Abstract
Carob has been widely grown in the Mediterranean region for a long time. It has been regarded as only a forest tree and has been neglected for other economic benefits. D-Pinitol is one of the major components in some plants, especially the Leguminosae family. Recently, carob fruit has become more popular because of its D-pinitol content. Therefore, the aim of this study was to evaluate the correlation of sugar profile (glucose, sucrose, and fructose) on the D-pinitol concentration of wild and cultivated types grown in Antalya, Turkey and to compare D-pinitol concentration between both types of carob pods. D-Pinitol concentrations as well as the correlation of sugar profile on it in 32 trees of cultivated and 38 trees of wild carob pods were determined. The results showed that the maximum D-pinitol concentration was 84.59 g/kg in wild type carob pods. Moreover, there was a correlation between sugar profile and D-pinitol for both cultivated and wild types of carob pods.
INTRODUCTION
Carob tree (Ceratonia siliqua L.) belongs to the family Leguminosae (syn. Fabaceae) of the order Rosales and is widely cultivated in the Mediterranean basin.[1] Turkey is one of the native lands of wild and cultivated carob that are grown mainly in the Mediterranean and Aegean region of Turkey. The fruit is a pod with pulp and seed, the pulp being 90% of its total dry weight.[2] Although there are no accurate statistics available about annual world production, about 315,000 tonnes of carob are produced per year.[3] Turkey contributes 4.8% of carob production with the other main producer and exporter countries, including Spain, Italy, Portugal, Morocco, Greece, and Cyprus.[4] Carob pod is a rich source of sugars, minerals, and polyphenolic antioxidants.[3,5,6] Recent studies have focused especially on the nutraceutical components of this fruit and carob has found to be effective in reducing LDL-cholesterol in blood[7] and in postprandial blood glucose in patients with Type II diabetes mellitus,[8] because Carob contains a compound named D-pinitol, a carbohydrate that has an insulin-like effect.[9] This sugar alcohol has been extracted from many plant sources, such as soybean,[10] Bougainvillea flower,[11] and ice plant.[12] Especially, soybean has been used to isolate D-pinitol in many studies.[13,14] Soybean produces large amount of D-pinitol (˜30 g/kg dry weight). Lately, carob has been highlighted because of its higher D-pinitol concentration.[15] Moreover, when carob fruit is compared with other plants for D-pinitol concentration, it has some advantages such as: cheap raw material, an easy extraction method, and rich D-pinitol concentrations.[6,16] Relationship between genotype and chemical profile (sugars, organic acids, anthocyanins, phenolics, etc.) of different fruits such as pomegranate, apricot, and strawberry has been studied by many researchers.[17–20] Both wild and cultivated forms of carobs have been exclusively grown in Turkey. The Mediterranean region has important plant genetic resources. It is assumed that the genetic variation among carob cultivars is rather low compared to the other fruit species, making wild carob highly important. Turkey and Morocco are very important as wild sources of carob. In Turkey, carobs have been propagated from seeds while cultivated varieties are easily propagated vegetatively by grafting. Wild carob is growing native. Main differences between cultivated and wild types carob, the former has larger pod size (pods are thick and long), more pulp and greater sugar content compared with the wild genotypes (pods are thin, long, and flat).[5]
The main aim of this study was to determine the D-pinitol concentrations of the pods of the wild and cultivated genotypes of carob fruits grown in the Mediterranean and Aegean regions of Turkey and to compare D-pinitol concentrations between the types of carob pods. Also, the sugar profile of carob fruit was investigated to see whether it had a correlation with D-pinitol concentrations in carob fruit.
MATERIALS AND METHODS
Carob fruits were randomly harvested from trees located near the coastline of the eastern to western Mediterranean region of Turkey in August 2008–August 2009, and 250 fruits were collected from each tree. The main carob growing areas (Mediterranean and Aegean) were surveyed and a total 70 promising wild and grafted carob genotypes were selected based on physical and chemical properties in order to show the authenticity of wild and cultivated carobs genotype was ensured.[5] Samples were taken from 32 trees of cultivated and 38 trees of wild genotypes and each sample contained 15–20 beans. After removing the seeds, the carob pods were comminuted into small particles (3–5 mm) and stored at +4°C until analysis. The pods were used for determination of D-pinitol and sugar concentrations. All samples were analyzed in duplicate.
For chemical analysis, pods were crushed and passed through a 35-mesh sieve. Then, 10 g of ground pods were mixed with 40 ml DI water in a beaker and homogenized (IKA-WERKE Ultra Turrax T 25 B, Staufen, Germany) for 5 min. Total dry matter was determined gravimetrically by placing 3 g of the samples in a drying oven at 70°C.[21] Soluble solid content (SSC) was measured at 25°C using an ATC-1E Abbe refractometer (Atago, Tokyo, Japan). pH was measured using a WTW 537 digital pH meter (WTW, Weilheim, Germany) at 25 ± 0.5°C. Total acidity was determined by titration with 0.1 N NaOH (Merck, Darmstadt, Germany) and calculated as percent of citric acid (anhydrous).[21] Crude fiber was analyzed and calculated according to Ozkaya.[22]
Sugar Profile and D-Pinitol Analyses
To determine D-pinitol and sugar content, 2 g of deseeded carob pods were weighed approximately, ground and diluted with MilliQ water (1:18), shaken gently and homogenized using an Ultraturrax macerator (IKA-WERKE Utra Turrax T25B, Staufen, Germany) at 24,000 rpm and centrifuged at 7000 rpm for 30 min at ambient temperature. Then the supernatant was diluted with MilliQ water (1:29). Diluted samples were passed through a 0.45-μm membrane filter (CHROMAFIL® PET-45/25, Macherey-Nagel, Düren, Germany) before analysis.
D-Pinitol and sugar analyses were determined by a Shimadzu LC-20AD HPLC solvent delivery system (Shimadzu, Tokyo, Japan). The HPLC system was equipped with a guard column (CARBOsep Coregel 87P, 4 × 20 mm, Transgenomic, Omaha NE, USA), connected to an analytical column (CARBOsep Coregel 87P, 7.8 × 300 mm, Transgenomic) and a Shimadzu RID-10A refractive index detector (Shimadzu). The columns were heated to 85°C with a Varian Mistral column oven (Varian, Palo Alto, CA, USA). MilliQ water as the mobile phase was allowed to flow at the rate of 0.6 mL min−1. The method used for chromatographic analysis of samples was offered by the manufacturer of the analytical column (Transgenomic, NE). Samples of 20 μL were injected using a Shimadzu SIL-20A autosampler (Shimadzu). D-Pinitol and sugar concentrations were calculated using standard curves obtained from injection of standard solutions prepared in MilliQ water in the range of 25–200 g/L as the external standards. The results were expressed in g/kg of dried weight and D-pinitol and sugar identification was made by comparing the relative retention times of sample peaks with standards.[6]
Data Analysis
All carob pod samples and analyses for content of D-pitinol and sugars were replicated twice. To evaluate the significance of the results between wild and cultivated types, the generalized linear model (GLM; with p < 0.05) and Tukey's honestly significant differences (HSD) multiple comparison module within Minitab Statistical Software Package (Release 13.3, Minitab Inc., State College, PA, USA) were used.
RESULTS AND DISCUSSION
This study was designed not only to show the content of sugars and D-pinitol for cultivated and wild genotypes of carob pods but also to determine the correlation between sugar profiles and D-pinitol in both types of carob pods.
The chemical analysis of both cultivated and wild types were shown in . Total dry matter and soluble solids of all species varied from 86.6 to 98.1 g/100 g and 51.0 to 83.5 g/100 g, respectively (). pH, total acidity, and total fiber contents were different among populations of each carob type dependent on years. Descriptive values belonging to the test groups were compared with a 2-sample t-test. According to the results, there were significant differences among cultivated and wild types in respect to total dry weight (wild higher), pH (wild higher), soluble solids (cultivated higher), and total fiber (wild higher) (p < 0.01). Soluble solids values were found to be lower in the wild genotype than in the domesticated genotypes. Marakis et al.[23] found that soluble solids were between 32 and 60% according to countries.
Table 1 Some descriptive properties of carob pods
Table 2 D-Pinitol and sugar concentrations of carob pods
D-Pinitol and Sugar Concentrations
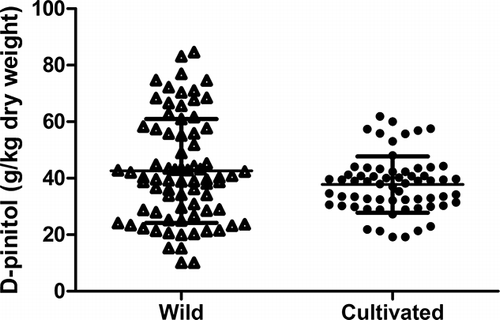
Distribution of D-pinitol concentrations of cultivated and wild types of carob pods were shown in Fig. 1. D-Pinitol concentrations of cultivated and wild genotypes of carob ranged from 19.2 to 61.9 g/kg and 10.1 to 84.6 g/kg, respectively (Fig. 1). The average D-pinitol concentration for cultivated carob pod was 37.8 g/kg, while it was 42.6 g/kg for wild types (). The maximum D-pinitol concentration was 84.6 g/kg in wild type carob pods (n = 38). Mean values of D-pinitol concentrations of both types did not differ significantly (p < 0.01). Soybean and dried soy were rich sources of D-pinitol, and contain approximately 5 and 20 g/kg of this compound, respectively.[24] Kim et al.[24] reported that carob pods contain 40 g/kg of chrio-inositol, which consists of 99% D-pinitol. Murakeozy et al.[25] found that concentrations of D-pinitol ranged in leaf samples of Limonium gmelini subsp. hungarica (Plumbaginaceae) from 2.91 to 15.13 g/kg dry weight. Some researchers determined that the presence of some cyclitols in honey was around 0.09–8.15 g/kg dry weight.[26] When the results were compared with the D-pinitol content of other plants, carob pods seem to be an important source with a rich content of D-pinitol.
The cultivated types had the highest average sucrose concentration (437 g/100 g) (). Conversely, the wild types had the highest fructose and glucose concentrations (97.8 and 64.5 g/100 g). In other words, there were a few cultivated and wild types that contained high sucrose, fructose, and glucose, and they were different from each other. The glucose was the lowest concentration of all sugars among the carob genotypes. The carob sugar profiles reported by several researchers demonstrated significant variations according to the country of origin and variety.[23] Sucrose has been determined to be the major sugar in carobs. These results were similar to the reported values for both cultivated and wild types of carob pods.[5]
Correlation of Sugar Profiles with D-Pinitol Content
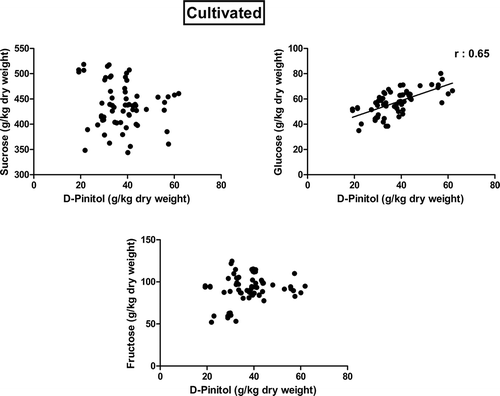
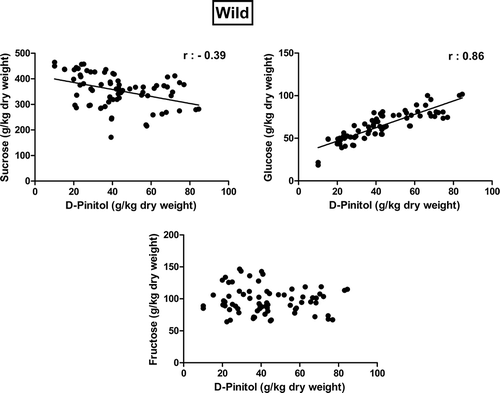
The correlation of sugar profile on D-pinitol for cultivated and wild types of carob pods were illustrated in Figs. 2 and 3. When the correlation of sugar profile on D-pinitol concentration was investigated, it was also interesting to notice the positive correlation (r = 0.650 and r = 0.860) between glucose and D-pinitol for both cultivated and wild types of carob pods (Figs. 2 and 3). It means that higher glucose concentrations in fruit resulted in higher D-pinitol concentrations. However, the correlation of sucrose and fructose concentration on D-pinitol was not significant (p > 0.01) for cultivated carob pods. On the other hand, sucrose concentrations in wild type carob pods had a negative correlation (r = −0.390) with D-pinitol concentration. When the sucrose concentration in wild carob pods was low, the D-pinitol concentration was decreased (Fig. 3).
CONCLUSION
This study showed that cultivated and wild types of carob pods are a rich source of D-pinitol and sugars. Moreover, there was a positive correlation between glucose and D-pinitol for both cultivated and wild types of carob pods. It means that higher glucose concentrations in fruit resulted in higher D-pinitol concentrations. As a result, it appears necessary to identify carob types with minimum and maximum content of D-pinitol and to determine the relationship between sugar profiles and D-pinitol content to give a better overview of the D-pinitol in the varieties. When compared with carob fruit and other plants as sources of D-pinitol, carob pods are the cheapest, they are grown naturally, have a high content of sugars, and an easy processing method. The results of this work open up the possibility of further investigation in many directions, including the optimization of extraction of D-pinitol from cultivated and wild types of carob pods with high concentration of D-pinitol.
ACKNOWLEDGMENTS
This study was supported in part by the Akdeniz University Research Foundation and COST FA0602 Action (Bioactive food components, mitochondrial function and health). The authors gratefully acknowledge the Scientific and Technical Research Council of Turkey for financial support (Project TOVAG-107O650); Associate Professor Dr. David Turner (School of Plant Biology, Faculty of Natural and Agricultural Sciences, The University of West Australia, Crawley, WA 6009) for reviews and comments on the manuscript; and Yenigun Food, Inc. (Antalya, Turkey) for supplying carob pods.
REFERENCES
- Battle , I. and Tous , J. 1997 . “ Carob Tree (Ceratonia siliqua L.) ” . In International Plant Genetic Resources Institute: Rome 10 – 39 . Italy
- Correia , P.J. and Martins-Loução , M.A. 2004 . The use of macronutrients and water in marginal Mediterranean areas: the case of carob-tree . Field Crops Research , 91 ( 1 ) : 1 – 6 .
- Makris , D.P. and Kefalas , P. 2004 . Carob pods (Ceratonia siliqua L.) as a source of polyphenolic antioxidants . Food Technology and Biotechnology , 42 ( 2 ) : 105 – 108 .
- Janick , J. and Paull , R.E. 2008 . “ The Encyclopedia of Fruit & Nuts ” . In CAB International , 387 – 392 . Cambridge , UK : Cambridge University Press .
- Biner , B. , Gubbuk , H. , Karhan , M. , Aksu , M. and Pekmezci , M. 2007 . Sugar profiles of the pods of cultivated and wild types of carob bean (Ceratonia siliqua L.) in Turkey . Food Chemistry , 100 : 1453 – 1455 .
- Tetik , N. , Turhan , I. , Oziyci , H.R. and Karhan , M. 2011 . Determination of D-pinitol in carob syrup . International Journal of Food Science & Nutrition , 62 ( 6 ) : 572 – 576 .
- Zunft , H.J.F. , Lüder , W. , Harde , A. , Haber , A. , Graubaum , J.J. and Gruenwald , J. 2001 . Carob pulp preparation for treatment for hypercholesterolemia . Advances in Therapy , 18 ( 5 ) : 230 – 236 .
- Kang , M.J. , Kim , J.I. , Yoon , S.Y. , Kim , J.C. and Cha , I.J. 2006 . Pinitol from soybeans reduces postprandial blood glucose in patients with type 2 diabetus mellitus . Journal of Medicinal Food , 9 ( 2 ) : 182 – 186 .
- Bates , S.H. , Jones , R.B. and Bailey , C.J. 2009 . Insulin-like effect of pinitol . British Journal of Pharmacology , 130 ( 8 ) : 1944 – 1948 .
- Smith , A.E. and Phillips , D.V. 1982 . Influence of sequential prolonged periods of dark and light on pinitol concentration in clover and soybean tissue . Pysiologia Plantarum , 54 ( 1 ) : 31 – 33 .
- Narayanan , C.R. , Joshi , D.D. , Mujumdar , A.M. and Dhekne , V.V. 1987 . Pinitol—A new anti-diabetic compound from the leaves of Bougainvillea spectabilis . Current Science , 56 ( 3 ) : 139 – 141 .
- Vernon , D.M. , Tarczynski , M.C. , Jensen , R.G. and Bohnert , H.J. 1993 . Cyclitol production in transgenic tobacco . The Plant Journal , 4 ( 1 ) : 199 – 205 .
- Streeter , J.G. 2001 . Simple partial purification of D-pinitol from soybean leaves . Crop Science , 41 ( 6 ) : 1985 – 1987 .
- Shin , Y.C. , Jeon , Y.C. , Kim , J.J. and Choi , C. 20030186401; 2003 . “ Method of recovering pinitol or chiro-inositol in high yield from soy fractions ” . In United States Patent
- Camero , B.M. and Merino , C.S. 6699511; 2004 . “ Method of obtaining pinitol from carob extracts ” . In United States Patent
- Turhan , I. , Bialka , L.K. , Demirci , A. and Karhan , M. 2010 . Ethanol production from carob extract by using Saccharomyces cerevisiae . Bioresource Technology , 101 ( 14 ) : 5290 – 5296 .
- Leccese , A. , Bartolini , S. and Viti , R. Genotype . 2011 . “ harvest season and cold storage influence on fruit quality and antioxidant properties of apricot ” . In International Journal of Food Properties DOI: 10.1080/10942912.2010.506019.
- Legua , P. , Melgarejo , P. , Martinez , J.J. , Martinez , R. and Hernandez , F. 2011 . “ Evaluation of Spanish pomegranate juices: Organic acids, sugars and anthocyanins ” . In International Journal of Food Properties DOI: 10.1080/10942912.2010.491931.
- Yu , C. , Ranieri , M. , Lv , D. , Zhang , M. , Charles , M.T. , Tsao , R. , Rekika , D. and Khanizadeh , S. 2010 . Phenolic composition and antioxidant capacity of newly developed strawberry lines from British Columbia and Quebec . International Journal of Food Properties , 14 ( 1 ) : 59 – 67 .
- Wanlapa , S. , Wachirasiri , K. , Sithisam-ang , D. and Suwannatup , T. 2011 . “ Potential of selected tropical fruit peels as dietary fiber in functional foods ” . In International Journal of Food Properties DOI: 10.1080/10942912.2010.535187.
- 1990 . “ AOAC. Official Methods of Analysis ” . In Ed.; Association of Official Analytical Chemists , 15th Washington DC
- Ozkaya , H. 1988 . Analytical Food Quality ControlFaculty of Agriculture Publications , 137 Ankara , Turkey : University of Ankara .
- Marakis , S. , Kalaitzakis , J. and Mitrakos , K. 1988 . “ Criteria for recognizing carob tree varieties ” . In Proc II International Carob Symposium 195 – 208 . Valencia , Spain
- Kim , J.I. , Kim , J.C. , Joo , H.J. , Jung , S.H. and Kim , J.J. 2005 . Determination of total chiro-inositol content in selected natural materials and evaluation of the antihyperglycemic effect of pinitol isolated from soybean and carob . Food Science and Biotechnology , 14 : 441 – 445 .
- Murakeozy , E.P. , Smirnoff , N. , Nagy , Z. and Tuba , Z. 2002 . Seasonal accumulation pattern of pinitol and other carbohydrates in Limonium gmelini subsp. hungarica . Journal of Plant Physiology , 159 : 485 – 490 .
- Sanz , M.L. , Sanz , J. and Martinez-Castro , I . 2004 . Presence of some cyclitols in honey . Food Chemistry , 84 : 133 – 135 .