMP were prepared according to the method described by Saetae et al.[Citation11] First, 200 g of DJM or JM was dispersed in 2000 mL 0.5 M NaCl solution with the pH 9.5. The suspension was stirred for 2 h at 50°C using a stirrer. Then, the suspension was centrifuged at 4000 rpm for 20 min. The supernatant was adjusted to pH 4.2 and centrifuged again at 4000 rpm for 20 min. The precipitates were adjusted to pH 7.2, then freeze-dried and stored at 4°C until used. The content of DJMP and JMP (92.26 and 91.12%) was determinated by the Kjeldahl method.
Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
SDS-PAGE was performed on a discontinuous buffered system according to the method described by Hojilla-Evangelista and Evangelista with some modifications,[Citation12] using a 12% separating gel and 5% stacking gel.
Molecular Weight Distribution, Surface Hydrophobicity
Molecular weight distribution profile of DJMP was determined using a Waters™ 600E Advanced Protein Purification System (Waters Corporation, Milford, MA, USA). Surface hydrophobicity (H0) was determined by the method described by Kato and Nakai[Citation13] with minor modifications. In brief, using 8-anilino-1-naphthalenesulfonic acid (ANS) as a fluorescent probe, the stock solutions of 16 mmol ANS and 1 mg/mL protein were prepared in 10 mM phosphate buffered saline (PBS; pH 7.2). Then, 5 mL of a different protein concentration (0.5 mg/mL to 0.03125 mg/mL) and 50 μL of ANS solution were added and shaken with a vortex mixer for 3 min. Fluorescence intensity (FI) was measured at 390 nm (excitation) and 470 nm (emission) at room temperature (25 ± 1°C) using a fluorescence spectrometer (F-7000 FL Spectrophotometer, Shimadzu, Kyoto, Japan). The initial slope of the FI versus protein concentration plot was calculated by linear regression analysis and used as an index of H0. First, 1 g of DJMP or JMP was suspended in 10 mL PBS (10 mM, pH 7.2). The suspension was vortexed for 30 s every 15 min for 1 h, then centrifuged at 4000 rpm for 20 min. The zeta potential of the supernatant was determined by using a Malvern Mastersizer ZEH3600 (Malvern Instruments Ltd., Worcestershire, UK) according to the method described by Malhotra and Coupland.[Citation14]
Total and Exposed Free Sulfhydryl
The contents of sulfhydryl groups were determined by the method described by Beveridge et al.[Citation15] with some modifications. Ellman’s reagent was prepared by dissolving 198 mg of DTNB in 50 mL of Tris–glycine buffer (0.086 M Tris, 0.09 M glycine, 4 mM edetate disodium [EDTA], pH 8.0). Then, 50 mg of protein samples were suspended in 1.8 mL of Tris–glycine buffer (total sulfhydryl) or with 8 M urea (exposed sulfhydryl). Next, 0.2 mL of the Ellman’s reagent was added. The suspension was incubated for 1 h at room temperature (25 ± 1°C) and vortexed for 30 s every 15 min, then centrifuged at 12,000 rpm for 10 min. The absorbance of the supernatant was determined at 412 nm against the reagent buffer as the blank. The protein contents of the protein samples were determined by Bradford.[Citation16] The sulfhydryl contents were calculated by using the extinction coefficient (13,600 M/cm) and expressed as μM/g of protein.
Disulfide Bond Content
The disulfide bond content was measured according to the methods reported by Thannhauser et al.[Citation17] with some modifications. The DTNB and sodium sulfite solution (NTSB) was prepared according to the method described by Thannhauser. In brief, 50 mg of the protein samples were previously dispersed in 1.8 mL of Tris-base buffer containing 0.1 M Na2SO3, 10 mM EDTA, and 3 M mercaptoethanol, then mixed with 0.2 mL of the NTSB test solution prepared prior to use. Absorbance at 412 nm was determined using the test NTSB solution as the reference. The extinction coefficient was 13,600 M/cm.
Secondary Structural by Fourier Transform-Infrared (FT-IR) Spectroscopy
All infrared spectra were obtained by an FT-IR spectrometer (Thermo Electron Corporation, Waltham, MA) according to the method described by Severcan et al.[Citation18] with some modifications. The spectra were recorded in the region between 4000 and 400 cm−1 with a spectral resolution of 4 cm−1 and an aperture of 5.0 mm. For each spectrum, 32 scans were averaged.
Thermal Properties
The thermal properties were examined with a Perkin Elmer Pyris 1 differential scanning calorimetry (DSC; Christiansburg, VA). A temperature ramp rate of 10°C/min was used in the range of 20 to 140°C. Thermal denaturation temperature (Td) and denaturation enthalpy[Citation8] were calculated from thermograms.[Citation19]
Functional Properties
The functional properties (protein solubility, water holding capacity, fat holding capacity, foaming properties, and emulsifying properties) were measured according to the methods reported by Hojilla-Evangelista and Evangelista[Citation12] with some modifications.
Solubility (PS)
First, 1 g of DJMP or JMP was suspended in 100 mL PBS (10 mM, pH 7.2). The suspension was stirred at room temperature for 30 min using a magnetic stirrer, and then centrifuged at 4000 rpm for 20 min. The protein content of the supernatant was determined by the method described by Bradford.[Citation16] PS was then calculated by the following formula:
Water holding capacity (WHC)
To begin, 1 g of DJMP or JMP was suspended in 10 mL PBS (10 mM, pH 7.2). The suspension was vortexed for 30 s every 15 min for 1 h, then centrifuged at 4000 rpm for 20 min to remove free water. WHC was calculated as the percentage increase of DJMP or JMP weight.
Fat holding capacity (FHC)
First, 0.5 g of DJMP or JMP was suspended in 10 mL of oil. The suspension was vortexed for 30 s every 15 min for 1 h, then centrifuged at 4000 rpm for 20 min to remove free oil. FHC was calculated as the percentage increase of DJMP or JMP weight.
Foaming properties
An aliquot of 0.5 g of DJMP or JMP was suspended in 50 mL PBS (10 mM, pH 7.2). The suspension were homogenized at a speed of 12,000 rpm for 2 min. Foaming capacity (FC) was the volume (mL) of foam produced in 2 min. Foam stability (FS) was the fraction of foam volume remaining (%) after standing for 60 min.
Emulsifying properties
First, 0.5 g of DJMP or JMP was suspended in 100 mL PBS (10 mM, pH 7.2) and vortexed for 1 min. The mixture was combined with 40 mL of soybean oil and homogenized at a speed of 12,000 rpm for 2 min. Then, 50 μL of the emulsion was taken from the bottom of the beaker at 0 and 30 min and diluted with 10.0 mL of 0.1% sodium dodecyl sulfate (SDS) solution. The absorbance was measured at 500 nm. The emulsifying activity (EA) was determined from the absorbance measured at 0 min. The emulsion stability (ES) was calculated by the following formula:
Statistical Analysis
All the values were expressed as means of triplicate determinations (standard deviation), and subjected to one way analysis of variance using SPSS (version 13.0; International Business Machines Corporation, Armonk, NY) software. Means were separated by Fisher’s protected least significant difference (p < 0.05).
RESULTS AND DISCUSSION
Molecular Weights Distribution
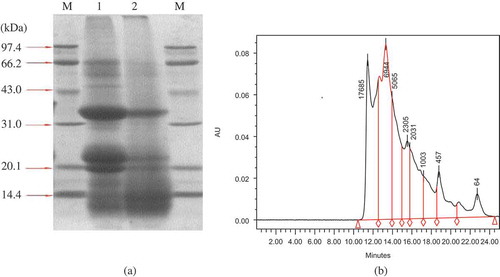
Electrophoresis (SDS-PAGE) of DJMP and JMP was performed to obtain information on the molecular weight and distribution pattern of the protein components (). The SDS-PAGE patterns of JMP contained three major bands, which contained proteins between 21.1 and 43 kDa, similar to the results of the reference.[Citation20] But the SDS-PAGE patterns of DJMP was not obvious, which may indicate that the molecular weight of DJMP was less than 14.4 kDa, showing the differences of various types of proteins differing in molecular weight caused by a detoxification process. The molecular weight distribution of DJMP (, >10 kDa, 23.31%; 10 kDa, 76.69%) determined by a Waters™ 600E Advanced Protein Purification System (Waters Corporation, Milford, MA, USA) confirmed the results of SDS-PAGE of DJMP.
Surface Hydrophobicity (H0)
According to the report of Mine,[Citation21] a higher H0 can reduce the energy barrier, which is helpful for the adsorption of proteins to air-water interfaces.[Citation22] Surface hydrophobicities of DJMP and JMP were presented in . H0 of DJMP (680.24) was significantly higher than that of JMP (374.24). The higher denaturation degree of protein could be attributed to promote availability of hydrophobic zones and unfold the protein molecules, which make more hydrophobic sites accessible to the binding of ANS fluorescence probe corresponding to increased H0.[Citation13,Citation23] This result suggested that more buried hydrophobic groups of DJMP were exposed after the detoxification treatment and the ordered structure of DJMP was lost.
TABLE 1 Effect of detoxification on H0, ZP, thermal characteristics, free sulfhydryl contents, and disulfide bond contents of DJMP and JMP
Zeta Potential (ZP)
The stability of the sol can be distinguished by ZP. The higher electrostatic repulsion in a system with higher zeta potential does not lead to the coagulation of the particles. The electrostatic repulsion and attraction between protein molecules is the important role for protein solubility, emulsifying, foaming, and gel network structure.[Citation24] As shown in , the values of ZP of DJMP and JMP were negative, which was beneficial for increasing the solubility and functional properties of DJMP and JMP.
Thermal Characteristics Analysis
The thermal characteristics (thermal denaturation temperature, Td; enthalpy change, ΔH) of DJMP and JMP were summarized in . As shown in , the Td and ΔH (79.20 ± 0.98°C; 3.31 ± 0.12 J/g) of DJMP were both lower than those of JMP (108.88 ± 2.17°C; 20.09 ± 1.01 J/g). The Td and ΔH are the measure for studying the thermodynamics of protein stability and they can provide a basic understanding of protein denaturation. A higher value of Td is usually associated with a higher thermal stability for proteins.[Citation25] Thus, the Td of DJMP decreased significantly as compared to that of JMP, suggesting that the detoxification process led to a noticeable decrease of thermal stability of DJMP. The ΔH represents the extent of ordered structure of protein. The ΔH of DJMP was also significantly decreased compared with that of JMP. This result indicated that the detoxification process induced unfolding of JMP and the ordered structure of DJMP was decreased, which was in agreement with the result of surface hydrophobicity.
TABLE 2 Effect of detoxification on the secondary structure of DJMP and JMP by FT-IR spectra
Sulfhydryl and Disulfide Bond Contents
Results for free sulfhydryl contents (total and exposed) and disulfide bond contents of DJMP and JMP were given in . The contents of exposed sulfhydryl, total sulfhydryl, and disulfide bond were significantly affected by the detoxification process. The sulfhydryl (Total, 19.30 ± 1.71; Exposed, 5.26 ± 0.37) and disulfide bond (7.02 ± 1.01) contents of DJMP were significantly lower than those of JMP (Total, 31.33 ± 2.47; Exposed, 10.27 ± 0.95; Disulfide bond, 10.53 ± 1.60), which was in agreement with the result of DSC (). The detoxification process unfolded the conformation of JMP, which might lead to the breakage of disulfide.[Citation25]
Secondary Structures
FT-IR spectra are remarkably sensitive to the secondary structure contents of proteins. The secondary structure of DJMP and JMP were estimated by FT-IR spectra. Commonly, the amide I region (1700 ± 1600 cm−1) is utilized for the analysis of secondary structure of proteins from FT-IR spectra. Therefore, to identify the bands from FT-IR spectra, curve fitting has to be applied.[Citation26] The secondary structure contents of DJMP and JMP were shown in . The DJMP was calculated composed of 19.53% α-helix, 32.05% β-sheet, 20.82% β-turn, and 27.7% random coil by the curve fitting program. And the secondary structure estimation of JMP revealed 18.6% α-helix, 33.64% β-sheet, 15.04% β-turn, and 32.72% random coil. DJMP contained more α-helix and β-turn, but less β-sheet and random coil than JMP. These changes may possibly be due to the detoxification process.
Functional Properties
Proteins solubility
Proteins solubility at the natural pH is the critical point for proteins with good functional properties.[Citation27,Citation28] Thus, it is important to study the solubility of DJMP and JMP at the natural pH. The solubility of DJMP (80.41%) was the highest in all the samples (JMP, 42.70%; soy protein isolate [SPI], 81%) at the natural pH, which was also higher than the observations reported by Lestari et al.[Citation29] and Saetae et al.[Citation11,Citation30] It may be that the detoxification treatment (65% ethanol) in our study was more moderate than that in the references (90% ethanol). The differences in solubility between DJMP and JMP may be attributed to the denatured proteins, which were caused by the step of detoxification treatment of ethanol (65%) washing. According to the results of molecular weights distribution and disulfide bond, the smaller and unfolding proteins could adsorb much more water by the exposed hydrophilic groups and the looser tertiary conformation might promote intramolecular hydration of proteins.[Citation31] Thus, the high solubility of DJMP would be beneficial for its utilization in food and nonfood applications without adjusting the pH.
Water and fat holding capacities (WHC, FHC)
DJMP was found to possess higher WHC of 2.16 g/g compared with those of JMP (1.65 g/g), but lower than SPI (4.84 g/g) (). This is likely due to the fact that the DJMP had a great ability to swell, dissociate, and unfold exposing additional binding sites, since ethanol (65%) washing in the step of detoxification might change the protein conformation and disrupt the internal structure and surface hydrophobicity of the protein. High WHC of DJMP makes them a potential ingredient in bread, cakes industries, and meat. From , FHC was 3.20 g/g of DJMP, and 2.69 g/g of JMP. These two values were both higher than 1.94 g/g of SPI. The differences in FHC between DJMP and JMP can be attributed to the denatured proteins, which can bind more oil through non polar groups.[Citation32]
TABLE 3 Effect of detoxification on functional properties of DJMP, JMP, and SPI
Foaming properties
Many food products are a type of foam products, such as cakes, bread, whipped cream, ice cream, soufflé, and so on. These products have a unique texture and taste mainly derived from the dispersion of fine bubbles. In most of these products, proteins play the role of the surfactant. Therefore, the protein foaming and foam stability have an important role for food products. DJMP, JMP, and SPI contain both hydrophobic groups and hydrophilic groups, which have a surface activity and can reduce the surface tension of water in the formation of foam when stirred. showed that, compared with JMP (50.75%) and SPI (39.13%), DJMP had the highest of FC (64.25%). But the FS of DJMP and JMP were inferior to that of SPI, similar to the observations reported by Lestari et al.[Citation29] and Saetae et al.[Citation11,Citation30] All those differences in foaming properties between DJMP and JMP can be attributed to ethanol (65%) washing when detoxifying, which can cause the structure of DJMP and JMP to change ( and ). Bubble formation of protein is closely related to its solubility, but foam stability closely depends on its viscosity, molecular interaction, and other physical characteristics.Citation55–35
Emulsifying properties
Emulsifying capacity is an indicator of ability for proteins to form oil/water emulsion. Emulsion stability is the ability to maintain the emulsion as stable. Protein is a surfactant, which can reduce the interfacial tension of water-oil to make it easy to emulsify. On the other hand, the proteins disperse in the discontinuous phase and continuous phase at the interface to resist the aggregation of the non-continuous phase, playing the role of emulsion stability. Emulsifying properties are affected by many factors, such as protein concentration, pH, solubility, and so on.[Citation36–38] showed that the EA and ES of DJMP (0.32, 91.36%) was higher than those of JMP (0.21, 83.21%), but a little lower than that of SPI (0.59, 97%). The results here are better than those reported by Lestari et al.[Citation29] and Saetae et al.[Citation11] Higher EA of DJMP compared to JMP in this study might be due to higher hydrophobicity of DJMP. Another possible reason may be that DJMP was composed of small protein moleculars (), which were caused by the moderate detoxification treatment of hydrolysis of enzymes (cellulase plus pectinase) followed by washing with ethanol (65%). Because of the unfolding and low reorientation abilities as compared with the large protein moleculars, the small moleculars of proteins were less efficient in reducing the interfacial tension.[Citation22]
CONCLUSIONS
The molecular fractions and structure of proteins was changed by the authors’ detoxification process of hydrolysis of enzymes (cellulase plus pectinase) followed by washing with ethanol (65%), which influenced the functional properties of proteins. The DJMP showed better protein solubility, water and fat absorption capacity, emulsifying activity and stability, foam capacity and stability than JMP at neutral pH conditions. The results revealed that the detoxification treatment the authors found was not only a promising way to detoxify Jatropha curcas meal, but also can be simultaneously enriched and improved functional properties of DJMP. It can also be concluded that suitable functional properties of DJMP could be produced from this new oil seed crop as a good source of a protein ingredient in food systems, such as salad dressing, mayonnaise, sausage, and meat products.
FUNDING
The authors acknowledge the funding and the technical services provided by Jiangnan University.
REFERENCES
- Manamperi, W.A.R.; Wiesenborn, D.P.; Chang, S.K.C.; Pryor, S.W. Effects of protein separation conditions on the functional and thermal properties of canola protein isolates. Journal of Food Science 2011, 76 (3), E266–E273.
- Berchmans, H.J.; Hirata, S. Biodiesel production from crude Jatropha curcas L. seed oil with a high content of free fatty acids. Bioresource Technology 2008, 99 (6), 1716–1721.
- Mohibbe Azam, M.; Waris, A.; Nahar, N. Prospects and potential of fatty acid methyl esters of some non-traditional seed oils for use as biodiesel in India. Biomass and Bioenergy 2005, 29 (4), 293–302.
- Liberalino, A.; Bambirra, E.; Moraes Santos, T.; Vieira, E. Jatropha curcas L. seeds: Chemical analysis and toxicity. Arquivos de Biologia E Tecnologia 1988, 31 (4), 539.
- Makkar, H.P.S.; Becker, K. Jatropha curcas, a promising crop for the generation of biodiesel and value-added coproducts. European Journal of Lipid Science and Technology 2009, 111 (8), 773–787.
- Ye, M.; Li, C.; Francis, G.; Makkar, H.P.S. Current situation and prospects of Jatropha curcas as a multipurpose tree in China. Agroforestry Systems 2009, 76 (2), 487–497.
- Joshi, C.; Mathur, P.; Khare, S.K. Degradation of phorbol esters by Pseudomonas aeruginosa PseA during solid-state fermentation of deoiled Jatropha curcas seed cake. Bioresource Technology 2011, 102 (7), 4815–4819.
- Martinez-Herrera, J.; Siddhuraju, P.; Francis, G.; Davila-Ortiz, G.; Becker, K. Chemical composition, toxic/antimetabolic constituents, and effects of different treatments on their levels, in four provenances of Jatropha curcas L. from Mexico. Food Chemistry 2006, 96 (1), 80–89.
- Aregheore, E.; Becker, K.; Makkar, H. Detoxification of a toxic variety of Jatropha curcas using heat and chemical treatments, and preliminary nutritional evaluation with rats. The South Pacific Journal of Natural Science 2003, 21 (1), 51–56.
- Xiao, J.; Zhang, H.; Niu, L.; Wang, X.; Lu, X. Evaluation of detoxification methods on toxic and antinutritional composition and nutritional quality of proteins in Jatropha curcas meal. Journal of Agricultural and Food Chemistry 2011, 59 (8), 4040–4044.
- Saetae, D.; Kleekayai, T.; Jayasena, V.; Suntornsuk, W. Functional properties of protein isolate obtained from physic nut (Jatropha curcas L.) seed cake. Food Science and Biotechnology 2011, 20 (1), 29–37.
- Hojilla-Evangelista, M.P.; Evangelista, R.L. Functional properties of protein from Lesquerella fendleri seed and press cake from oil processing. Industrial Crop and Products 2009, 29 (2–3), 466–472.
- Kato, A.; Nakai, S. Hydrophobicity determined by a fluorescence probe method and its correlation with surface properties of proteins. Biochimica et Biophysica Acta (BBA)—Protein Structure and Molecular Enzymology 1980, 624 (1), 13–20.
- Malhotra, A.; Coupland, J.N. The effect of surfactants on the solubility, zeta potential, and viscosity of soy protein isolates. Food Hydrocolloids 2004, 18 (1), 101–108.
- Beveridge, T.; Beveridge, T.; Toma, S.J.; and Nakai, S. Determination of SH- and SS-groups in some food proteins using Ellman’s reagent. Journal of Food Science 1974, 39, 49–51.
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 1976, 72 (1–2), 248–254.
- Thannhauser, T.W.; Konishi, Y.; Scheraga, H.A. Sensitive quantitative analysis of disulfide bonds in polypeptides and proteins. Analytical Biochemistry 1984, 138 (1), 181–188.
- Severcan, M.; Severcan, F.; Haris, P.I. Estimation of protein secondary structure from FTIR spectra using neural networks. Journal of Molecular Structure 2001, 565, 383–387.
- Zhu, K.X.; Sun, X.H.; Chen, Z.C.; Peng, W.; Qian, H.F.; Zhou, H.M. Comparison of functional properties and secondary structures of defatted wheat germ proteins separated by reverse micelles and alkaline extraction and isoelectric precipitation. Food Chemistry 2010, 123 (4), 1163–1169.
- Hamarneh, A.I.; Heeres, H.J.; Broekhuis, A.A.; Picchioni, F. Extraction of Jatropha curcas proteins and application in polyketone-based wood adhesives. International Journal of Adhesion and Adhesives 2010, 30 (7), 615–625.
- Mine, Y. Effect of dry heat and mild alkaline treatment on functional properties of egg white proteins. Journal of Agricultural and Food Chemistry 1997, 45 (8), 2924–2928.
- Voutsinas, L.P.; Cheung, E.; Nakai, S. Relationships of hydrophobicity to emulsifying properties of heat denatured proteins. Journal of Food Science 1983, 48 (1), 26–32.
- Thompson, L.; Liu, R.; Jones, J. Functional properties and food applications of rapeseed protein concentrate. Journal of Food Science 1982, 47 (4), 1175–1180.
- Chan, W.M.; Ma, C.Y. Acid modification of proteins from soymilk residue (okara). Food Research International 1999, 32 (2), 119–127.
- Harwalkar, V.; Ma, C.Y. Study of thermal properties of oat globulin by differential scanning calorimetry. Journal of Food Science 1987, 52 (2), 394–398.
- Byler, D.; Brouillette, J.; Susi, H. Quantitative studies of protein structure by FT-IR spectra deconvolution and curve-fitting. Spectroscopy 1986, 1 (3), 29–32.
- Kim, S.Y.; Park, P.S.W.; Rhee, K.C. Functional properties of proteolytic enzyme modified soy protein isolate. Journal of Agricultural and Food Chemistry 1990, 38 (3), 651–656.
- Cao, X.; Li, C.; Wen, H.; Gu, Z. Extraction technique and characteristics of soluble protein in germinated brown rice. International Journal of Food Properties 2010, 13 (4), 810–820.
- Lestari, D.; Mulder, W.J.; Sanders, J.P.M. Jatropha seed protein functional properties for technical applications. Biochemical Engineering Journal 2010, 55 (3), 297–304.
- Saetae, D.; Suntornsuk, W. Toxic compound, anti-nutritional factors and functional properties of protein isolated from detoxified Jatropha curcas seed cake. International Journal of Molecular Sciences 2010, 12 (1), 66–77.
- Zhao, G.; Liu, Y.; Zhao, M.; Ren, J.; Yang, B. Enzymatic hydrolysis and their effects on conformational and functional properties of peanut protein isolate. Food Chemistry 2011, 127 (4), 1438–1443.
- Ishino, K.; Kudo, S. Conformational transition of alkali-denatured soybean 7S and 11S globulins by ethanol. Agricultural and Biological Chemistry 1980, 44 (3), 537–543.
- Waniska, R.; Kinsella, J. Foaming properties of proteins: Evaluation of a column aeration apparatus using ovalbumin. Journal of Food Science 1979, 44 (5), 1398–1402.
- Wang, M.; Hettiarachchy, N.; Qi, M.; Burks, W.; Siebenmorgen, T. Preparation and functional properties of rice bran protein isolate. Journal of Agricultural and Food Chemistry 1999, 47 (2), 411–416.
- Adebowale, Y.A.; Schwarzenbolz, U.; Henle, T. Protein isolates from bambara groundnut (Voandzeia subterranean L.): Chemical characterization and functional properties. International Journal of Food Properties 2011, 14 (4), 758–775.
- Pearce, K.N.; Kinsella, J.E. Emulsifying properties of proteins: Evaluation of a turbidimetric technique. Journal of Agricultural and Food Chemistry 1978, 26 (3), 716–723.
- Qi, M.; Hettiarachchy, N.; Kalapathy, U. Solubility and emulsifying properties of soy protein isolates modified by pancreatin. Journal of Food Science 1997, 62 (6), 1110–1115.
- Chobert, J.M.; Bertrand-Harb, C.; Nicolas, M.G. Solubility and emulsifying properties of caseins and whey proteins modified enzymically by trypsin. Journal of Agricultural and Food Chemistry 1988, 36 (5), 883–892.