Abstract
Ovotransferrin is one of the major allergens in egg white. To define the properties of ovotransferrin, a simple protocol for ovotransferrin purification was developed and a broad physicochemical and immunological assay were used for the characterization. The prepared ovotransferrin revealed a single band on sodium dodecyl sulfate polyacrylamide gel electrophoresis, and had an estimated purity of more than 97%. The identity of the prepared protein identified by mass spectrometry analysis demonstrated that it was iron-free ovotransferrin with a molecular weight of 77,726 Da and pI value of 6.85, respectively, and its nature folded structure was kept well as defined by circular dichroism spectroscopy. Western blotting and enzyme-linked immunosorbent assays showed strong immune reactions between the prepared ovotransferrin and rabbit polyclonal antibody against ovotransferrin/egg-allergic patients' sera, suggesting that the prepared ovotransferrin bound to IgG and IgE strongly. In addition, 24 of the potential linear IgE binding epitopes on the prepared ovotransferrin were predicted using a web-based allergen database for food safety. The preparation method of ovotransferrin in the present work might provide scientific support to the authenticity of ovotransferrin for developing certified reference materials.
INTRODUCTION
Egg allergy is recognized as a common problem all over the world, and its prevalence has been an increasing trend.[Citation1] The molecules in egg that are capable of eliciting an allergic response in sensitive people are known as egg allergens. Currently, pure egg allergens are required as tools for diagnosis and treatment of egg allergies, as well as certified reference materials for their quantification in foods. Moreover, to better understand the relationship between allergenicity and protein structure, stability, food matrix, and processing, a well-defined food allergen is highlighted.
The dominating allergens from egg are the four egg white proteins: ovomucoid (OVM, Gal d 1, 11% of egg white protein), ovalbumin (OA, Gal d 2, 54% of egg white protein), ovotransferrin (OVT, Gal d 3, 12% of egg white protein), and lysozyme (Lys, Gal d 4, 3.5% of egg white protein).[Citation2 − Citation7] Among these egg allergens, OVT, also called conalbumin, which can be recognized by 22% of egg-allergic patients,[Citation8] is a heat-labile protein with a molecular weight (MW) of 78 kDa, and it contains 2.6% of carbohydrate content and 15 disulfide bonds without free sulfhydryl groups.[Citation9 − Citation12] Generally speaking, OVT is known for its antimicrobial activity, antioxidant property, immunomodulating effects, and novel anticancer activity,[Citation13 − Citation18] and it is widely used as a functional ingredient in foods, especially in infant foods.
The properties of prepared OVT were once characterized by Jacobsen and his colleagues,[Citation1] however, the OVT was contained with degradation fragments derived from itself and with more ferric-OVT than existed in egg white because of their purification protocol. For this reason, the characteristics of prepared OVT showed part of the characterizations of impurities. Therefore, in this study, we aimed to prepare OVT with high purity, and characterized its properties using a broad physicochemical and immunological array.
MATERIALS AND METHODS
Materials and Chemicals
Fresh hen eggs were purchased from a local market. Commercial OVT, complete Freund's adjuvant, incomplete Freund's adjuvant, Tween-20, goat anti-rabbit IgG conjugated with horseradish peroxidase (HRP), anti-human IgE-peroxidase antibody produced in goat, О-phenylene diamine (OPD), and 3,3′,5,5′-tetramethylbenzidine (TMB) were obtained from Sigma Chemical Co., (St. Louis, MO, USA). All other chemicals used in the experiments were of analytical grade.
Pretreatment of Egg White
Pretreatment of egg white was performed according to a modified method of Croguennec and his colleagues.[Citation19] After the separation from egg yolk, 65 ml of egg white was first diluted with 2 volumes of distilled water and the mixture was adjusted to pH 6.0 with 2 mol/L hydrochloric acid, followed by being gently stirred for 3.5 h at 4°C enabling ovomucin precipitation. The mixture developed a white and gelatinous precipitate, which was later removed by centrifugation at 3000 × g for 5 min at 4°C. The supernatant was adjusted to pH 8.0 with 3 mol/L sodium hydroxide, followed by centrifugation at 10,000 × g for 20 min at 4°C in order to remove insoluble materials. The supernatant was collected as “mucin-free” egg white (“mucin-free” EW) for further use.
Ovotransferrin Isolation by Ion Exchange Column Chromatography
The chromatography used to purify OVT was performed with DEAE-Sepharose Fast Flow anion exchanger purchased from GE (Schenectady, NY, USA). The gel was prepared in a XK 50/20 column (30 × 1.2 cm) (Pharmacia Biotech AB, Uppsala, Sweden) carried out following the procedure the producer provided. The column was previously equilibrated with 0.05 mol/L Tris-HCl buffer (pH 8.0), followed by loading 4 ml of the “mucin-free” EW onto the column. Non-retained proteins were washed out by the same buffer, and the proteins that retained on the column were eluted by a linear gradient using 0–0.5 mol/L sodium chloride in 0.05 mol/L Tris-HCl buffer (pH 8.0) at a rate of 1.0 ml/min. The absorbance of the fractions was determined at 280 nm, while the purity was identified by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).
Polyacrylamide Gel Electrophoresis
SDS-PAGE was performed as described by Laemmli[Citation20] with a Bio-Rad Mini Protean II system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). SDS-protein samples were heated at 100°C for 5 min. Electrophoresis was carried out at 10 mA in 5% stacking gel and 30 mA in 12% separating gel for 1 h using an electrophoretic buffer of Tris-Glycine containing 0.1% SDS. The gel was stained with Coomassie Blue R250 solution for 1 h, followed by destaining with a solution of 87.5% distilled water, 5% methanol, and 7.5% acetic acid. The identity of the band was then measured by mass spectrometry (MS).
Identity Analysis
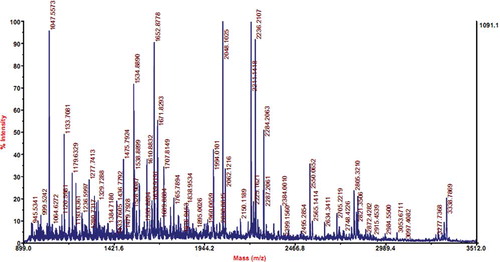
The accurate molecular weight and sequence analysis of the prepared OVT was performed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) analysis on an Applied Biosystems 4700 MALDI-TOF/TOF Analyzer (Applied Biosystems Inc., Framingham, MA, USA) after an in-solution tryptic digest of the purified protein. A 0.4-μl aliquot of the concentrated tryptic peptide mixture in 0.1% trifluoroacetic acid (TFA) was mixed with 0.4 μl of α-cyano-4-hydroxycinnamic acid (CHCA) matrix solution (0.026 mol/L CHCA in 50% ACN/0.1% TFA) and spotted onto a 384 well of MALDI steel target plate (Bruker Daltonik GmbH, Bremen, Germany). Each sample was spotted in triplicate on the plate and was left to air dry. After air drying, the crystallized spots were analyzed on the Applied Biosystems 4700 MALDI-TOF/TOF Analyzer (Applied Biosystems Inc.). MS calibration was automatically performed by a peptide standard kit (Applied Biosystems Inc.) containing Angiotensin II (MW 1046.5420), Angiotensin I (MW 1296.6853), Substance P (MW 1347.7361), Bombesin (MW 1619.8230), ACTH clip 1-17 (MW 2093.0868), ACTH clip 18-39 (MW 2465.1990), and Somatostatin 28 (MW 3147.4714). All mass spectra were recorded in the reflector positive mode using a laser operated at a 200 Hz repetition rate with a wavelength of 355 nm. The accelerated voltage was operated at 20 kV. Neither baseline subtraction nor smoothing was applied to recorded spectra. MS and MS/MS data were analyzed and peak lists were generated using GPS Explorer 3.6 (Applied Biosystems Inc.). MS peaks were selected between 900 and 3500 Da and filtered with a signal to noise ratio greater than 50. MS and MS/MS data were analyzed using a MASCOT™ 2.1 search engine (Matrix Science, London, UK) to search against the Homo sapiens protein sequence database downloaded from the International Protein Index (IPI) database and SwissProt database. All identifications presented were significant (p < 0.05).
Circular Dichroism (CD) Analysis
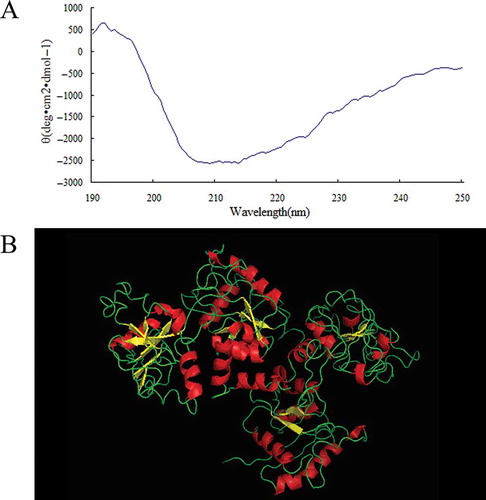
To define the secondary structure of the prepared OVT, Far-UV CD spectroscopy was performed in the spectral range from 190 to 250 nm. Protein concentrations of 3.40 × 10−6 mol/L were used for analysis. Spectrum was recorded on a JASCO J810 spectropolarimeter (Jasco, Groß-Umstadt, Germany) at room temperature. A scan speed at 100 nm per min, a bandwidth of 1 nm, and a response time of 1–4 s were used. The CD spectrum was measured three times and the results were averaged, followed by the molar residue ellipticity (θ) being expressed as deg·cm2·dmol−1, and the contents of different secondary structures were calculated with JASCO secondary structure software.
Preparation of Antisera Against Ovotransferrin
Antisera against OVT were produced from four female New Zealand rabbits (2.5 kg ± 0.3 kg) by injection of the prepared OVT. Two other rabbits were used as control. All rabbits were accommodated for a week after arrival at the facility (Institute of Occupational Medicine of Jiangxi, the permission number is “SYXK (Gan) 2008-003”), and they were maintained under standard animal housing conditions with free access to food and water. The four rabbits were pre-bled and received 1 ml of protein solution emulsified with an equal volume of complete Freund's adjuvant by subcutaneous injections. Control rabbits were injected with normal saline (0.9% w/v sodium chloride). Two weeks after the first injection, a booster immunization was given with the same amount of antigens using incomplete Freund's adjuvant. The rabbits were re-stimulated four times with the same amount as the secondary immunization at a two weeks' interval. One week after the last injection, the rabbits were bled to produce the blood samples, which were stored at 4°C overnight to form a clot. Antisera were collected from each blood sample by 30 min centrifugation at 3500 × g at 4°C after clot formation, followed by storing at −70°C until use.
Egg Allergic Patients' Sera
Human sera were collected from four egg allergic patients. Their specific IgE level against egg proteins and total IgE level were determined by AllergyScreen (MEDIWISS Analytic, Moers, Germany). The four patients were numbered p1–p4, respectively, with an average age of 9.375 years. Among them, p1 and p4 had a case history of anaphylactic reactions to egg, p2 had an eczema, and p3 had atopic dermatitis, whose total IgE level was more than 200 kU/L, higher than the others (<200 kU/L, data not shown). gives a brief summary of each patient's clinical history. All serum samples were kindly provided by Dr. Luo from the Department of Paediatrics, The First Affiliated Hospital of Guangxi Medical University (Guangxi, China) and stored at −80°C until use. As negative controls, sera of three healthy people without egg allergy were used, numbered c1–c3, respectively.
Table 1 Clinical history of egg white allergic patients' sera
Assays for Measurement of IgG Binding Ability of Ovotransferrin
To determine the IgG binding ability of the prepared OVT, an indirect enzyme-linked immunosorbent assay (ELISA) was developed. One hundred microliters of the prepared OVT (1.92 × 10−8 mol/L) in 50 mM sodium carbonate buffer, pH 9.6, was added to each well of a 96-well ELISA microplate. The plate was incubated overnight at 4°C, followed by washing for three times with phosphate buffered saline (PBS) containing 0.05% Tween-20 (PBST), and the plate was blocked with 5% skimmed milk in PBS for 1 h at 37°C, followed by washing for three times with PBST. Then, 100 μl of antisera diluted serially in PBS were added to the wells and incubated for 1 h at 37°C. After further washing the wells for three times, 100 μl of goat anti-rabbit IgG conjugated with horseradish peroxidase (HRP) diluted with PBST to 1:10,000 was transferred to the wells, followed by incubation for 1 h at 37°C. Once again, the plate was further washed for three times with PBST, and the color was developed in 100 μl of О-phenylene diamine (OPD) dissolved in citrate buffer by incubation for 20 min at 37°C. The reaction was stopped by adding 50 μl of 2 mol/L sulphuric acid to each well, and the optical density was determined at 490 nm using a Bio-Rad Microplate Reader (Bio-Rad Laboratories, Inc.).
In addition, an indirect competitive ELISA was carried out to evaluate antigenicity of OVT. This assay was performed almost the same as above except the step of the addition of the rabbit sera. At this step, 50 μl of antisera against the prepared OVT (diluted to 1:100,000) and 50 μl of various concentrations of commercial OVT (1.28 × 10−6 mol/L, 1.28 × 10−7 mol/L, 1.28 × 10−8 mol/L, 1.28 × 10−9 mol/L, 1.28 × 10−10 mol/L) were pre-incubated in another uncoated microtiter plate for 1 h at 37°C, then the mixture of each pre-incubated sample was transferred to the coated wells for competition with the prepared OVT at 37°C for 1 h. The other procedures were the same as described in indirect ELISA. The inhibition curve was plotted in a form of (B0 - B)/B0 × 100% vs. logC, where B and B0 are the absorbance of the competitor at the standard point and at zero concentration of the competitor, respectively.
Indirect ELISA to Determine IgE Binding Ability of Purified Ovotransferrin
The assay protocol was similar to the IgG binding ability test above. ELISA microplate was coated, blocked, and washed, followed by incubation at 37°C for 2 h with human serum (diluted to 1:20 in PBS). The plate was washed and incubated at 37°C for 1 h with anti-human IgE-peroxidase antibody produced in goat diluted to 1:1200 in PBS. Following washing three times with PBST, the plate was developed in 100 μl substrate solution of 3,3′,5,5′-tetramethylbenzidine (TMB) by incubating for 15 min at 37°C. The reaction was terminated by the addition of 50 μl of 2 mol/L sulphuric acid, and the optical density was determined at 450 nm using the Bio-Rad Microplate Reader (Bio-Rad Laboratories, Inc.).
Western Blotting
The protein samples were electrophoresed as described above and electrotransferred to polyvinylidene fluoride (PVDF) membranes with a current of 50 mA for 2 h. After PVDF membranes were blocked with 5% skimmed milk in tris-buffered saline (TBS, 0.02 mol/L Tris-HCl, 0.5 mol/L NaCl, pH 7.5) for 1 h at 37°C, the pooled rabbits antiserum against the prepared OVT diluted to 1:10,000 in TBS was added and incubated for 1 h at 37°C. The membrane was washed three times for 10 min with 0.05% Tween-20 in TBS, followed by incubation for 1 h at 37°C with goat anti-rabbit IgG/HRP diluted to 1:5000 in TBS. Finally, the immunoblotting images were developed on the membrane using 6 mg methoxy-naphthol dissolved in 2 ml of methanol and 10 ml of TBS with 6 μl of 30% H2O2 after the membrane was washed for three times with TBST.
Prediction of IgE-Binding Epitopes of the Prepared Ovotransferrin
The information on IgE epitopes on the prepared OVT was predicted using an online database ADFS (allergen database for food safety, http://allergen.nihs.go.jp/ADFS/) by blasting the sequence with 91 epitope-known allergens.
Statistical Analysis
All the experiments were performed in triplicate. Data were analyzed by using analysis of variance (SPSS version 10.0 for windows; SPSS Inc., Chicago, IL, USA).
RESULTS AND DISCUSSION
Ovotransferrin Isolation
In many previous studies, OVT was purified from egg white using anion exchange chromatography. Unfortunately, all of the operators faced a major problem that almost all egg white proteins were bound to resin sites, resulting in few resin sites available for OVT.[Citation19,Citation21,Citation22] Among the egg white proteins, ovomucin is easy to precipitate into the column and has a significant negative effect on the isolation of OVT. Consequently, we fractionated a “mucin-free” EW as starting material for ion exchange chromatography by adjusting the pH to 6.0 in order to precipitate ovomucin. Part of the lysozyme and ovalbumin were also co-precipitated during a “mucin-free” step, while the content of OVT was hardly influenced (data were not shown). Moreover, many other factors drive a column-based separation, such as elution buffer, sodium chloride gradient, and fluid linear velocity. To find the optimal elution condition, plenty of exploring experiments were carried out, and finally, the linear gradient elution using 0–0.5 mol/L sodium chloride in 0.05 mol/L Tris-HCl buffer (pH 8.0) at a rate of 1.0 ml/min was found to be optimal for preparing OVT with high purity.
An amount of 4 ml of “mucin-free” EW containing about 202 mg of total protein was applied to the DEAE-Fast Flow anion-exchange chromatography column, and it was then eluted as described in the methods. The elution profiles in a linear gradient using 0–0.5 mol/L sodium chloride in 0.05 mol/L Tris-HC1 buffer (pH 8.0) shows four major peaks (). Peak a appeared at 0.048 mol/L of sodium chloride, and the total content of collected protein was 21.28 mg. SDS-PAGE analysis of proteins contained in each peak () reveals that peak a had a single protein band with an estimated MW of 78 kDa, and corresponded to the reported MW of OVT.[Citation11]
Figure 1 DEAE-Sepharose Fast Flow anion-exchange chromatography for purifying OVT. (a) The elution profile of “mucin-free” egg white on DEAE-Sepharose Fast Flow column in a linear gradient using 0–0.5 mol/L sodium chloride in 0.05 mol/L Tris-HCl buffer (pH 8.0). (b) SDS-PAGE patterns of protein fractions on 12% separating gel stained with Coomassie Blue R250. Lane M: prestained markers; lane 1: peak a; lane 2: peak b; lane 3: peak b′; lane 4: peak c; lane 5: peak d.
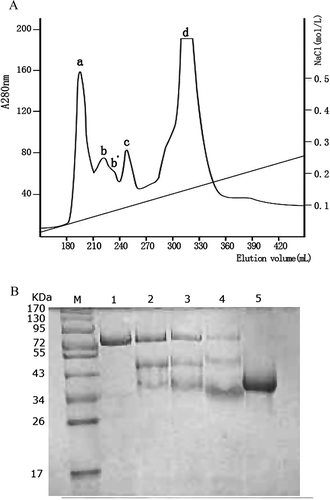
The purity of isolated OVT, compared with commercial OVT (Sigma, purity >95%), was analyzed by SDS-PAGE shown in . When the same amount of 3 μg protein were loaded onto the gel, there were extra bands located both in higher and lower position of OVT in the commercial OVT sample (, lane 2) , while only one band existed in the prepared OVT sample (, lane 1). Moreover, the purity of prepared OVT was estimated to be more than 97%, and the yield for OVT purification was 87.57% ().
Table 2 The yield of OVT by ion exchange chromatography
Figure 2 SDS-PAGE patterns of prepared and commercial OVT (stained with Coomassie Brilliant Blue R250). Lane M: prestained marker; lane 1: prepared OVT; lane 2: commercial OVT (Sigma, purity >95%). The loading amount of each OVT sample was 3 μg.
All the results demonstrated that this method was efficient for egg OVT purification with high purity, high recovery rate, relatively low cost, and easy performance. It could conclude that a single step anion exchange chromatography for purifying OVT was successfully developed, and it is suitable for laboratory use of OVT preparation.
Identity of the Prepared Ovotransferrin
The identity of the prepared OVT was identified by MS analysis. MALDI-TOF mass spectrometry () shows that the protein sample collected from peak a () had a molecular weight of 77,726 Da and pI value of 6.85, respectively. Sequence blasting analysis shows that the testing sample had high homology with human lactotransferrin and TRFE_CHICK, an OVT precursor in Gallus gallus (chicken), with the scores of 173 and 1199, respectively. Because the protein sample was extracted from hen's egg, the prepared protein was identified to be OVT. Moreover, since the protein pI values 6.4, 6.6, and 6.8 corresponded to the OVT contain 2, 1, and no ferric ions, respectively, as reported by Desert et al.,[Citation23] we could conclude that the prepared OVT in this work was iron-free OVT and represented the main form existing in egg white.
The Structure Characterization of the Prepared Ovotransferrin by CD Spectroscopy
The secondary structure of the prepared OVT was measured by CD. gives the prepared OVT a CD profile of an α-helical structure with double negative peaks at 208 and 222 nm, respectively, and a positive peak at around 192 nm, a β-sheet structure with a maximum at 195 nm and a negative minimum at 215 nm, and a random coil structure with the unsmooth line. The estimated α-helix, β-sheet, β-turn, and random coil content of the prepared OVT calculated by JASCO secondary structure software are 17.9, 28.8, 17.3, and 36.0%, respectively. Although the secondary structure contents may have a little discrepancy with Kurokawa et al.'s report,[Citation24] the α-helical and β-sheet structure contents indicates that the prepared OVT was folded. In addition, since the prepared OVT was iron free, and the sequence identified by MS analysis had 100% identity with the apo-OVT 1AIV (PDB DOI number: 10.2210/pdb1aiv/pdb) when blasted with the reported sequences in PDB database, the three dimensional (3-D) structure of the prepared OVT was presumed to be the same as 1AIV shown in .
Immunological Characterization of the Prepared Ovotransferrin
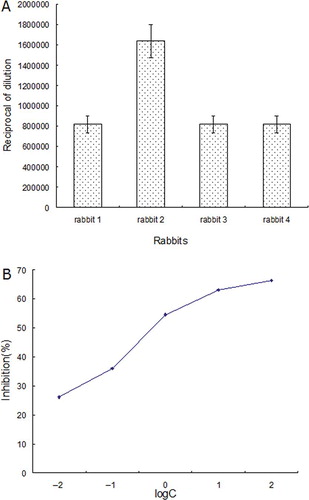
The IgG binding to the prepared OVT was defined by indirect ELISA, western blotting, and indirect competitive ELISA, respectively. shows the rabbits antisera IgG binding to the prepared OVT by ELISA assays. In indirect ELISA, the antisera were diluted serially in PBS, and then added to the wells to react with the prepared OVT. The dilution of antisera for the last positive reaction with the prepared OVT was shown in . From the bars, it was found that the prepared OVT could react with rabbit antisera IgG even when the dilution was higher than 1:819,200, suggesting that the prepared OVT had a strong IgG binding (). Regarding the indirect competitive ELISA (), commercial OVT was used as the competitor and the concentration necessary for achieving 50% ELISA competition was 0.78 × 10−8 mol/L, indicating the IgG binding reactivity to OVT was very sensitive and strong. It also suggests that the prepared OVT may be a good reagent for quantifying OVT content in food by using indirect competitive ELISA assay.
Figure 5 The IgG binding properties of prepared OVT. (a) The highest dilution of rabbits antisera reacted to the prepared OVT defined by ELISA. Data are expressed as mean ± standard deviation (X ± SD). (b) Indirect competitive ELISA curve of OVT. A series of concentrations of the commercial OVT from 10−2 to 100 μg/ml (from 1.28 × 10−10 mol/L to 1.28 × 10−6 mol/L) was used as the competitor. Data are represented as mean ± standard deviation (X ± SD). (Color figure available online.)
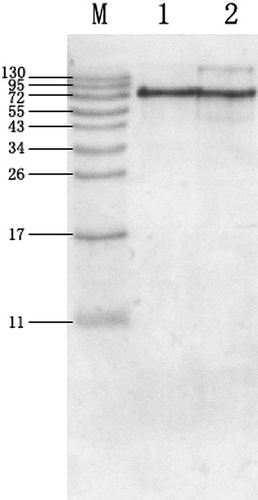
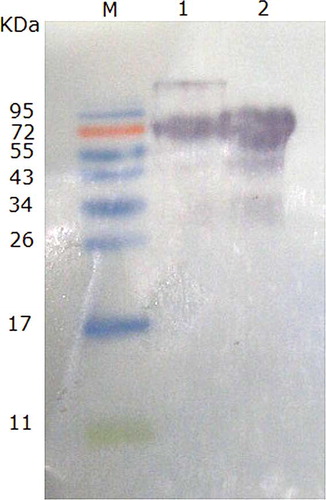
Western blot analysis using rabbit polyclonal antibody against OVT shows positive reactions with both commercial OVT and the prepared OVT (), supporting that the prepared OVT had IgG binding capacity. Since commercial OVT contaminates with other proteins, as shown in , additional IgG binding images existed in the commercial OVT sample. Strangely, some smaller immunoblotting images appeared in the prepared OVT, too. One possible reason was that OVT was easy to break down into smaller fragments, which could also bind to the antibody.[Citation1]
Figure 6 Western blotting of commercial OVT and the prepared OVT reacted with rabbits polyclonal antisera. M: prestained marker; 1: commercial OVT (Sigma); 2: prepared OVT. (Color figure available online.)
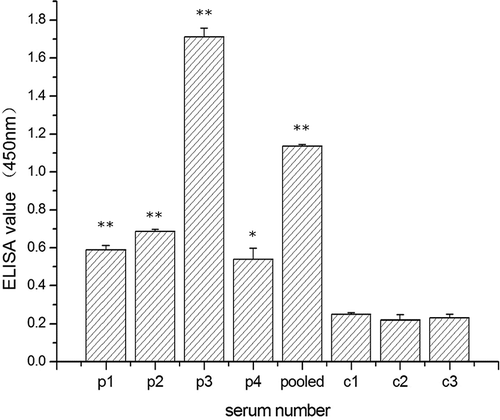
To evaluate the IgE binding to the prepared OVT, sera samples from four egg allergic patients and three non allergic individuals as controls were chosen for use. In comparison with the control sera, all patient sera and their pooled sera showed significant positive IgE binding reaction to the prepared OVT (), suggesting that the prepared OVT contain the IgE-binding epitopes. The reaction potencies varied largely among the four sera samples. The IgE binding reaction between the prepared OVT and the sera of p3 was the highest, followed by the pooled sera, and the sera of p1, p2, and p4 showed similar lower IgE binding to the prepared OVT. The individual differences of the IgE binding reaction may be due to the different recognition of the four patients' sera by the prepared OVT. Moreover, when compared with the control group, the IgE binding activities of the prepared OVT were all highly significant (p < 0.001) with each single patient's serum and their pooled sera, except the binding with the sera of p4 (p < 0.05), suggesting that the prepared OVT had a strong binding to the specific IgE.
Figure 7 The IgE binding to the prepared OVT defined by ELISA. Four individual sera from egg-allergic patients and their pooled sera were used for testing the IgE binding to the prepared OVT, and three normal human sera were used as control. p1–p4 represent egg allergy patients numbered 1, 2, 3, and 4, respectively; pooled represents the sera pool of the four patients; c1–c3 represent three normal people as control. Data are represented as mean ± standard deviation (X ± SD). The asterisk indicates that the IgE of the patient sera binding to prepared OVT is significantly different from that of the control sera (p < 0.05). Double asterisks means the significant level is p < 0.001.
It is well demonstrated that IgE-binding epitopes on allergens are the hallmark of allergic reactions, and an epitope is the part of a macromolecule that is recognized by antibodies, including linear epitopes and conformational epitopes. Since allergens usually lose conformational integrity during digestion, linear epitopes are considered to be more important in class I food allergy.[Citation25] Currently, ADFS database (allergen database for food safety) is free for use, containing 1285 of registered allergen sequences and 91 of epitope-known allergens, and it also involves information on allergen structures and many computational tools for defining allergenicity. Therefore, linear epitopes on OVT can be simply predicted using a web-based ADFS database. After blasting the sequence of the prepared OVT with 608 sequences of the 91 epitope-known allergens in ADFS database, 24 peptides within OVT are matched with the known IgE epitopes, and the location and amino acids sequence of each peptide are shown in . The matched peptides indicate that the 24 peptides are the potential linear IgE epitopes on the prepared OVT. It might be the first time to report the IgE epitopes on OVT. Nevertheless, an experimental work is strongly recommended, if possible, to confirm the predicted IgE epitopes on OVT due to no reliable way to exactly predict B-cell epitopes based solely on the sequence or the three-dimensional structure of a protein.[Citation26]
Table 3 The predicted linear epitopes on purified OVT
Reliable diagnosis of egg allergy and allergen testing in food is dependent on the analytes used, and only well-characterized materials could meet this need. In the present study, the characteristics of OVT outlined here provide a detailed property of protein structure and immunological reaction, resulting in better understanding OVT as an egg allergen. The defined OVT may be used to set up allergen specific diagnostic assays and to screen egg-allergic patients' sera for the development of patient-tailored OVT-specific immunotherapy. Moreover, this prepared OVT could be employed for the reliable determination of OVT in foods.
CONCLUSION
An easily performed purification procedure was developed to purify OVT, and detailed physicochemical and immunological properties, including purity and identity, protein folding, IgG binding and IgE binding, and predicted epitopes of the prepared OVT were well defined in this study. The purification procedure outlined here provides OVT with a high purity and high recovery rate of 97 and 87.57%, respectively, and maintained its immunological reactivities throughout the purification procedure. The availability of such information of OVT will be useful to standardize the OVT for developing certified reference material.
ACKNOWLEDGMENTS
The work was supported by the National Natural Science Foundation of China (No. 31060215), National High Technology Research and Development Program of China (863 Program, No. 2013AA102205), Specialized Research Fund for the Doctoral Program of Higher Education, the Research Program of State Key Laboratory of Food Science and Technology (Nos. SKLF-ZZA-201302 and SKLF-ZZB-201302), and Jiangxi Provincial Science and Technology Support Project (No. 20122BBG701702).
REFERENCES
- Jacobsen , B. , Hoffmann-Sommergruber , K. , Have , T.T. , Foss , N. , Briza , P. , Oberhuber , C. , Radauer , C. , Alessandri , S. , Knulst , A.C. , Fernandez-Rivas , M. and Barkholt , V. 2008 . The panel of egg allergens, Gal d 1-Gal d 5: Their improved purification and characterization . Molecular Nutrition and Food Research , 52 ( 2 ) : 176 – 185 .
- Anet , J. , Back , J.F. , Baker , R.S. , Barnett , D. , Burley , R.W. and Howden , M.E. 1985 . Allergens in the white and yolk of hen's egg . A study of IgE binding by egg proteins. International Archives of Allergy and Applied Immunology , 77 ( 3 ) : 364 – 371 .
- Bernhisel-Broadbent , J. , Dintzis , H.M. , Dintzis , R.Z. and Sampson , H.A. 1994 . Allergenicity and antigenicity of chicken egg ovomucoid (GaldI) compared with ovalbumin (GaldII) in chicken with egg allergy and in mice . Journal of Allergy and Clinical Immunology , 93 : 1047 – 1059 .
- Hoffman , D.R. 1983 . Immunochemical identification of the allergens in egg white . Journal of Allergy and Clinical Immunology , 71 ( 5 ) : 481 – 486 .
- Langeland , T. 1982 . A clinical and immunological study of allergy to hen's egg white. III. Allergens in hen's egg white studied by crossed radio-immunoelectrophoresis (CRIE) . Allergy , 37 ( 7 ) : 521 – 530 .
- Langeland , T. 1983 . A clinical and immunological study of allergy to hen's egg white . IV. Specific IGE-antibodies to individual allergens in hen's egg white related to clinical and immunological parameters in egg-allergic patients. Allergy , 38 ( 7 ) : 493 – 500 .
- Mine , Y. and Yang , M. 2008 . Recent advances in the understanding of egg allergens: Basic, industrial, and clinical perspectives . Journal of Agricultural and Food Chemistry , 56 ( 13 ) : 4874 – 4900 .
- Djurtoft , R. , H.S. , Pedersen , B. , Aabin and Barkholt , V. 1991 . Studies of food allergens: Soybean and egg proteins . Advances in Experimental Medicine and Biology , 289 : 281 – 293 .
- Dev , S.R.S. , Orsat , V. , Gariepy , Y. , Raghavan , G.S.V. and Ruiz-Feria , C. 2010 . Selected post-heating properties of microwave or hot water heated egg white for in-shell pasteurization . International Journal of Food Properties , 13 ( 4 ) : 778 – 788 .
- Ibraham , H.R. 2000 . Ovotransferrin: Chemistry and Antimicrobial Function , 211 – 226 . Boca Raton , Florida : CRC Press .
- Mine , Y. 1995 . Recent advances in the understanding of egg-white protein functionality . Trends in Food Science & Technology , 6 ( 7 ) : 225 – 232 .
- Williams , J. , Elleman , T.C. , Kingston , I.B. , Wilkins , A.G. and Kuhn , K.A. 1982 . The primary structure of hen ovotransferrin . European Journal of Biochemistry , 122 ( 2 ) : 297 – 303 .
- Giansanti , F. , Rossi , P. , Massucci , M.T. , Botti , D. , Antonini , G. , Valenti , P. and Seganti , L. 2002 . Antiviral activity of ovotransferrin discloses an evolutionary strategy for the defensive activities of lactoferrin . Biochemistry and Cell Biology , 80 ( 1 ) : 125 – 130 .
- Ibrahim , H.R. and Kiyono , T. 2009 . Novel anticancer activity of the autocleaved ovotransferrin against human colon and breast cancer cells . Journal of Agricultural and Food Chemistry , 57 ( 23 ) : 11383 – 11390 .
- Ibrahim , H.R. , Hoq , M.I. and Aoki , T. 2007 . Ovotransferrin possesses SOD-like superoxide anion scavenging activity that is promoted by copper and manganese binding . International Journal of Biological Macromolecules , 41 ( 5 ) : 631 – 640 .
- Mine , Y. 2007 . Egg proteins and peptides in human health—Chemistry, bioactivity and production . Current Pharmaceutical Design , 13 ( 9 ) : 875 – 884 .
- Nakai , S. , Chanput , W. and Theerakulkait , C. 2010 . Introduction of new computer softwares for classification and prediction purposes of bioactive peptides: Case study in antioxidative tripeptides . International Journal of Food Properties , 13 ( 5 ) : 947 – 959 .
- Valenti , P. , Visca , P. , Antonini , G. and Orsi , N. 1985 . Antifungal activity of ovotransferrin towards genus Candida . Mycopathologia , 89 ( 3 ) : 169 – 175 .
- Croguennec , T. , Nau , F. , Pezennec , S. , Piot , M. and Brule , G. 2001 . Two-step chromatographic procedure for the preparation of hen egg white ovotransferrin . European Food Research and Technology , 212 ( 3 ) : 296 – 301 .
- Laemmli , U.K. 1970 . Cleavage of structural proteins during the assembly of the head of bacteriophage T4 . Nature , 227 ( 5259 ) : 680 – 685 .
- Awade , A.C. , Moreau , S. , Molle , D. , Brule , G. and Maubois , J.L. 1994 . Two-step chromatographic procedure for the purification of hen egg white ovomucin, lysozyme, ovotransferrin and ovalbumin and characterization of purified proteins . Journal of Chromatography A , 677 ( 2 ) : 279 – 288 .
- Vachier , M.C. , Piot , M. and Awade . A.C. Isolation of hen egg white lysozyme . ovotransferrin and ovalbumin, using a quaternary ammonium bound to a highly crosslinked agarose matrix. Journal of Chromatography B: Biomedical Sciences and Applications 1995 , 664 ( 1 ) 201 – 210 .
- Desert , C. , Guerin-Dubiard , C. , Nau , F. , Jan , G. , Val , F. and Mallard , J. 2001 . Comparison of different electrophoretic separations of hen egg white proteins . Journal of Agricultural and Food Chemistry , 49 ( 10 ) : 4553 – 4561 .
- Kurokawa , H. , Mikami , B. and Hirose , M. 1995 . Crystal-structure of diferric hen ovotransferrin at 2.4 Angstrom resolution . Journal of Molecular Biology , 254 ( 2 ) : 196 – 207 .
- Harrer , A. , Egger , M. , Gadermaier , G. , Erler , A. , Hauser , M. , Ferreira , F. and Himly , M. 2010 . Characterization of plant food allergens: An overview on physicochemical and immunological techniques . Molecular Nutrition and Food Research , 54 ( 1 ) : 93 – 112 .
- Reimer , U. , Reineke , U. and Schutkowski , M. 2009 . “ Prediction of linear B-cell epitopes ” . In Methods in Molecular Biology, Epitope Mapping Protocols , 335 – 344 . Humana Press, New York .