Abstract
The antioxidant activities of ethanol, methanol, chloroform, ethyl acetate, and petroleum ether extracts from the fruit of Piper wallichii (Miq.) Hand.-Mazz. were investigated using different in vitro antioxidant assays. The methanol extract showed the most potent scavenging activity on 2,2-diphenyl-1-picryl hydrazyl (DPPH), Ferric reducing antioxidant power (FRAP), 2,2-azino-bis-(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) hydrogen peroxide, hydroxy radical, and reducing power assays with EC50 values of 46.70 ± 0.85, 41.7 ± 0.74, 45.23 ± 2.02, 49.30 ± 1.15, 40.39 ± 1.92, and 50.21 ± 1.2 μg/ml, respectively. The total phenolic content and the total flavonoids content were also determined. The methanol extract showed the highest total phenolic content and total flavonoids content, followed by the chloroform extract. This study revealed that the methanol extract had the strongest antioxidant activities, which were correlated with its high level of phenolic and flavonoids content.
INTRODUCTION
Reactive oxygen species (ROS) and free radicals have drawn increasing attention in the past decade.[Citation1] Current life style causes over production of ROS and free radicals, such as super oxide anion radical (O2 − •), hydrogen peroxide (H2O2), hydroxyl radical (OH•) are introduced into the body as byproducts in normal metabolic functions or also from the environment. These molecules/radicals are exacerbating factors in cellular injury and the aging process.[Citation2] The consumption of fruit and vegetables has been inversely associated with morbidity and mortality from degenerative and coronary heart diseases.[Citation3] The protection that fruit and vegetables provide against diseases has been attributed to the various antioxidants contained in them.[Citation4] Particularly polyphenols show protective effects on brain degenerative processes[Citation5] and have anti-inflammatory[Citation6] and anticarcinogenic[Citation7] effects. Plant volatile and nonvolatile secondary metabolites have wide applications in dietary regimens, food flavoring and preservation, folk medicine, and the fragrance industry.[Citation8]
Piper wallichii (Miq.) Hand.-Mazz. is a dioeciously, climbing shrub grown in the plains of foothills in semi evergreen and moist deciduous forests between 100–800 m elevations. The plant is distributed in India, Bhutan, Nepal, and China. The fruits are used medicinally against cold fever, cough, and as a uterine stimulant. The fruits are added in curry for flavor and in pickle as preservatives by the tribal people.[Citation9] The fruits possess bitter acridic in taste. It has also cardiac and antibiotic properties. A few compounds were isolated from P. wallichii (Miq.) Hand.-Mazz.[Citation10]
In recent times, researchers have been trying to isolate powerful and non-toxic natural antioxidants from edible plants not only to prevent autoxidation and lipid peroxidation, but also to replace synthetic antioxidants. The fruits of Piper wallichii (Miq.) Hand.-Mazz. have been used more as medicinal purposes rather than food by the local tribes of North East India, which had attracted us to investigate the antioxidant activities in the fruit. As we know, no report is available in the literature on the antioxidant potential of Piper wallichii (Miq.) Hand.-Mazz. fruit. The main objectives of this study were (i) to determine phenolic and flavonoid content of the fruits, (ii) to evaluate the antioxidant activities in terms of effective concentration at 50% inhibition/scavenging in different methods, and (iii) establishing its correlation with phenolic and flavonoids content.
MATERIALS AND METHODS
Plant Material
The plant material was collected from the Lohit district of Arunachal Pradesh, India. The specimen voucher has been deposited in NERIST, Nirjuli, Arunachal Pradesh. The plant with female spikes and fruits is shown in and .
Extraction Process
The fruits (1.6 kg fresh) at maturity of uniform size were collected and dried under shade to obtain a 400-g dry sample. The dry samples were powdered in a Willy Mill to 60 mesh size and used for solvent extraction. The dry fruits (400 g) of the plant were extracted in petroleum ether, chloroform, ethyl acetate, methanol, and ethanol, respectively. The solvents were removed by using a rotary evaporator (Buchi, Flawil, Switzerland) under reduced pressure at 40°C and stored at 4°C until used for evaluation of antioxidant activity, total phenolic, flavonoid content, peroxide scavenging, hydroxyl radical scavenging, and reducing power assays.
Chemicals and Reagents
Ascorbic acid, aluminum chloride, 2,2′-diphenyl-1-picryl hydrazyl (DPPH), gallic acid, 2,4,6-tripyridyl-s-triazine (TPTZ), ferric chloride, potassium persulphate, Folin-Ciocalteu's phenol reagent, and 2,2-azino-bis-(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) were obtained from Sigma-Aldrich (Bangalore, India). All other reagents and chemicals of analytical grade were procured from Merck (Mumbai, India) and local sources of India and milli-Q quality water were used.
Determination of Total Phenolic Content (TPC)
TPC was determined as per the method described by Folin-Ciocalteu method.[Citation11] Briefly, 100 μl of the filtered extract were mixed with 900 μl of distilled water and 1 ml of Folin-Ciocalteu reagent. After 5 min, 2 ml of saturated sodium carbonate (75 gm/l) and 2 ml of water were added. The absorbance of the resulting blue colored solution was measured at 765 nm after incubation at 30°C for 1.5 h with intermittent shaking. Quantitative measurements were performed based on a standard calibration curve of five points, 20, 40, 60, 80, and 100 mg/100 ml of gallic acid in 80% methanol. The total phenol content was expressed as gallic acid equivalent (GAE) in milligram per gram of dry sample.
Determination of Total Flavonoid Content (TFC)
TFC was determined by the colorimetric method[Citation12] with slight modification. To start, 50 mg of each extract was dissolved in 10 ml of 80% aqueous methanol and filtered through Whatman filter paper No. 42 (125 mm). In a 10-ml test tube, 0.3 ml of extracts, 3.4 ml of 30% methanol, 0.15 ml of 0.5 M NaNO2, and 0.15 ml of 0.3M AlCl3.6H2O were added and mixed. After 5 min, 1 ml of 1M NaOH was added. The mixture was measured at 506 nm. The standard curve for total flavonoid was made using rutin as the standard solution (0–100 mg/l) under the same procedure as above. The total flavonoid was expressed as milligrams of rutin equivalents per gram of dried sample.
Antioxidant Activity
Each sample was dissolved in 95% methanol at a concentration 1 mg/ml and then diluted to prepare the series concentrations for antioxidant assays. Ascorbic acid was used as the reference chemical in all assays.
DPPH Radical Scavenging Activity Assay
Antioxidant activity was determined by the 1,1-diphenyl-2-picrylhydrazyl (DPPH) method[Citation13] in which the donation capacity of the extracts was measured by bleaching of the purple-colored solution of the DPPH radical. This assay was based on the ability of the antioxidant to scavenge the DPPH cation radical. This method determines the hydrogen donating capacity of the molecule and does not produce oxidative chain reactions or react with free radical intermediates. Briefly, to 100 μl of sample extract or standard, 2.9 ml of 0.1 mM DPPH methanolic solution was added. The mixture was shaken vigorously and kept at room temperature for 30 min. Then the discoloration of DPPH was measured against the blank at 517 nm. The scavenging activity was estimated based on the percentage of DPPH radical scavenged using the following equation:
The antiradical activity was expressed as EC50 (μg/ml), the concentration required to cause 50% DPPH inhibition.
Ferric Reducing Antioxidant Power (FRAP) Assay
FRAP was determined in the sample extracts according to the method described by Iris et al.[Citation14] with some modifications. The method was based on the ability of the sample to reduce Fe3+ to Fe2+ ions. In the presence of TPTZ, the Fe2+–TPTZ complex exhibits blue color, which was read at 593 nm. Briefly, 3.0 ml of FRAP reagent was added to the appropriate concentrations of the sample extract (a few samples were diluted further to get into the standard range) and after incubation for 4 min at room temperature the absorbance was measured at 593 nm. The antioxidant effect was calculated by using the following equation:
ABTS Radical Cation Scavenging Activity Assay
The ABTS radical cation scavenging activity was measured according to the method developed by Re et al.[Citation15] with slight modifications. In brief, ABTS solution (7 mM) was reacted with potassium persulfate (2.45 mM) solution and kept overnight in the dark to yield a blue-green colored solution containing ABTS radical cation. Prior to use in the assay, the resulting blue-green ABTS+ • solution was diluted with 50% methanol for an initial absorbance of about 0.700 ± 0.02 at 734 nm, with the temperature control set at 30°C. Free radical scavenging activity was assessed by mixing 100 μl of test sample with 2.9 ml of ABTS radical solution microcuvette. The decrease in absorbance was measured exactly 1 min after mixing the solution, then up to 6 min. The final absorbance was noted. The scavenging effect was calculated according to the following equation:
The antioxidant capacity of the test sample was expressed as EC50, the concentration necessary for a 50% reduction of ABTS. The radical stock solution was freshly prepared daily.
Hydrogen Peroxide Scavenging Activity Assay
Hydrogen peroxide scavenging activity was measured according to a slightly modified method of Zhao et al.[Citation16] First, 1 ml of H2O2 (0.1 mM) and 1 ml of 100 μg/ml concentration of the extracts or standard antioxidants were mixed with 100 μl ammonium molybdate (3%), 10 ml H2SO4 (2 M), and 7 ml KI (1.8 M). The mixed solution was titrated with Na2S2O3 (5 mM) until the yellow color disappeared. The abilities to scavenge the hydrogen peroxide were calculated using the following equation:
Hydroxyl Radical Scavenging Assay
Hydroxyl radical scavenging activity of extracts was assayed by the method developed by Halliwell and Gutteridge.[Citation17] The reaction mixture contained 500 ml of 2-deoxyribose(2.8 mM) in phosphate buffer (50 mM, pH 7.4), 200 ml of premixed ferric chloride (100 mM), and EDTA (100 mM) solution (1:1; v/v), 100 ml of hydrogen peroxide (200 mM) without or with the extract solution (100 ml). The reaction was triggered by adding 100 ml of 300 mM ascorbate and incubated for 1 h at 37°C. A solution of tert butyl alcohol (TBA) in 1 ml (1%; w/v) of 50 mM NaOH and 1 ml of 2.8% (w/v; aqueous solution) trichloroacetic acid (TCA) was added. The mixture was heated for 15 min on a boiling water bath and then cooled. The absorbance was measured at 532 nm. The scavenging activity of the hydroxyl radical was calculated according to the following equation:
Reducing Power Assay
The method of Oyaizu[Citation18] was used to assess the reducing power of different extracts. A total of 2 ml of different extracts of concentration 10 mg/ml was mixed with 2 ml of 0.2 M phosphate buffer (pH 6.6) and 2 ml of 1% potassium ferricyanide [K3Fe(CN)6] and incubated in a water bath at 50°C for 20 min. Then 2 ml of 10% trichloroacetic acid was added to the mixture, which was centrifuged at 650 g for 10 min. The supernatant (2 ml) was then mixed with 2 ml distilled water and 0.4 ml of 0.1% ferric chloride solution. After 10 min, the intensity of the blue-green colored solution was measured at 700 nm. The EC50 value (μg/ml) is the extract concentration at the absorbance 0.5 (a.u.) for the reducing power and was calculated from the graph of absorbance at 700 nm against extract concentration.
Statistical Analysis
All assays were analyzed in triplicate and the results are expressed as means ± standard deviation (n = 3). The ANOVA test was used to analyze the differences among EC50 of various extracts for different antioxidant assays with least significant difference (LSD) P < 0.01 as a level of significance. The EC50 values were calculated using the Graph Pad Prism software (La Jolla, USA).
RESULTS AND DISCUSSION
Total Phenolic Content (TPC)
TPC was estimated by the Folin-Ciocalteu colorimetric method using gallic acid as a standard phenolic compound as shown in . The results revealed that methanol was the best solvent for extracting phenolic compounds followed by chloroform, ethanol, and ethyl acetate. A variation in the total phenolic content ranged from 30.47 ± 1.05 to 75.37 ± 1.75 (mgGAE/g d.w.) from the petroleum ether to methanolic extracts, respectively (). The results were in accordance with the reported[Citation19] results. It was shown that the phenolic contents of the plant were influenced by a number of intrinsic (genus, species, cultivar) and extrinsic (agronomic, environmental, handling and storage) factors.[Citation20]
Table 1 Phenolic and flavonoid content of different extract of Piper wallichii (Miq.) Hand.-Mazz
Total Flavonoid Content (TFC)
Flavonoids are a group of polyphenolic compounds naturally present in most of the yellow edible fruits and vegetables. They constitute most of the yellow and red colors in the fruits.[Citation21] Flavonoids from the fruit and vegetables samples were widely studied as components had the potential to provide multiple health benefits. Epidemiological and clinical studies have provided evidence of a potential role for flavonoids in lowering the risk of coronary heart disease, cardiovascular diseases, free radical scavenging capacity, neurodegenerative diseases, osteoporosis, and lung cancer.[Citation22] The amount of TFC in different solvent extracts is shown in . The results revealed that methanol was the best solvent for extractability of flavonoids 22.55 ± 0.87 (mg RE/g d.w.). These findings are in agreement with the available literature on the flavonoid content of the plant Ficus microcarpa L.[Citation19] The rich flavonoid content in plants could be a good source of antioxidant that can help to protect from lipid peroxidation.[Citation23]
DPPH Radical Scavenging Activity
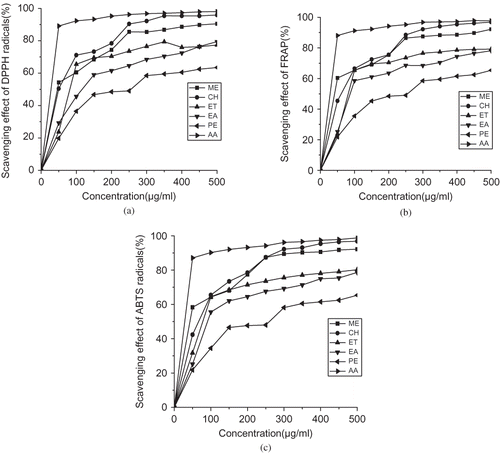
The DPPH method is used worldwide in the quantification of free radical scavenging activity in vitro in biological systems. In the present study, the investigation of antioxidant capacity was measured as the cumulative capacity of the compounds present in the sample to scavenge stable organic free radicals with a drop of violet colors, which gave the absorbance maxima within 517 nm using the DPPH reaction. This method is sensitive to light, pH, temperature, and solvent.[Citation24] The variation of scavenging activity in different solvents is shown in . The extract showed dose dependent DPPH radical-scavenging activities. It was generally observed that the DPPH radical-scavenging effect increased as the concentration of the solvent extract was increased, to a certain extent, and then leveled off, even with the further increases in the concentration. The EC50 values of scavenging DPPH radicals for the methanol and chloroform were 46.70 ± 0.85 and 50.21 ± 1.75 μg/ml, respectively. The study revealed that methanol and chloroform extract had free radical scavenging or inhibiting activities, acting possibly as a primary antioxidant.
Ferric Reducing Antioxidant Power (FRAP)
The FRAP assay is one of the most simple, rapid, inexpensive tests and is very useful for routine analysis. The FRAP assay is developed for direct test of total antioxidant power of a sample. The antioxidant effect by FRAP assay with variation of concentration is shown in . The results imply that all the tested extracts inhibit the radical in a concentration dependent manner. The methanol and chloroform extracts exhibited higher inhibition activities in comparison to petroleum ether and ethyl acetate extract. The EC50 values obtained from methanol extract (41.70 ± 0.74 μg/ml) were significantly different from the EC50 values obtained from petroleum ether extract (>250 μg/ml) (). However, a difference in EC50 was observed between the extracts and ascorbic acid, which was used as standard antioxidant.
Table 2 Antioxidant effect (EC50) on DPPH radicals, FRAP assay, ABTS radicals, hydrogen peroxide radicals, hydroxyl radicals assay of different extracts of Piper wallichii (Miq.) Hand.-Mazz. fruit
ABTS Radical Cation Scavenging Activity
ABTS+ • is a well known nitrogen centered synthetic radical and is widely used to determine antioxidant activity. The ABTS radical was generated by reaction with potassium persulphate and when extracts are added to ABTS radical, it is converted to a non radical. The scavenging effect of ABTS radicals with variation of concentration is shown in . The methanol and chloroform extracts exhibit high scavenging abilities when reacted with ABTS radicals, whereas the petroleum ether extract showed smaller effect at higher concentration. The EC50 values obtained from methanol extract (45.23 ± 2.02 μg/ml) was significantly different from the EC50 values obtained from petroleum ether extract (>250 μg/ml). The ABTS radical scavenging activity of methanol and chloroform extracts of this plant was less than that of ascorbic acid (). Moreover, a difference in EC50 was also noted between the extracts of the plant and the ascorbic acid.
Hydrogen Peroxide Scavenging Radicals
It is well established that hydrogen peroxide is not dangerous as it is, but may well be used for its ability to form the hydroxyl radical, thereby emphasizing the importance of its elimination. Indeed, it has already been proven that dietary phenols protect mammalian and bacterial cells from cytotoxicity induced by hydrogen peroxide.[Citation25] Thus, the observed hydrogen peroxide scavenging activity could be due to the presence of phenols in the extracts. From the EC50 values, it was seen that the hydrogen peroxide scavenging activities of chloroform (40.83 ± 1.85 μg/ml) and methanol (49.30 ± 1.15 μg/ml) extracts were more effective than that of petroleum ether extract(>250 μg/ml). The EC50 values of all extracts were found to be quite different from the EC50 values obtained for ascorbic acid.
Hydroxyl Radical Scavenging Activity
The hydroxyl radical is known to be the most reactive oxygen radical and it severely damages adjacent bio-molecules in the body, such as protein and DNA, resulting in cell damage. This damage causes aging, cancer, and several other diseases.[Citation26] The removal of hydroxyl radical is, therefore, probably one of the most effective defensive mechanism of a living body against various diseases. In this study, the Fenton reagent (Fe2+ + H2O2 → Fe3+ + OH− + OH−), a source of hydroxyl radical, was used to evaluate the scavenging activities of different extracts towards hydroxyl radical. Hydroxyl radical scavenging activity of an extract is directly related to the antioxidant activities and was found to be in the order of ME > CH > ET > EA > PE for the studied plant. The hydroxyl radical scavenging activity for methanol, ethanol, and chloroform extracts do not differ significantly from each other. The EC50 value for the methanol extract was 40.39 ± 1.92 μg/ml, while for the petroleum ether extract was >250 μg/ml. Since the hydroxyl radical scavenging activity is directly related to propagation lipid peroxidation, they seem to be good scavengers of active oxygen species, thereby reducing the rate of the reaction.
Reducing Power
Earlier studies have indicated that the reducing power of certain plant extracts was associated with their antioxidant activity.[Citation27] The reducing power assay, therefore, is often used to evaluate the ability of an antioxidant to donate an electron. In this study, the ability of various solvent extracts to reduce Fe3+ to Fe2+ was determined. In the reducing power assay, the presence of reductants (antioxidants) in the extracts would result in the reduction of Fe3+/ferric cyanide complex to the ferrous form by donating an electron. Increasing absorbance at 700 nm indicates an increase in reducing ability. The sequence for reducing power was ME > CH > ET > EA > PE extracts. The EC50 values for the methanol and chloroform extract were 50.21 ± 1.2 and 56.12 ± 1.1 μg/ml, respectively, while for the PE extract it was ≫250 μg/ml ().
Correlation with EC50 Values of Antioxidant Activities and Phytochemicals Content
Through correlation analysis for phytochemical contents with EC50 values of radical scavenging activity and/or antioxidant ability of extracts, the phenolic content exhibited a positive correlation (R 2 > 0.69) with DPPH, FRAP, ABTS radicals, and reducing power assay (). This result suggested that the phenolic compounds contributed significantly to the antioxidant capacity of the investigated plant species. This result was consistent with the finding of many research groups who reported such positive correlation between total phenolic content and antioxidant activity.[Citation19,Citation28,Citation29] However, a non significant correlation was found in phenolic and flavonoids content with that of hydroxyl radical, hydrogen peroxide scavenging abilities. The Folin-Ciocalteu assay provided a crude estimate of the total phenolic compounds present in an extract. It is not specific to polyphenols, but many interfering compounds may react with the reagent, giving elevated apparent phenolic concentrations.[Citation30] Moreover, various phenolic compounds respond differently in these assays, depending on the number of phenolic groups they have,[Citation11] and total phenolic content did not incorporate necessarily in all the antioxidants that may be present in an extract. Hence, this may explain the equivocal correlation between total phenolic content and antioxidant activity of different extracts as appeared in . However, the correlation between antioxidant activity and flavonoid content was not so significant except for the DPPH radical scavenging assay.
Table 3 Correlation between the EC50 values of antioxidant activities and phenolic and flavonoids content of Piper wallichii (Miq.) Hand.-Mazz
CONCLUSION
The results obtained carry considerable value with respect to the antioxidant activities of Piper wallichii (Miq.) Hand.-Mazz. fruits. The activity of these extracts attributed to the phenolic and flavonoid content. Consequently, the results suggest that the extracts can be utilized as an effective source of antioxidants. It can be concluded that fruits of Piper wallichii (Miq.) Hand.-Mazz. consumed as a foodstuff in different areas of India, can be used as an accessible source of natural antioxidants with consequent health benefits. There is also a need for further study of its extracts and isolated compounds.
ACKNOWLEDGMENTS
The authors are thankful to CSIR, New Delhi for providing financial support under EMPOWER scheme and to Dr. P. G. Rao, Director, North East Institute of Science and Technology, Jorhat, Assam for providing facilities and valuable advice.
REFERENCES
- Singla , R. , Ganguli , A. and Ghosh , M. 2010 . Antioxidant activities and polyphenolic properties of raw and osmotically dehydrated dried mushroom (Agaricus bisporous) snack food . International Journal of Food Properties , 13 : 1290 – 1299 .
- Gulcin , I. , Tel , A.Z. and Antioxidant , Kirecci, E. 2008 . antimicrobial, antifungal, and antiradical activities of Cyclotrichium Niveum (BOISS.) Manden and Scheng . International Journal of Food Properties , 11 : 450 – 471 .
- Block , G. , Patterson , B. and Fruit , Subar, A. 1992 . vegetables and cancer prevention: A review of the epidemiological evidence . Nutrition & Cancer , 18 : 1 – 29 .
- Gey , K.F. 1990 . The antioxidant hypothesis of cardiovascular disease: Epidemiology and mechanisms . Biochemical Society Transactions , 18 : 1041 – 1045 .
- Herkin , E.N. and Guzel , S. 2010 . Total antioxidant capacity and total phenol content of selected commercial fruit juices in Turkey . International Journal of Food Properties , 13 : 1373 – 1379 .
- Subbaramaiah , K. , Chung , W.J. , Michaluart , P. , Telang , N. , Tanabe , T. , Inoue , H. , Jang , M. , Pezzute , J.M. and Dannenberg , A.J. 1998 . Resveratrol inhibits cyclooxygenase-2 transcription and activity in phorbol ester-treated human mammary epithelial cells . Journal of Biological Chemistry , 273 : 21875 – 21882 .
- Kuroda , Y. and Hara , Y. 1999 . Antimutagenic and anticarcinogenic activity of tea polyphenols . Mutation Research , 436 : 69 – 97 .
- Huang , D. , Ou , B. and Prior , R.L. 2005 . The chemistry behind antioxidant capacity assays . Journal of Agriculture and Food Chemistry , 53 : 1841 – 1856 .
- Gajurel , P.R. , Rethy , P. , Kumar , Y. and Singh , B. 2008 . Piper Species (Piperaceae) of North East India (Arunachal Pradesh) , 23 – A . India : Bishen Singh Mahendra Pal Singh: Dehra Dun .
- Virinder , S.P. , Jain , S.C. , Bisht , K.S. , Jain , R. , Taneja , P. , Jha , A. , Tyagi Om , D. , Prasad , A.K. , Wengel , J. , Olsens , C.E. and Boll Per , M. 1997 . Phytochemistry of genus Piper. Phytochemistry , 46 ( 4 ) : 591 – 673 .
- Singleton , V.L. and Rossi , J.A. 1965 . Colorimetry of total phenols with phosphomolybdic phosphotungstic acid reagents . American Journal of Enology and Viticulture , 16 : 144 – 158 .
- Park , Y.-S. , Jung , S.T. , Kang , S.G. , Heo , B.K. , Arancibia-Avila , P. , Toledo , F. , Drzewiecki , J. , Namiesnik , J. and Gorinstein , S. 2008 . Antioxidants and proteins in phosphor-treated kiwifruits . Food Chemistry , 107 ( 2 ) : 640 – 648 .
- Aoshima , H. , Hideaki , T. , Hirofumi , K. and Yoshinobu , K. 2004 . Aging of whiskey increases 1, 1-diphenyl-2-picryl hydrozyl radical scavenging activity . Journal of Agricultural and Food Chemistry , 52 ( 16 ) : 5240 – 5244 .
- Iris , F. , Benzie , F. and Strain , J.J. 1999 . Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration . Methods in Enzymology , 299 : 15 – 27 .
- Re , R. , Pellegrini , N. , Proteggente , A. , Pannala , A. , Yang , M. and Rice-Evans , C. 1999 . Antioxidant activity applying an improved ABTS radical cation decolorization assay . Free Radical Biology & Medicine , 26 : 1231 – 1237 .
- Zhao , G.R. , Xiang , Z.J. , Ye , T.X. , Yuan , Y.J. and Guo , Z.X. 2006 . Antioxidant activities of Salvia miltiorrhiza and Panax notoginseng . Food Chemistry , 99 : 767 – 774 .
- Halliwell , B. and Gutteridge , J.M.C. 1981 . Formation of thiobarbituric acid reactive substances from deoxyribose in the presence of iron salts: The role of superoxide and hydroxyl radicals . FEBS Letters , 128 : 347 – 352 .
- Oyaizu , M. 1986 . Antioxidant activity of browning products of glucosamine fractionated by organic solvent and thin-layer chromatography . Nippon Shokulin Kogyo Gakkaishi , 35 : 771 – 775 .
- Ao , C. , Li , A. , Elzaawely , A.A. , Xuan , D.T. and Twata , S. 2008 . Evaluation of antioxidant and antibacterial activities of Ficus microcarpa L. fill extract . Food Control , 19 : 940 – 948 .
- Thomas-Barberan , F. and Espin , J.C. 2001 . Phenolic compounds and related enzymes as determinants of quality of fruits and vegetables . Journal of the Science of Food Agriculture , 81 : 853 – 876 .
- Erlund , I. 2004 . Review of the flavonoids quercetin, hesperetin and naringenin. Dietary sources, bioactivities, bioavailability and epidemiology . Nutrition Research , 24 : 851 – 874 .
- Yao , L.H. , Jiang , Y.M. , Shi , J. , Tomas-Barberan , F.A. , Dutta , N. , Singanusong , R. and Chen , S.S. 2004 . Flavonoids in food and their health benefits . Plant Foods for Human Nutrition , 59 : 113 – 122 .
- Sharififar , F. , Dehghn-Nudeh , G. and Mirtajaldini , M. 2009 . Major flavonoids with antioxidant activity from Teucrium polium L . Food Chemistry , 112 : 885 – 888 .
- Ozcelik , C. , Lee , J.H. and Min , D.B. 2003 . Effects of light, oxygen and pH on the absorbance of 2, 2-diphenyl-1-picrylhydrazyl . Journal of Food Science , 68 : 487 – 490 .
- Nakayama , T. 1994 . Suppression of hydroxyperoxide-induced cytotoxicity by polyphenols . Cancer Research , 54 : 1991 – 1993 .
- Yashikawa , T. , Naito , Y. and Kondo , M. 1997 . “ Free radicals and disease ” . In Food and Free Radicals , Edited by: Hiramatsu , M. , Yoshikawa , T. and Inoue , M. 11 – 19 . New York : Plenum Press . InEds.
- Meir , S. , Kanner , J. , Akiri , B. and Hadas , S.P. 1995 . Determination and involvement of aqueous reducing compounds in oxidative defense systems of various senescing leaves . Journal of Agricultural and Food Chemistry , 43 : 1813 – 1819 .
- Cai , Y. , Luo , Q. , Sun , M. and Corke , H. 2004 . Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer . Life Sciences , 74 : 2157 – 2184 .
- Zheng , W. and Wang , S.Y. 2001 . Antioxidant activity and phenolic compounds in selected herbs . Journal of Agricultural and Food Chemistry , 49 : 5165 – 5170 .
- Prior , R.L. , Wu , X. and Schaich , K. 2005 . Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements . Journal of Agricultural and Food Chemistry , 53 : 4290 – 4302 .