Abstract
Profile of phenolic compounds of Tilia rubra subsp. caucasica was measured by high performance liquid chromatography coupled with ultraviolet and tandem mass spectroscopy. Three different extraction methods (methonolic, selective extraction, and acidic hydrolysis) were used to evaluate phenolic composition and antioxidant capacity in three different parts of T. rubra. The antioxidant activities of the species were investigated in terms of total phenolics and flavonoids, and cupric reducing antioxidant capacity and 1,1-diphenyl-2-picrylhydrazyl scavenging assays. Different phenolic compounds related to antioxidant activities of three different parts and three different extraction ways of T. rubra were determined by high performance liquid chromatography-ultraviolet and high performance liquid chromatography-mass spectroscopy. Gallic and protocatechuic acid were the main phenolic compounds in the all extracts and parts of Tilia rubra subsp. caucasica by high performance liquid chromatography-ultraviolet ranging from 356.20 to 159.83 and 1873.90 to 720.80 μg phenolic compound/g dry sample, respectively. Epicatechin, luteolin, and rhamnazin were detected by high performance liquid chromatography-mass spectroscopy.
INTRODUCTION
The continuity of life depends on the oxidation reactions. Likewise the metabolism of food, as well as other biological processes, involves these reactions. These reactions can produce free radicals that have some negative effects. They bring about damages in the cell. Antioxidants, such as warriors, play an important role against these damages. Living organisms have complicated systems of multiple types of antioxidants. Vitamins C and E, glutathione, some enzymes, like catalyses, superoxide dismutase, and some peroxides, are examples of antioxidants. When levels of antioxidants decrease or inhibitions occur on antioxidant enzymes, oxidative stress occurs. Killed or damaged cells and many human diseases[Citation1] are the results of oxidative stress.
Edible or non-edible plants are sources of natural antioxidants. They contain thousands of different phenolic compounds that have antioxidant effects.[Citation2 − Citation4] Due to fights against many human diseases, plants have become indispensable in alternative medicine.[Citation5] One of the members of alternative medicine is lime, which it is called in Britain, and linden or basswood in North America.[Citation6] They come from the Tiliaceae family and tilia genus of about 30 species of trees. Their species are well known around the world for their properties, such as sedatives, tranquilizers, diuretics, and expectorants, and for diaphoretic activities in traditional medicine, especially used as herbal tea. After brewing, a unique and nice smell, which belongs to this genus, comes into being.[Citation7]
Several methods have been developed in the last two decades to evaluate the antioxidant capacity of biological samples. A direct relationship has been found between the total phenolics and antioxidant capacity of many natural samples. The antioxidant activity of phenolics is related to a number of different mechanisms, such as free radical scavenging, hydrogen donation, singlet oxygen quenching, metal ion chelation, and acting as a substrate for radicals, such as DPPH, ABTS, superoxide, and hydroxide.[Citation8]
Phenolic compounds can be determined either qualitative or quantitative by high performance liquid chromatography (HPLC), which is an important analytical technique for separating and quantifying components in complex liquid mixtures. By choosing the appropriate equipment (i.e., column and detector), this method is applicable to samples with components ranging from small organic and inorganic molecules and ions to polymers and proteins with high molecular weights Reversed phase liquid chromatography in combination with UV or MS detection is the method of choice for analysis of the phenolic content of the plant extracts.
Although some phenolic substances of many members of the genus tilia were reported elsewhere, this is most probably the first detailed report about effects of different extraction methods on phenolic compositions in Tilia rubra subsp. caucasica. The purpose of this study was to evaluate the phenolic compounds in Tilia rubra subsp. caucasica by high performance liquid chromatography-ultraviolet (HPLC-UV) and high performance liquid chromatography-ultraviolet-mass spectroscopy/mass spectroscopy (HPLC-UV-MS/MS).
MATERIALS AND METHODS
Chemicals and Instrumentation
Standard (purity >99.0%) phenolic compounds for HPLC analysis as follows: gallic acid, protocatechuic acid, p-hydroxy benzoic acid, vanillic acid, caffeic acid, chlorogenic acid, syringic acid, epicatechin, p-coumaric acid, ferulic acid, benzoic acid, o-coumaric acid, trans-cinnamic acid, abscisic acid, catechin, rutin, quercetin, luteolin, apigenin, kaempferol, rhamnetin, propylparaben; as internal standard (IS): sodium carbonate, ammonium acetate, potassium acetate, cupric chloride, neocuproine (2,9-dimethyl-1,10-phenanthroline), aluminum nitrate nonahydrate and DPPH (1,1-Diphenyl-2-picrylhydrazyl radical, 2,2-Diphenyl-1-(2,4,6-trinitrophenyl) hydrazyl) were supplied from Sigma-Aldrich (St. Louis, MO, USA) and Merck (Darmstadt, Germany). Used solvents of methanol, acetic acid, acetonitrile, diethyl ether, and ethyl acetate were obtained from Sigma-Aldrich and Merck. Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2 carboxylic acid) and Folin-Ciocalteu's phenol reagent were from Fluka Chemie GmbH (Buchs, Switzerland).
Sample Preparation
Samples of the species were collected from Soğukçeşme village (270–300 m) in Rize, Turkey (July 2010). Voucher species were identified by Professor Salih Terzioğlu from the Department of Faculty of Forestry, Karadeniz Technical University, Trabzon, Turkey. The specimens were dried at room temperature for later experiments. The plant was divided into three parts: blossom, leaf, and trunk. About 5 g of dried powder of the samples were extracted with 30 mL methanol in a flask attached to the condenser, in a sonicator apparatus (Elma® Transsonic Digital, Singen/Htw, Germany) at 60°C for 3 h. Then, 10 mL of the sample were separated from each extract to determine antioxidant activities. What was left of each extract was divided into three parts to carry out different extraction methods to determine phenolic substance with HPLC-UV. First, the methanol extracts were analyzed directly. The second method was named selective extraction and the methanol extract was evaporated until it dries with a rotary evaporator (IKA® Nerke GmbH & Co., Staufen, Germany) at 50°C. The residues were dissolved in 10 mL of distilled water and applied liquid-liquid extractions. The mixtures were extracted with 5 mL of diethyl ether and 5 mL of ethyl acetate three times consecutively. Organic phases were picked up in the same flask and evaporated till drying under reduced pressure in a rotary evaporator at 40°C. The residues were weighed and dissolved in methanol for HPLC analysis. The last method is named acidic hydrolyze and after the methanol extract was evaporated until dryness with rotary evaporator at 50°C, then the residues were dissolved with 10 mL of 2 M HCI solution and hydrolyzed at a constant temperature of 90°C for 2 h. The acidic mixtures after cooling were applied to liquid-liquid extractions in the same way as the second section of the methanol extracts. The organic solvents were evaporated until they dried under reduced pressure in a rotary evaporator at 40°C. The residue was weighed and dissolved in methanol for HPLC analysis.
Determination of Phenolic Compounds by HPLC-UV
HPLC-UV analyses of phenolic compounds were performed on a reverse phase Zorbax Eclipse XDB-C18 column (4.6 × 150 mm, 5 μm), using a gradient program with two solvent systems,[Citation5,Citation9] (A: 80% acetonitrile in methanol, B: 2% acetic acid in distilled water). The elution gradient was performed from high polarity and low pH to low polarity and high pH. Gradient: 0–2 min, 95% B; 2–8 min, 95–90% B; 8–11 min, 90–85% B; 11–13 min, 85–75% B; 13–17 min, 75–70% B; 17–30 min, 70–65% B; 30–33 min, 65–0% B; 33–38 min, 0–0% B; 38–40 min 95% B; 40–48 min, 95% B. The injection volume was 50 μL and the column temperature was regulated at 30°C in a column oven. A flow rate of 1 mL/min was used and detection was performed at 280 and 315 nm. In this system, 17 phenolic compounds were analyzed at 280 and 315 nm simultaneously. Internal standard (IS) technique was applied to the analysis to increase the repeatability. Propylparaben was a suitable IS for this system.[Citation10] Values of limit of detection (LOD) and limit of quantification (LOQ) were calculated for identification and quantification of phenolic compounds in the extracts ().
Table 1 The standard chromatogram values of seventeen individual phenolic substances and an integral standard
Determination of Phenolic Compounds by HPLC-UV-MS-MS
A Quattro ESI triple quadrupole mass spectrometer (Micromass, Manchester, UK) connected with a HPLC-UV system (Waters, Manchester, UK) was used for HPLC-UV-MS-MS analyses. A Luna C18 column (250 × 2.00 mm i.d., 5 μm) (Phenomenex, Torrance, CA, USA) column was used for all analyses. Gradient elution was used for liquid chromatographic (LC) separation. Mobile phase A: 2% acetic acid in water; mobile phase B: 70% acetonitrile (ACN) in water; and for cleaning stage mobile phase C mobile: 100% acetonitrile. Gradient: 0–3 min, 95% A; 3–8 min, 95–92% A; 8–15 min, 92–82% A; 15–20 min, 82–80% A; 20–25 min, 80–62% A; 25–35 min, 62–25% A; 35–40 min, 0% A, 0% B, 100% C; 40–50 min, 95% A; 50–65 min, 95% A. Injection volume was 20 μL and column temperature was 25°C.
Seventeen phenolic compounds, gallic acid, protocatechuic acid, p-hydroxy benzoic acid, catechin, vanillic acid, chlorogenic acid, epicatechin, caffeic acid, syringic acid, p-coumaric acid, ferulic acid, rutin, quercetin, luteolin, apigenin, kaempferol, and rhamnetin, were used to identify the phenolic compounds in methanol extracts of three parts of the plant. To tune the mass spectrometer for full scan MS analysis, 5 ppm of phenolic standard-mix in 2% acetic acid in 50% acetonitrile/water solution was used. Optimized negative electrospray ionization (ESI) spectra were obtained using the following conditions: capillary voltage, 2.8 kV; cone voltage, 25 V; desolvation gas (N2) flow, 400 L/h; cone gas flow 50 L/h; desolvation temperature, 350°C; capillary temperature, 110°C; collision energy, 1. The spectra were recorded in the range of m/z 100–900. To tune the mass spectrometer for MS-MS analysis, 5 ppm rutin in 2% acetic acid in 50% acetonitrile/ water solution was used.
Determination of Total Phenolic Content
Total phenolic contents were analyzed with Folin-Ciocalteu's phenol reagent method, using gallic acid as the standard.[Citation11] First, 20 μL of various concentrations of gallic acid and 20 μL of methanolic samples (1 mg/mL), 400 μL of 0.5 Folin-Ciocalteu regents and 680 μL of distilled water were mixed and the mixture was vortexed. Following 3 min incubation, 400 μL of Na2CO3 (10%) solution was added, and after vortexing, the mixture was incubated for 2 h. After the incubation period at room temperature, absorbance of the mixtures was measured at 760 nm. The concentration of total phenolic compounds was calculated as g of gallic acid equivalents per kg of dry weight, by using a standard curve for gallic acid in the concentration range between 0.015 and 0.5 mg/mL (r 2 = 0.998). All extracts obtained from three parts of Tilia rubra subsp. caucasica showed the same total phenolic content.
Determination of Flavonoid Content
The total flavonoid content was determined by the aluminum complexation method.[Citation12] In this method, the extracted solutions were prepared in a different concentration (0.5 mL) and mixed with 0.1 mL of 10% aluminum nitrate, 0.1 mL of 1 mol/L potassium acetate, and 4.3 mL of 80% ethyl alcohol. The samples were kept at room temperature for 40 min and the absorbance was measured at 415 nm. Quercetin was used as the standard to produce the calibration curve. The meanings of three readings were used and the total flavonoid content expressed in mg of quercetin equivalents (mg/g).
Scavenging of Free Radical (DPPH) Assay
Radical scavenging activity of methanolic extracts against 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical measured was spectrophotometrically at 517 nm. First, the method was used by Blois[Citation13] developed and modified by Brand-Williams et al.[Citation14] and Molyneux.[Citation15] The assay is based on the color change of DPPH solution from purple to yellow as the radical is deactivated by antioxidants.[Citation16] Briefly, various concentrations 0.75 mL of parts of Tilia rubra subsp. caucasica extracts were mixed with 0.75 mL of 0.1 mM of DPPH in methanol. Radical scavenging activity was measured by spectrophotometry. The values are expressed as SC50 (mg sample per mL), the concentration of the samples that causes 50% scavenging of DPPH radical. SC50 of Trolox was used as a standard to compare DPPH results with others.
Cupric Reducing Antioxidant Capacity (CUPRAC) Assay
The method, which is CUPRAC of Apak et al.,[Citation17] is based on the measurement of absorbance changing at 450 nm. To start, 1 mL of CuCl2 solution (1.0 × 10−2 M), 1 mL of neocuproine ethanolic solutions (7.5 × 10−3 M), and 1 mL NH4CH3COO buffer solution were added to a test tube and mixed; (x) mL of phenolic extract followed by (1.1 – x) mL of water were added and vortexed. After incubating about 30 min, measurements were taken at 450 nm.
Statistical Analysis
Values shown in the tables refer to ± standard deviations of three parallel measurements. The SC50 values were calculated from linear regression analysis (Microsoft Excel, Microsoft Corporation©, Albuquerque, NM, USA). Data were tested using SPSS Version 16.0 (SPSS, Chicago, IL, USA). Statistical analysis of the results was based on Mann–Whitney U-test and Pearson correlation analyses. Differences of p < 0.05 were considered significant.
RESULT AND DISCUSSION
HPLC Analysis of Phenolic Compounds
shows the standard chromatogram values of 17 individual phenolic substances, and an integral standard (IS) in the three parts, blossom, leaf, and trunk of Tilia rubra subsp. caucasica species, have been carried out by HPLC-UV and HPLC-MS. Seventeen phenolic compounds were analyzed and 14 compounds were determined: gallic acid, protocathechuic acid, p-hydroxy benzoic acid, caffeic acid, chlorogenic acid, syringic acid, p-coumaric acid, ferulic acid, benzoic acid, o-coumaric acid, abscisic acid, catechin, rutin, and quercetin by HPLC-UV. However, vanillic acid, epicatechin, and trans-cinnamic acid were not detected in any parts of them and all extractions (). We also used three different extraction methods on three different parts of the species and generally acid hydrolysis extracts have the highest phenolics. Among all parts of the species, gallic acid ranging from 159.83 to 356.20 μg/g dry sample and protocathechuic acid ranging from 720.80 to 1873.90 μg/g dry sample were the main compounds that were defined. When three extraction methods were compared in detail, some differences could be seen about phenolics.
Table 2 Phenolic constituents of Tilia rubra subsp. caucasica determined by HPLC-UV
In blossom sections, six phenolics in methanolic analysis, seven phenolics in selective extraction, and eight phenolics in acidic hydrolyses were determined. Gallic acid (246.54–346.02 μg/g dry sample), protocatechuic acid (1054.23–1369.34 μg/g dry sample), p-hydroxy benzoic acid (45.10–92.57 μg/g dry sample), abscisic acid (2.00–3.67 μg/g dry sample), and quercetin (31.81–127.45 μg/g dry sample) were identified in each three extractions in blossom. Meanwhile, catechin (341.82 μg/g dry sample) and chlorogenic acid (6.46 μg/g) were seen only in acidic hydrolysis; p-coumaric (18.90 μg/g dry sample) and o-coumaric acid (18.18 μg/g dry sample) were seen only in selective extraction.
In leaves, equal numbers of components were found in the each extraction method. But they were not the same. Even though Rutin (69.50 μg/g dry sample) was determined only in methanolic extraction, p-hydroxybenzoic (20.10–52.52 μg/g dry sample) and caffeic acid (23.92–45.46 μg/g dry sample) were defined in selective extraction and acidic hydrolysis. Ferulic acid was determined to be nearly the same value in methanolic extraction (55.91 μg/g dry sample) and selective extraction (56.86 μg/g dry sample). Five compounds: gallic acid (308.33–356.20 μg/g dry sample), protocathechuic acid (1588.30–1873.90 μg/g dry sample), catechin (223.27–354.20 μg/g dry sample), chlorogenic acid (11.71–576.96 μg/g dry sample), and quercetin (3.74–12.44 μg/g dry sample)were defined in all extraction methods for leaves analysis.
Finally, in the trunk, maximum components were identified, especially in methanolic extract. Particularly, benzoic acid (9.00 μg/g dry sample) and p-coumaric were found at the first time in all parts and all extractions.
Table 3 Phenolic compounds of Tilia rubra subsp. caucasica of determined by HPLC-UV-MS
Our study was supported by several studies in the literature. Some of the phenolic compounds were investigated in different tilia species and protocathequic acid was determined as the main phenolic substance by Demiray et al. and Štěrbová, et al.[Citation7,Citation18]
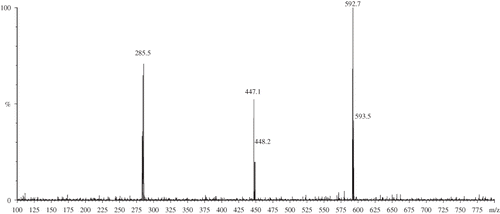
We also have performed HPLC-MS analysis to characterize unknown phenolic compounds, and according to HPLC-MS analysis, epicatechin was detected in all parts unlike HPLC-UV analysis. There are some phenolic compounds, such as procyanidin dimer, quercetin hexose, quercetin pentose, luteolin hexose, luteolin-deoxyhexose-hexose, luteolin-hexose-deoxyhexose, and rhamnazin, that have been characterized based on their mass spectrums and relatively their retention order in comparison with the literature. Protocatechuic acid, procyanidin dimer, epicatechin, rutin, quercetin hexose, and rhamnazin were detected in all parts. p-Hydroxy benzoic acid and luteolin-deoxyhexose-hexose were detected only in blossom extracts; chlorogenic acid, quercetin, and luteolin were detected only in leaf extracts; catechin and quercetin-pentose were not detected in blossom extracts unlike leaf and trunk extracts (). Protocatechuic acid has a retention time of 8.28 min and an [M-H]− ion at 153. The MS spectrum of [M-H] − ion shows an ion at 109 m/z indicating a loss of a 44 amu ion that corresponds to the decarboxylation.[Citation19] p-Hydroxy benzoic acid has a retention time of 13.79 min and an [M-H] − ion at 137. The fragment at 93 m/z also corresponds to the decarboxylation, a result of the loss of 44 amu. Catechin has a retention time at 16.8 min and an [M-H] − ion at 289. The fragment at 245 amu (a loss of 44 amu) suggests a loss of –CH2–CHOH– group from C3 and C4 from ring C, together with the hydroxyl group on C3, leading to [M-45] − .[Citation19,Citation20] Chlorogenic acid has a retention time at 18.74 min and an [M-H] − ion at 353. The mass difference of, for example, 162 amu observed between 353 and fragment ion at 191 suggests a loss of caffeoyl group.[Citation21] Epicatechin has a retention time of 21.9 min and a negative molecular ion, [M-H] − , at 289,[Citation19 − Citation21] and it has also a fragment ion at 245 amu as catechin. Two procyanidins (oligomeric catechins) were detected in the extracted ion chromatogram of the ion at m/z 577 (). This molecular ion indicates a procyanidin dimer consisting of catechin/epicatechin units linked by a C4–C8 or C4–C6 interflavonoid bond. They have a retention time at 20.03 and 24.8 min, respectively. The MS-MS spectrum of the [M-H]− ion of the procyanidin dimer shows ions at 289 and 245 serving as a confirmation for catechin/epicatechin units.[Citation22,Citation23] Rutin is the quercetin-deoxyhexose-hexoses type that has an [M-H] − ion at 609 and retention time of 29.8 min. The MS-MS spectrum of the [M-H] − ion of the diglycoside shows an ion at 300, a free radical form of quercetin, serving as a confirmation for quercetin aglycon.[Citation24,Citation25] Flavonoid-glycosides have a higher polarity than flavonoid aglycones due to the presence of glycoside moieties and as such elute earlier in the gradient (e.g., rutin, or quercetin-rutinoside, which elutes at 29.8 min). Quercetin-hexose has a retention time of 30.35 min and an [M-H] − ion of 463 amu. The mass difference of 162 amu suggests a loss of a hexose unit.[Citation19] Quercetin-pentose (eluting at 31.1 min) has a molecular ion of 433 amu. The mass difference of 132 amu in the MS-MS spectrum of the [M-H] − ion suggests a loss of a pentose group.[Citation26] Quercetin has a retention time of 34.9 min and the [M-H] − is 301 amu. Luteolin-deoxyhexose-hexose has a retention time of 30.98 min with a molecular ion of 593 amu. The mass difference (of 308 amu) between 593 and the fragment ion at 285 suggests a loss of both hexose and deoxyhexose.[Citation26] Luteolin-hexose-deoxyhexose has a retention time of 34.75 min with a molecular ion of 593 amu. The mass difference of 146 between 593 and the fragment ion at 447 suggests a loss of deoxyhexose and then 162 amu between 447 and the fragment ion at 285 suggests a loss of hexose ().[Citation27] Luteolin is a common flavon that has a retention time of 35.3 min and an [M-H] − ion of 285 amu.[Citation27] Rhamnazin is an o-methyl flavonol having two methoxy groups. As methyl groups decrease the polarity, rhamnazin is eluted last in the chromatograms. The retention time of rhamnazin is 37.28 min and it has a [M-H] − ion of 329 amu.
Plant phenolics are the largest class of plant secondary metabolites, which, in many cases, serve in plant defense mechanisms to counteract reactive oxygen species in order to survive and prevent molecular damage and damage by microorganisms, insects, and herbivores.[Citation28] Many studies of plant phenolic compounds have been carried out concerning responses to various stresses.[Citation29,Citation30]
In this study, besides HPLC-UV and HPLC-UV-MS/MS analysis, spectrophotometric assays, which are the way to determine the contents of phenolics and flavonoids as only quantitative, were used. These spectrophometric results gave some idea about blossom, leaf, and trunk's antioxidant effects in methanolic extract. All results were expressed as standard (gallic acid for total phenolic content, quercetin for total flavonoid content, and trolox for DPPH assay) equivalent such as the other studies.[Citation31 − Citation33] Ranges of the result of total phenolics were 1.70–1.76 mg GAE/100 g of sample by using Folin-Ciocalteu method (). Leaves had the highest value about total flavonoids (6.6 mg quercetin/100 g dry sample) like results of total phenolics. When results were compared with each other, a high correlation was seen (r 2 = 0.97) ().
Table 4 Antioxidant activities, total phenolics and flavonoids of parts of Tilia rubra subsp. caucasica
Table 5 Correlation coefficients, r 2, for relationships between spectrophometric assay
Antioxidants, on interaction with DPPH, transfer electron or hydrogen atoms to DPPH, and thus neutralize its free-radical character.[Citation34] DPPH free radical-scavenging activities of phenolic extracts of part of tilia sections are shown in . The SC50 value is expressed as mg/mL and represents the concentration of phenolic extracts that is required for 50% of free radicals inhibition. Lower SC50 value indicates higher antioxidant activity. The DPPH scavenging activity of extracts and fractions, expressed in the term of SC50, was in the range of 0.106–0.231 mg/mL, with the strongest antioxidant potency for phenolic extracts of tilia leaves. The radical scavenging of extracts was lower than that of Trolox (0.004 mg/mL). There is a reverse high correlation between total phenolics and SC50 values (r 2 = −0.98) and between total flavonoids and SC50 values (r 2 = −0.90) (). These results showed that tilia leaves might contain the strongest free radical-scavenger compounds.
Leaves of tilia having the highest total polyphenol and flavonoid contents showed the highest cupric reducing power. Correlation values were calculated between 0.90 and 1.00 (). The cupric reducing power of these samples, expressed as Trolox equivalent antioxidant capacity (TEAC), were found to be 0.272–0.552 mmol Trolox/g ().
Briefly, according to the HPLC-UV, HPLC-UV-MS/MS, and spectrophotometrical analysis, the studied plants are a good source of powerful antioxidants, such as phenolics. And results also showed us that types of extraction are extremely important. In the same part of a plant, a component can be determined by an extraction procedure; the same component cannot be determined by another extraction procedure.
ABBREVIATIONS
| DPPH | = |
1,1-Diphenyl-2-picrylhydrazyl |
| CURRAC | = |
Cupric reducing antioxidant capacity |
| GAE | = |
Gallic acid equivalents |
| HPLC | = |
High performance liquid chromatography |
| UV | = |
Ultraviolet |
| MS | = |
Mass spectroscopy |
| SC | = |
Scavenging activity |
| LOD | = |
Limit of detection |
| LOQ | = |
Limit of quantification |
| IS | = |
Internal standard |
| Trolox | = |
6-Hydroxy-2, 5, 7, 8-tetramethylchroman-2 carboxylic acid |
| TPTZ | = |
2,4,6-Tripyridyl-striazine |
ACKNOWLEDGMENTS
Huseyin Şahin would like to thank TUBİTAK BİDEB for the financial support given to him. The authors would also like to thank Professor Salih Terzioğlu for identifying the tilia genus and Faculty of Pharmacology, Karadeniz Technical University for helping with the HPLC device.
REFERENCES
- Astley , S. B. 2003 . Dietary antioxidants—Past . present and future? Trends in Food Science and Technology , 14 : 93 – 98 .
- Hakkinen , S. H. , Heinonen , M. , Karenlampi , S. , Mykkanen , H. M , Ruuskanen , J. and Torronen , R. 1999 . Screening of selected flavonoids and phenolic acids in 19 berries . Food Research International , 32 : 345 – 353 .
- Shaiban , M. , Al-Mamary , M. and Al-Habori , M. 2006 . Total antioxidant activity and total phenolic content in Yemeni smoked, cheese . Malaysian Journal Nutrition , 12 : 87 – 92 .
- Arabshahi-Delouee , S. and Urooj , A. 2007 . Application of phenolic extracts from selected plants in fruit juice . International Journal of Food Properties , 10 : 479 – 488 .
- Kolaylı , S. , Şahin , H. , Ulusoy , E. and Tarhan , Ö. 2010 . Phenolic composition and antioxidant capacities of Helichrysum plicatum . Hacettepe Journal of Biology and Chemistry , 38 : 269 – 276 .
- Yildirim , A. , Mavi , A. , Oktay , M. , Kara , A.A. , Algur , O.F. and Bilaloglu , V. 2000 . Comparison of antioxidant and antimicrobial activities of tilia (Tilia argenta Desf Ex DC), sage (Salvia triloba L.) and black tea (Camellia sinensis) extracts . Journal of Agricultural and Food Chemistry , 48 : 5030 – 5034 .
- Demiray , S. , Pintado , M.E. and Castro , P.M.L. Evaluation of phenolic profiles and antioxidant activities of Turkish medicinal plants: Tilia argentea, Crataegi folium leaves and Polygonum bistorta roots . World Academy of Science, Engineering and Technology, 2009 , 54 312 – 317 .
- Kolayli , S. , M. , Küçük , C. , Duran , Candan , F. and Dinçer , B. 2003 . Chemical and antioxidant properties of Laurocerasus officinalis Roem. (cherry laurel) fruit grown in the Black Sea region . Journal of Agricultural and Food Chemistry , 51 : 7489 – 7494 .
- De Villiers , A. , Lynen , F. , Crouch , A. and Sandra , P. 2004 . Development of a solid-phase extraction procedure for the simultaneous determination of polyphenols, organic acids and sugars in wine . Chromatographia , 59 : 403 – 409 .
- Öztürk , N. , Tunçel , M. and Tunçel , N.B. 2007 . Determination of phenolic acids by a modified HPLC: Its application to various plant materials . Journal of Liquid Chromatography and Related Technologies , 30 : 587 – 596 .
- Singleton , V.L. and Rossi , J.L. 1965 . Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents . American Journal of Enology and Viticulture , 16 : 144 – 158 .
- Marcucci , M.C. , Woisky , R.G. and Salatino , A. 1998 . Use of aluminum chloride in the flavonoids quantification of propolis samples . Mensagem Doce , 46 : 3 – 9 .
- Blois , M.S. 1958 . Antioxidant determinations by use of stable free radical . Nature , 181 : 1199 – 1200 .
- Brand-Williams , W. , Cuvelier , M.E. and Berset , C. 1965 . Use of a free radical method to evaluated antioxidant activity . LWT–Food Science and Technology , 26 : 25 – 30 .
- Molyneux , P. 2004 . The use of the stable free radical diphenylpicrylhyrazyl (DPPH) for estimating antioxidant activity . Songklanakarin Journal of Science and Technology , 26 : 211 – 219 .
- Karagözler , A.A. , Erdağ , B. , Çalmaz , E.Y. and Uygun , A.D. 2008 . Antioxidant activity and proline content of leaf extracts from Dorystoechas hastate . Food Chemistry , 111 : 400 – 407 .
- Apak , R. , Güçlü , K. , Özyürek , M. and Karademir , S.E. 2004 . Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method . Journal of Agricultural and Food Chemistry , 52 : 7970 – 7981 .
- Štěrbová , D. , Matějícek , D. , Vlček , J. and Kubán , V. 2004 . Combined microwave-assisted isolation and solid-phase purification procedures prior to the chromatographic determination of phenolic compounds in plant materials . Analytica Chimica Acta , 513 : 435 – 444 .
- Bravo , M.N. , Silva , S. , Coelho , A.V. , Boas , L.V. and Bronze , M.R. 2006 . Analysis of phenolic compounds in muscatel wines produced in Portugal . Analytica Chimica Acta , 563 : 84 – 92 .
- Borbalán , Á. , Zorro , L. , Guillén , D.A. and Barroso , C.G. 2003 . Study of polyphenol content of red and white grape varieties by liquid chromatography-mass spectrometry and its relationship to antioxidant power . Journal of Chromatography A , 1012 : 31 – 38 .
- Clifford , M.N. , Johnston , K.L. , Knight , S. and Kuhnert , N. 2003 . Hierarchical scheme for LC-MS identification of chlorogenic acids . Journal of Agricultural and Food Chemistry , 5 : 2900 – 2911 .
- Zywicki , B. , Reemtsma , T. and Jekel , M. 2002 . Analysis of commercial vegetable tanning agents by reversed-phase liquid chromatography-electrospray ionization-tandem mass spectrometry and its application to wastewater . Journal of Chromatography A , 970 : 191 – 200 .
- Benavides , A. , Montoro , P. , Bassarello , C. , Piacente , S. and Pizza , C. Catechin derivatives in Jatropha macrantha trunks: Characterization and LC/ESI/MS/MS quali-quantitative analysis . Journal of Pharmaceutical and Biomedical Analysis 2006 , 40 639 – 647 .
- Alonso-Salces , R.M. , Ndjoko , K. , Queiroz , E.F. , Ioset , J.R. , Hostettmann , K. , Berrueta , L.A. , Gallo , B. and Vicente , F. On-line characterization of apple polyphenols by liquid chromatography coupled with mass spectrometry and ultraviolet absorbance detection . Journal of Chromatography A 2004 , 1046 89 – 100 .
- Silva , B.A. , Ferreres , F. , Malva , J.O. and Dias , A.C.P. 2005 . Phytochemical and antioxidant characterization of Hypericum perforatum alcoholic extracts . Food Chemistry , 90 : 157 – 167 .
- Häkkinen , S. and Auriola , S. 1998 . High-performance liquid chromatography with electrospray ionization mass spectrometry and diode array ultraviolet detection in the identification of flavonol aglycones and glycosides in berries . Journal of Chromatography A , 829 : 91 – 100 .
- Lai , J.P. , Lima , Y.H. , Sua , J. , Shen , H.M. and Ong , C.N. 2007 . Identification and characterization of major flavonoids and caffeoylquinic acids in three compositae plants by LC/DAD-APCI/MS . Journal of Chromatography B , 848 : 215 – 225 .
- Robards , K. , Prenzler , P.D. , Tucker , G. , Swatsitang , P. and Glover , W. 1999 . Phenolic compounds and their role in oxidative processes in fruits . Food Chemistry , 66 : 401 – 436 .
- Cannac , M. , Pasqualini , V. , Greff , S. , Fernandez , C. and Ferrat , L. 2007 . Characterization of phenolic compounds in Pinus laricio needles and their responses to prescribed burnings . Molecules , 12 : 1614 – 1622 .
- Alonso , M. , Rozados , M.J. , Vega , J.A. , Perez-Gorostiaga , P. , Cuinas , P. and Fonturbel , M. 2002 . Biochemical responses of Pinus pinaster trees to fire-induced trunk girdling and crown scorch: Secondary metabolites and pigments as needle chemical indicators . Journal of Chemical Ecology , 28 : 687 – 700 .
- Kolayli , S. , Kara , M. , Tezcan , F. , Erim , F.B. , Sahin , H. , Ulusoy , E. and Aliyazicioglu , R. 2010 . Comparative study of chemical and biochemical properties of different melon cultivars: Standard, hybrid, and grafted melons . Journal of Agricultural and Food Chemistry , 58 : 9764 – 9769 .
- Küçük , M. , Kolayli , S. , Karaoglu , S.A. , Ulusoy , E. and Baltaci , C. 2007 . Biological activities of three different Turkish honey. Food Chemistry , 100 : 526 – 534 .
- Gülçin , İ. 2009 . Antioxidant activity of L-adrenaline: An activity-structure insight . Chemico-Biological Interactions , 179 : 71 – 80 .
- Naik , G.H. , Priyadarsini , K.I. , Satav , J.G. , Banavalıkar , M.M. , Sohoni , P.P. and Biyani , M.K. 2003 . Comparative antioxidant activity of individual herbal components used in Ayurvedic medicine . Phytochemistry , 63 : 97 – 104 .