Abstract
Chemical constituents, total phenolic content, total oxidant status, total antioxidant status, lipid hydroperoxides, total free –SH levels, and antimicrobial activity of essential oil obtained from the Ferulago sandrasica (Umbelliferae) were investigated. The essential oil was obtained by hydrodistillation using a Clevenger-type apparatus. The chemical constituents were analyzed by gas chromatography‐mass spectrometry. The main components of the essential oil were ocimene (30.5%), carene-δ-3 (27.4%), and α-pinene (17.8). The antimicrobial activity was tested by a disc diffusion method against E. coli MC 400, E. coli ATCC 25922, E. coli 0157 H7, E. colaecea ATCC 23355, E. feacalis ATCC 19433, P. aeruginosa NRRL B-2679, S. aureus ATCC 25923, B. nischenoformis NRRL B-1001, S. aureus ATCC 33862, B. cereus NRRL B-3711, B. subtilis NRRL B-209, M. luteus NRRL B-1013, L. monocytogenes ATCC 7644, B. subtulis ATCC 6633.
INTRODUCTION
Natural antioxidants provide health benefits associated with their ability to prevent damage due to biological degeneration. The availability of appropriate and complete food composition data is crucial. Due to the chemical diversity of the antioxidant compounds present in foods, no complete databases of food antioxidant content are yet available. In addition, levels of individual antioxidants in food do not necessarily reflect their total antioxidant capacity, which could also depend on synergic and redox interactions among the different antioxidant molecules (minerals, fiber, vitamins, and phytochemicals) present in the food.[Citation1]
The definition of oxidative stress implies increased oxidant production and/or a decreased antioxidant capacity in animal cells characterized by the release of free radicals, resulting in cellular degeneration. The imbalance between the rate of free radical production and the antioxidant defense causes cellular damage resulting in lipid peroxidation.[Citation2] Free sulfydryl (–SH) groups of proteins are mainly responsible for their antioxidant response, and they can serve as a sensitive indicator of oxidative stress.[Citation3]
Ferulago W. Koch is a medium-sized genus of Umbelliferae comprising about 45 species distributed across part of Europe (W, Central, SW, S, SE, E), Asia (SW, Middle, the Caucasus), and Africa (N, NW).[Citation4] Ferulago species are related in the traditional medicine to Ferula ones, so that they have been used as sedatives, tonic, digestive, in the treatment of intestinal worms and haemorrhoids, as an aphrodisiac, vermifuge, and for carminative disorders.[Citation5] To the best of our knowledge, there are no publications on phenolic content, oxidant/antioxidant status of F. sandrasica.
MATERIALS AND METHODS
Plant Material and Isolation Procedures
Ferulago sandrasica samples at the flowering stage were collected from the Sandras region (1850 m), Beyağaç-Denizli, Turkey, where it is endemic. The taxonomic identification of plant materials was confirmed by Dr. Ali Celik, Department of Biology, Pamukkale University, Denizli, Turkey. Due to its unique nature, we conducted our research with a careful collection, thereby using limited material in order to avoid giving damage to this species. Collected plant materials were dried in the shade, the leaves of plants were separated from the stem, and ground in a grinder with a 3-mm diameter mesh. The voucher specimen has been deposited at the Herbarium of the Department of Biology, Pamukkale University, Denizli, Turkey (Voucher No: AC 176). The essential oil of dried parts of F. sandrasica was obtained via hydrodistillation by using a Clevenger type apparatus for 8 h. The oils were dried over anhydrous sodium sulphate and stored +4°C until required (yield 0.62%).
Gas Chromatography-Mass Spectroscopy (GC-MS) Analysis
The essential oil was analyzed by GC-MS (GC-MS-QP2010 Plus, Shimadzu, Japan). The analysis of the essential oil was also performed using a TRB-5MS capillary column (Teknokroma, Sant. Cugat, Spain; 30 m × 0.25 mm × 0.25 mm film thickness). Helium was used as a carrier gas at a flow rate of 1 to 2 mL/min and constant pressure. The oven temperature was programmed from 50 to 300°C at a rate of 5°C/min. Diluted samples (1/100 in ethyl acetate, v/v) of 1.0 μL were injected by an auto-sampler in the split mode (1/100). Identification of essential oil compounds was based on comparison of their relative retention time and mass spectra with those of commercial standards (for the main components) and retention indices (RI) relative to a C8–C32 n-alkane mixture. The results were also confirmed by computer matching of mass spectra. The relative percentage of the essential oil constituents was calculated from the GC peak areas.
Total Antioxidative Capacity (TAC)
Plasma TAC levels were determined using a novel automated colourimetric measurement method developed by Erel.[Citation6] In this method, the hydroxyl radical, the most potent biological radical, is produced by the Fenton reaction and reacts with the colourless substrate O-dianisidine to produce the dianisyl radical, which is bright yellowish-brown in colour. Upon the addition of a plasma sample, the oxidative reactions initiated by the hydroxyl radicals present in the reaction mix are suppressed by the antioxidant components preventing the colour change and thereby providing an effective measure of the total antioxidant capacity of the sample. The results were expressed as mmol Trolox equivalent/L.
Total Phenolic Content (TPC)
TPC was determined by the Folin–Ciocalteu micro-method.[Citation7] A 200 μL aliquot of oil solution was mixed with 900 μL of distilled water and 100 μL of Folin–Ciocalteu's reagent followed by 800 μL of 75 g L−1 Na2CO3 solution. The mixture was incubated in a shaking incubator at 40°C for 2 h and its absorbance at 760 nm was measured. Gallic acid was used as the standard for the calibration curve. Total phenolic content was expressed as gallic acid equivalent (GAE)/L.
Total Oxidant Status (TOS) of Plasma
TOS of serum was determined using a novel automated measurement method developed by Erel.[Citation8] Oxidants present in the sample oxidize the ferrous ioneo-dianisidine complex to ferric ion. The oxidation reaction is enhanced by glycerol molecules, which are abundantly present in the reaction medium. The ferric ion makes a coloured complex with xylenol orange in an acidic medium. The colour intensity was measured spectrophotometrically related to the total amount of oxidant molecules present in the sample. The assay was calibrated with hydrogen peroxide and the results were expressed as μmol H2O2 equivalent/L.
Lipid Hydroperoxides (LOOHs)
Plasma lipid hydroperoxides were measured by an automated xylenol orange method.[Citation9] Oxidation of Fe (II) to Fe (III) by lipid hydroperoxides, under acidic conditions, was followed by complexation of Fe (III) by xylenol orange. It has been carried out automatically, with two reagents, in a two-end-point mode with bichromatic detection at 570 and 700 nm. The results were expressed as μmol H2O2 equivalent/L.
Total Free Sulfhydryl (TFS) Groups
TFS of samples were assayed according to the method described by Elman[Citation10] and modified by Hu et al.[Citation11] Briefly, 1 ml of buffer containing 0.1 M Tris, 10 mM EDTA, pH 8.2, and 50 μl serum was added to cuvettes, followed by 50 μl 10 mM 5,5’-dithio-bis(2-nitrobenzoic acid) (DTNB) in methanol. Blanks were run for each sample as a test, but there was no DTNB in the methanol. Following incubation for 15 min at room temperature, sample absorbance was read at 412 nm on a Cecil 3000 spectrophotometer (Cecil Instruments, Cambridge, UK). Sample and reagent blanks were subtracted. The concentration of sulfhydryl groups was calculated using reduced glutathione as the free sulfhydryl group standard.
Microbial Strains
The antimicrobial activity of F. sandrasica essential oil was tested using a panel microorganism that included Gram-positive and Gram-negative bacteria. Tested microorganisms were Escherichia coli MC 400, E. coli ATCC 25922, E. coli 0157 H7, E. colaecea ATCC 23355, E. feacalis ATCC 19433, Pseudomonas aeruginosa NRRL B-2679, Staphylococcus aureus ATCC 25923, S. aureus ATCC 33862, Bacillus cereus NRRL B-3711, B. subtulis ATCC 6633, B. subtilis NRRL B-209, B. nischenoformis NRRL B-1001, Micrococcus luteus NRRL B-1013, and Listeria monocytogenes ATCC 7644.
Evaluation of Antibacterial Activity
To determine antimicrobial activity of the essential oil, the disc diffusion method was employed.[Citation12] Bacterial strains were cultivated on Müeller Hinton Broth (OXOID, Unipath Limited, Basingstoke, UK). Briefly, a suspension of the tested microorganism (0.1 ml of 108 cells/ml) was spread on the solid media plates. Then 6-mm sterilised discs (No. 2668, Schleicher and Schuell, Dassel, Germany) were soaked with 0.1 and 0.25 μl of the pure liquid oil placed on the inoculated plates and, after staying at 4°C for 2 h, were incubated at 37°C for 24 h for bacteria. The diameters of the inhibition zones were measured in millimetres. Levofloxacin was used as a positive control: 1 mg of levofloxacin was dissolved in 1 ml sterilized and distilled water and then the sterilized discs were soaked with 25 μl of this solution. All tests were carried out in triplicate.
RESULTS AND DISCUSSION
Chemical Constituents
There are many reports on the chemical composition and antioxidant activity of the essential oils isolated from medicinal plants.[Citation13–17 Citation Citation Citation Citation17 To the best of the authors’ knowledge, there is no publication on the chemical constituents, phenolics, and antioxidant activity of F. sandrasica. The results obtained by GC-MS analysis of the essential oil obtained from F. sandrasica is given in . The oil was colourless, with a weak perfumery odour. Twenty-three compounds amounting to 99.9% were identified in F. sandrasica oil. Data reported in show that the main components of F. sandrasica oil were ocimene (30.5%), carene-δ-3 (27.4%), and α-pinene (17.8%). Monoterpene hydrocarbons (84.8%) dominated the chemical constituents of the investigated oil and sesquiterpens (9.9%), and other hydrocarbons (5.2%) are present in very small quantities.
Table 1 Chemical compounds and composition of the essential oil from Ferulago sandrasica (percentages <0.1% as traces)
Medicinal plant parts are commonly rich in phenolic compounds, such as flavonoids, phenolic acids, stilbenes, tannins, coumarins, lignans, and lignins.[Citation18] They have multiple biological effects including antioxidant activity. The antioxidant properties of phenolic acids and flavonoids are due to their redox properties, ability to chelate metals, and quenching of singlet oxygen.[Citation19]
Determination of Antibacterial Activity of the Essential Oil
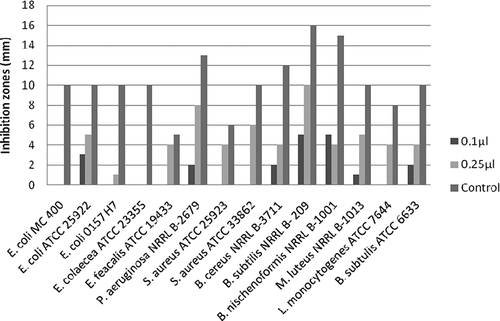
At 0.10 and 0.25 μg/mL concentrations, the antimicrobial activity of essential oil of F. sandrasica tested were determined by a disc diffusion method using 14 bacteria. The results of in vitro antimicrobial activity assay showed that oil possessed broad antimicrobial activity against the microorganisms tested. As clearly seen in , the essential oil demonstrated varying degrees of antibacterial activity, depending on the oil concentration and the tested bacterial strains. The oil showed inhibition zones with diameters of 1–10 mm, depending on the susceptibility of the tested microorganisms. Based on one report, pinene-type monoterpene hydrocarbons (α-pinene and β-pinene) had slight activity against a panel of microorganisms.[Citation20]
Ocimene, found in appreciable amounts in this study, has been found to have antibacterial activity.[Citation21] These findings are in agreement with the results presented here.
Results from the antimicrobial assay indicated that Gram-positive B. subtilis NRRL B-209 was the most sensitive strain to F. sandrasica oil, with an inhibition zone of 5 and 15 mm at 0.1 and 0.25 μg/mL, respectively. On the other hand, E. colaecea ATCC 23355 and E. coli MC 400 showed more resistance against the oil than other bacterial strains. It is also noteworthy that synergistic and/or antagonistic effects may be taken into account for the activity observed in complex systems, such as essential oils.
Table 2 Oxidant-antioxidant status of the essential oil from Ferulago sandrasic a
Determination of the Total Phenolics Content (TPC), Total Oxidant Status (TOS), Total Antioxidant Capacity (TAC), Measurement of Lipid Hydroperoxides (LOOHs), and Measurement of Total Free Sulfhydryl (TFS) Groups
The results of TPC, TOS, TAC, LOOHs, and TFS of F. sandrasica oil are given in . Erel[Citation6] showed that the results of TAC determination method, applied in this study, were highly and significantly correlated with previously described 2,2’-azino-bis-(3-ethylbenzothiazoline-6-sulphonicacid)-based methods and also with most of the other total antioxidant activity determination methods. In the present study, we worked the total antioxidant and oxidant parameters instead of individual antioxidant compounds, which act in combination with each other, affecting total antioxidant capacity producing synergistic or antagonistic effects. As in the case of the total oxidant status, knowledge of total antioxidant capacity, which is the cumulative capacity of antioxidant components to scavenge free radicals, was claimed to be useful for epidemiologic purposes by many researchers.[Citation22] The antioxidant activity may be due to different mechanisms, such as prevention of chain initiation, decomposition of peroxides, prevention of continued hydrogen abstraction, free-radical scavenging, reducing capacity, and binding of transition metal ion catalysts.[Citation23] Plasma TAC is an accurate index of oxidative stress and denotes total plasma defenses against reactive oxygen species.[Citation19] Oxidative stress due to free radicals is related to the pathogenesis of many chronic disorders, including cancer, inflammation, and neurological diseases.[Citation24] Typical phenolics and particularly phenolic acids are a major class of phenolic compounds, widely occurring in the plant kingdom, especially in fruits and vegetables. Since the phenolic compounds are very important constituents of plants and known as powerful chain-breaking antioxidants, total phenolic content of the F. sandrasica oil was investigated and expressed as mmol GAE equivalent/L, as shown in . The amount of total phenolics, measured by Folin–Ciocalteu method[Citation7] was found to be 2.67 mmol GAE equivalent/L. This result suggested that phenolic compounds may be responsible for the antioxidant activity. However, it is obvious that the total phenolic content measured by the Folin–Ciocalteu procedure does not give a full picture of the quality or quantity of the phenolic constituents in the extracts.[Citation25]
Plant phenolics can delay the onset of lipid oxidation and decomposition of hydroperoxides in food products as well as in living tissues. These antioxidants can be concentrated either as crude extracts or individual phenolic compounds to be used in food products, especially highly unsaturated oils.[Citation26] As stressed by Khelifa and Steghens, lipid hydroperoxides appear to be good candidates as initial biomarkers of oxidative stress and they are a large family of the first by-products of oxidized lipids, and their quantification could become a useful biomarker.[Citation9] Lipid hydroperoxides were found to be 172 μmol H2O2 equivalent/L.
TOS is also a significant data for understanding the antioxidant-oxidant balance of the plant essential oil that was found in this study as 201 μmol H2O2 equivalent/L.
Thiols are very susceptible to oxidation and sulfhydryl groups are known to scavenge aqueous peroxyl radicals; they are considered to be one of the most important plasma sacrificial antioxidants.[Citation27] When the organism is exposed to oxidative stress, –SH groups are among the first antioxidants that are consumed.[Citation28] Free sulfydryl (–SH) groups of proteins are mainly responsible for their antioxidant response, and they can serve as a sensitive indicator of oxidative stress.[Citation3]
CONCLUSIONS
It is believed that the results of the present study will contribute to the recent increase in research on using natural products in many areas, such as food, cosmetics, pharmacy, alternative medicine, and natural therapy. Further studies should be carried out for the evaluation of the in vivo potential of this oil in animal models and isolation and identification of individual phenolic compounds, as well. To the best of our knowledge, we herein present the first report on chemical constituents and oxidant/antioxidant status of the F. sandrasica oil.
REFERENCES
- Murcia , M.A. , Jimenez , A.M. and Martinez-Tome , M. 1999 . Vegetables antioxidant losses during industrial processing and refrigerated storage . Food Research International , 42 ( 8 ) : 1046 – 1052 .
- Halliwell , B. and Gutteridge , J.M.C. 1999 . Free Radicals in Biology and Medicine, 3rd Edited by: Halliwell , B. and Gutteridge , J.M.C. 246 – 250 . Oxford , UK Clarendon PressOxford, UK
- Radi , R. , Bush , K.M. , Cosgrove , T.P. and Freeman , B.A. 1991 . Reaction of xanthine oxidase-derived oxidants with lipid and protein of human plasma . Archives of Biochemistry and Biophysics , 286 ( 1 ) : 117 – 125 .
- Pimenov , M.G. and Leonov , M.V. 1993 . The Genera of the Umbelliferae: A Nomenclator , 159 Kew , UK : Royal Botanic Gardens .
- Baytop , T. 1999 . Therapy with Medicinal Plants in Turkey—Past and Present, 2nd , 348 Istanbul , Turkey : Nobel Tip Basimevi .
- Erel , O.A. 2004 . A novel automated method to measure total antioxidant response against potent free radical reactions . Clinical Biochemistry , 37 ( 2 ) : 112 – 119 .
- Slinkard , K. and Singleton , V.L. 1977 . Total phenol analyses: Automation and comparison with manual methods . American Journal of Enology and Viticulture , 28 ( 1 ) : 49 – 55 .
- Erel , O.A. 2005 . A new automated colorimetric method for measuring total oxidant status . Clinical Biochemistry , 38 ( 12 ) : 1103 – 1111 .
- Arab , K. and Steghens , J.P. 2004 . Plasma lipid hydroperoxides measurement by an automated xylenol orange method . Analytical Biochemistry , 325 ( 1 ) : 158 – 163 .
- Ellman , G.L. 1958 . Tissue sulfhydryl groups . Archives of Biochemistry and Biophysics , 82 ( 1 ) : 70 – 77 .
- Hu , M.L. , Louie , S. , Cross , C.E. , Motchnik , P. and Halliwell , B. 1993 . Antioxidant protection against hyochlorous acid in human plasma . Journal of Laboratory and Clinical Medicine , 121 ( 2 ) : 257 – 262 .
- Murray , P.R. , Baron , E.J. , Pfaller , M.A. , Tenover , F.C. and Yolke , R.H. 1995 . Manual of Clinical Microbiology, 6th , 465 Washington , DC : ASM .
- Arslan , I. and Celik , A. 2008 . Chemical composition and antistaphylococcal activity of Salvia chrysophylla Stapf. naturally distributed in Denizli province (Turkey) and its vicinity . Pakistan Journal of Botany , 40 ( 4 ) : 1799 – 1803 .
- Celik , A. , Aydinlik , N. and Arslan , I. 2011 . Phytochemical constituents and ınhibitory activity towards methicillin-resistant Staphylococcus aureus strains of Eryngium species (Apiaceae) . Chemistry & Biodiversity , 8 ( 3 ) : 454 – 459 .
- Celik , A. , Mercan , N. , Arslan , I. and Davran , H. 2008 . Chemical composition and antimicrobial activities of essential oils from Nepetacadmea . Chemisty of Natural Compounds , 44 : 119 – 120 .
- Erge , H.S. and Karadeniz , F. 2011 . Bioactive compounds and antioxidant activity of tomato cultivars . International Journal of Food Properties , 14 ( 5 ) : 968 – 977 .
- Bozalan , N.K. and Karadeniz , F. 2011 . Carotenoid profile, total phenolic content, and antioxidant activity of carrots . International Journal of Food Properties. , 14 ( 5 ) : 1060 – 1068 .
- Cai , Y. , Luo , Q. , Sun , M. and Corke , H. 2004 . Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer . Life Sciences , 74 ( 17 ) : 2157 – 2184 .
- Rice-Evans , C. and Miller , N.J. 1994 . Total antioxidant status in plasma and body fluids . Methods Enzymology , 234 : 279 – 293 .
- Dorman , H.J.D. and Deans , S.G. 2000 . Antimicrobial agents from plants: Antibacterial activity of plant volatile oils . Journal of Applied Microbiology , 88 ( 2 ) : 308 – 316 .
- Juteau, F.; Jerkovic, I.; Masotti, V.; Milos, M.; Mastelic, J.; Bessiére, J.M.; Viano, J. Composition and antimicrobial activity of the essential oil of Artemisia absinthium from Croatia and France. Planta Med. 2003, 69, 158–161.
- Celik , A. , Herken , E.N. , Arslan , I. , Özel , M.Z. and Mercan , N. 2010 . Screening of the constituents, antimicrobial and antioxidant activity of endemic . Origanumhypericifolium O. Schwartz & P.H. Davis. Natural Product Research , 24 ( 16 ) : 1568 – 1577 .
- Mao , L.C. , Pan , X. , Que , F. and Fang , X.H. 2006 . Antioxidant properties of water and ethanol extracts from hot air-dried and freeze-dried daylily flowers . European Food Research and Technology , 222 : 236 – 241 .
- Tsubota , K. 2007 . Oxidative stress and inflammation: Hypothesis for the mechanism of aging . Nippon Ganka Gakkai Zasshi , 111 ( 3 ) : 193 – 205 .
- Wu , X. , Beecher , G.R. , Holden , J.M. , Haytowitz , D.B. , Gebhardt , S.E. and Prior , R.L. 2004 . Lipophilic and hydrophilic antioxidant capacities of common foods in the United States . Journal of the Agricultural and Food Chemistry , 52 ( 12 ) : 4026 – 4037 .
- Wettasinghe , M. and Shahidi , F. 1999 . Antioxidant and free radical scavenging properties of ethanolic extracts of defatted borage (Boragoofficinalis L.) seeds . Food Chemistry , 67 : 339 – 414 .
- Wayner , D.D.M. , Burton , G.W. , Ingold , K.U. , Barclay , L.C. and Locke , S.J. 1987 . The relative contributions of vitamin E, urate, ascorbate and proteins to the total peroxyl radical-trapping antioxidant activity of human blood plasma . Biochimica Biophysica Acta , 924 : 408 – 419 .
- Frei , B. , Stocker , R. and Ames , B.N. 1992 . Molecular Biology of Free Radical Scavenging Systems , 23 – 45 . New York : Cold Spring Harbor .