Abstract
Methanolic extract of Musa paradisiaca inflorescence and its different solvent fractions, petroleum ether, ethyl acetate, butanol, and water were studied for their total phenolic and flavonoid content, free radical scavenging, and antiglycation activities, and these properties were compared with standard antioxidant compounds. Ethyl acetate fraction exhibited higher antioxidant and antiglycation properties than other fractions. IC50 values of ethyl acetate fraction for 1,1-diphenyl-2-picrylhydrazyl method, 2,2-azinobis-3 ethylbenzothiazoline-6-sulphonic acid method, superoxide radical scavenging, hydroxyl radical scavenging, nitric oxide radical scavenging, and antiglycation activities were 9.80, 13.50, 26.40, 19.71, 25.73, and 31.00 μ g/ml, respectively. Total phenolic content of ethyl acetate (21.52 mg GAE/g) was significantly higher than other fractions. There was positive linear correlation between antioxidant activity and total phenolic content.
INTRODUCTION
The role of free radicals in many disease conditions has been well established. Reactive oxygen species (ROS), such as superoxide anion (O2 1), hydrogen peroxide (H2O2), and hydroxyl radical (OH), are constantly formed in the human body by normal metabolic actions. Their action is opposed by a balanced system of antioxidant defenses, including antioxidant compounds and enzymes. Upsetting this balance causes oxidative stress, which can lead to cell injury and death.[Citation1] Epidemiological and in vitro studies strongly suggest that food containing phytochemicals with antioxidants have protective effects against many diseases, including cardiovascular diseases, diabetes, and cancer.[Citation2] Plants contain a diverse group of phenolic compounds.[Citation3] Diets rich in fruits and vegetables are reported to be protective against chronic diseases like cancer and heart disease. These protective effects are generally attributed to the presence of various functional components, such as phenolic compounds, vitamin C, vitamin E, provitamins, minerals, and fibers. Many of these chemicals have been claimed to contribute to the antioxidant activities of the plants, and the antioxidant activity is mainly due to their redox properties derived from various possible mechanisms: free radical scavenging activity, transition-metal-chelating activity, and singlet oxygen quenching capacity. Phenolic compounds are beneficial for human health and can decrease the risk of degenerative diseases by reducing oxidative stress and inhibiting macromolecular oxidation.[Citation4,Citation5] Hyperglycemia is a well-recognized pathogenic factor in diabetes. Chronic hyperglycemia leads to nonenzymatic glycation of biological proteins with irreversible formation of reactive advanced glycation end products (AGEs). This process occurs in vivo through the covalent binding of aldehyde or ketone groups of reducing sugars to free amino groups of proteins, forming AGEs. The AGEs have a propensity to generate ROS.[Citation6] In addition, glucose and other aldehydes, whether free or protein-bound, can undergo autoxidation reactions to yield radicals and other reactive intermediates (e.g., H2O2 and other peroxides), which can also contribute to AGEs formation.[Citation7]
Musa paradisiaca is a herbaceous flowering plant belonging to the family musaceae. It is one of the tropical plants that have been consumed for centuries by humans and animals as a nutritious food. The banana's inflorescence and inflorescence stalk are widely used for culinary purposes in southern parts of India.[Citation8] In Ayurveda, a traditional system of medicine in India, Musa paradisiaca, is cited for treatment of many disorders. Its leaves can be used in the treatment of cough and bronchitis. Roots are used to arrest hemoptysis, and possess strong astringent and anthelmintic properties.[Citation9] The fruits can improve renal and muscular activities, reduce the risk of kidney cancer and hypertension. It is also used for the treatment of diarrhea, stomach ache, lack of appetite, gastric ulcer, mental shock, and strengthening the immune system and bones.[Citation10] The inflorescence and inflorescence stalk have been reported to possess antidiabetic activity and are also used in naturopathy for reducing obesity.[Citation11,Citation12] The juice of the flowers mixed with curd is administered in dysmenorrhea and menorrhagia. Epidemiological studies have suggested positive associations between the consumption of phenolic-rich foods or beverages and the prevention of diseases.[Citation13] In the present study, an attempt was made to evaluate the antioxidant, antiglycation, and radical scavenging activities of methanolic extract and its different solvent fractions of M. paradisiaca (Nendran) inflorescence by different in vitro models.
MATERIALS AND METHODS
Chemicals
1,1-Diphenyl-2-picrylhydrazyl (DPPH), 2,2-azinobis-3 ethylbenzothiazoline-6-sulphonic acid (ABTS), Trolox (6-hydroxy-2,5,7,8-tetramethyl chroman-2-carboxylic acid), ascorbic acid, curcumin, catechin, and amino guanidine were purchased from Sigma Aldrich Chemical Co. (St. Louis, MO, USA). Thiobarbituric acid (TBA), ethylene diamine tetra acetic acid (EDTA) and nicotinamide adenine dinucleotide (NADH), deoxyribose, nitro blue tetrazolium (NBT), phenazine methosulphate (PMS), trichloroacetic acid (TCA), potassium persulphate, and gallic acid were of analytical grade and were purchased from Sisco Research Lab (Mumbai, India).
Plant Material and Extraction
Inflorescence of Musa paradisiaca (MPI) were freshly collected from farms at Adoor, Pathanamthitta District, Kerala, India. The plant material was collected after the fruits were developed. Inflorescence of MPI were shade dried, coarse powdered, and extracted with methanol using a Soxhlet apparatus. The extract was filtered through Whatman No. 1 filter paper and concentrated in vacuum at 40°C in a rotary evaporator (Heidolph, Schwabach, Germany). The methanolic extract (ME) was mixed with equal volume of water and successively fractionated with various solvents viz., petroleum ether (PE), ethyl acetate (EA), butanol (BU), and water (WA).[Citation14] These fractions were collected and screened for the antioxidant and antiglycation activities.
Preliminary Phytochemical Analysis
The methanolic extract and different solvent fractions of MPI were subjected to preliminary phytochemical testing for the detection of major classes of chemical compounds.[Citation15]
Evaluation of In Vitro Antioxidant Activities
DPPH radical scavenging activity
The antioxidant activity of MPI was measured in terms of hydrogen donating or radical scavenging ability using the stable DPPH method.[Citation16] To 2.8 ml of methanolic 1,1-diphenyl-2-picrylhydrazyl (DPPH 1.4 mM) and 0.2 ml of extracts at various concentrations were added, mixed well and incubated for 30 min at room temperature. The absorbance was measured at 517 nm using a UV-visible spectrophotometer (Shimadzu UV–Vis Spectrophotometer, Model 1240, Shimadzu, Kyoto, Japan). Gallic acid was used as the reference standard.
ABTS radical scavenging activity
The scavenging activity MPI extracts on 2,2-azinobis-3 ethylbenzothiazoline-6-sulphonic acid (ABTS) radical cation was measured according to the method of Re et al.[Citation17] ABTS (7 mM) and potassium persulphate (2.45 mM) were mixed and kept in the dark at room temperature overnight for generating the ABTS radical cation. This was diluted with distilled water to obtain an absorbance of 1.45–1.5 nm at 734 nm. The diluted ABTS radical cation solution was added to 50 μ l of MPI extracts at different concentrations and Trolox was used as the standard. After 90 min, the absorbance was measured at 734 nm.
Superoxide anion scavenging activity
Superoxide anion scavenging activity of MPI extracts were assessed by NBT–PMS method.[Citation18] To varying concentrations of MPI extracts, Tris-HCl buffer (16 μM, pH 8.0), NADH (78 μM), NBT (338 μM), and PMS (30 μM) were added and incubated at 25°C. After 5 min, the absorbance was measured at 560 nm. Ascorbic acid was used as the positive control.
Hydroxyl radical scavenging activity
The hydroxyl radical scavenging activity was measured by the deoxyribose method[Citation19] and compared with catechin. The reaction mixture, which contained MPI extracts, deoxyribose (3.75 mM), H2O2 (1 mM), potassium phosphate buffer (20 mM, pH 7.4), FeCl3 (0.1 mM), EDTA (0.1 mM), and ascorbic acid (0.1 mM), were incubated in a water bath at 37°C for 1 h. Then, 1 ml of thiobarbituric acid (1% w/v) and 1 ml of trichloro acetic acid (2.8% w/v) were added to the mixture and heated in a water bath at 100°C for 20 min. The absorbance of the resulting mixture was measured at 532 nm.
Nitric oxide radical scavenging activity
Nitric oxide radical scavenging activity of extracts was determined by the sodium nitroprusside method.[Citation20] Initially, 2 ml of 10 mM sodium nitroprusside was mixed with 0.5 ml of MPI extracts at various concentrations and incubated for 150 min. Then, 0.5 ml of the incubation mixture was taken and mixed with 0.5 ml of Griess reagent; the absorbance was then read at 540 nm after 30 min. Curcumin was used as the positive control.
Determination of total phenolic content
Total phenolic content (TPC) of the extracts were determined using the Folin–Ciocalteu method and using gallic acid as the reference compound (20–100 μg/ml concentration).[Citation21] To 100 μl of extracts, 500 μl of Folin–Ciocalteu reagent and 1 ml of sodium carbonate (20%) were added and incubated at 37°C for 90 min. The absorbance was measured at 760 nm. The TPC of MPI extracts were expressed as milligram gallic acid equivalent per gram.
Determination of total flavonoid content
Total flavonoid content (TFC) was determined by the method of Jia et al.[Citation22] First, 1 ml of 5% sodium nitrite and MPI extracts were mixed and kept for 6 min followed by the addition of 1 ml of 10% aluminium chloride. After 6 min, 10 ml of 4.3% sodium hydroxide was added and the total volume was made up to 25 ml with distilled water. After keeping 15 min at room temperature, absorbance was measured at 510 nm. The TFC of MPI extracts were expressed as milligram catechin equivalents per gram of extract (mg CE/g DW).
Total reducing power
The reductive potential of the extract was determined by the method of Oyaizu et al.[Citation23] The different concentrations of extracts and standard in 1 ml of distilled water were mixed with phosphate buffer (0.2 M, pH 6.6) and potassium ferricyanide (1% w/v). The mixture was incubated at 50°C for 20 min and then 10% of TCA was added to the mixture, and then subjected to centrifugation for 10 min. The upper layer of the solution was taken and then mixed with distilled water and 0.1% FeCl3. The absorbance was read at 700 nm. Ascorbic acid was the reference standard. An increased absorbance of the reaction mixture indicated increased reducing power.
Antiglycation activity
Antiglycation potential was determined by the method of Matsuura et al.[Citation24] Albumin (1 mg/ml final concentration) was incubated with glucose (500 mM final concentration) in the presence of different concentrations of extracts. Phosphate buffered saline (PBS) was used as the control. The final volume of the reaction mixture was 1 ml. The mixture was kept at 37°C for 7 days. After 7 days, the reaction was stopped by adding 10 μL of 100% (w/v) trichloroacetic acid (TCA). The reaction mixture was kept at 4°C for 10 min and subjected to centrifugation at 10,000× g. The precipitate was dissolved in alkaline PBS (pH 10) and estimated the relative amount of glycated bovine serum albumin (BSA) based on fluorescence intensity by a spectrofluorimeter. The excitation and emission wavelengths used were 370 and 440 nm, respectively. Aminoguanidine was used as the standard inhibitor.
Statistical Analysis
The experimental results were expressed as means ± SD of three parallel measurements. The results were processed using Microsoft Excel 2003 and the data were subjected to one way analysis of variance (ANOVA) and the significance of differences between samples means were calculated by Duncan's multiple range tests using SPSS for Windows, Standard Version 11.5 (SPSS Inc., Chicago, IL, USA).
RESULTS AND DISCUSSION
Preliminary Phytochemical Analysis
Qualitative phytochemical analysis of ME and its different solvent fractions () revealed the presence of phenolics, flavonoids, terpenoids, alkaloids, tannins, steroids, saponins, and reducing sugars.
Table 1 Preliminary phytochemical screening of methanolic extract and different solvent fractions of Musa paradisiacal inflorescence
Antioxidant Activities
The 1,1-diphenyl-2-picrylhydrazyl radical (DPPH . ) is a stable free radical, with an absorption maximum at 517 nm. When reduced to hydrazine derivative by an antioxidant via electron or hydrogen atom transfer reactions, this absorption maximum decreases. The DPPH . radical scavenging activity of the ME and its different solvent fractions of M. paradisiaca inflorescence were tested through this method and the results were compared with gallic acid. IC50 values is defined as the concentration required to scavenge 50% of the available free radicals. The ME, PE, EA, BU, and WA of MPI exhibited a significant dose dependent inhibition of DPPH activity. The IC50 values of these extracts were 28.50, 65.00, 9.80, 61.00, and 52.00 μg/ml, respectively, and that of reference compound gallic acid was 1.50 μg/ml (). DPPH has been extensively used as a free radical to evaluate reducing substances and is used for investigating the free radical scavenging actions of compounds. Phenolic compounds in plants are viewed as powerful antioxidants and their effects on DPPH are due to their hydrogen-donating ability.[Citation25]
Table 2 IC50 values of DPPH radical, ABTS radical, superoxide radical, hydroxyl radical, and nitric oxide radical, scavenging activities of Musa paradisiaca inflorescence [IC50 value μg/ml (mean ± SD)]
The most widely used colorimetric method is 2,2-azinobis 3-ethylbenzothiazoline-6-sulfonate (ABTS .+ ), in which a colorless molecule, reduced ABTS, is oxidized to a characteristic blue-green ABTS . +. When the colored ABTS .+ is mixed with any substance that can be oxidized, it is reduced to its original colorless form. ABTS radical scavenging assay measures the relative antioxidant ability to scavenge the radical as compared with a standard compound trolox (IC50 8.5 μg/ml) (Trolox equivalent antioxidant capacity, TEAC). Compared to ME and other fractions, the EA fraction exhibited a maximum TEAC (IC50 13.50 μg/ml) followed by ME (35.50 μg/ml), PE (96.80 μg/ml), BU (73.00 μg/ml), and WA (69.00 μg/ml) (). This method is widely used to evaluate antioxidant activities in foods and biological systems.[Citation26]
Superoxide radical scavenging activities of extracts were measured by autoxidation of hydroxylamine in the presence of NBT (nitroblue tetrazolium). The reduction of NBT in the presence of antioxidants was measured. A decrease in absorbance at 560 nm indicates the consumption of superoxide anion in the reaction mixture. Ascorbic acid (IC50 15.50 μg/ml) was used as the reference standard. ME, PE, EA, BU, and WA possess IC50 of 44.70, 105.00, 26.40, 112.00, and 97.00 μg/ml, respectively (). The EA fraction shows the highest superoxide radical scavenging activity compared to ME and other fractions. Superoxide anions play important roles in the formation of ROS, such as hydrogen peroxide, hydroxyl radical, and singlet oxygen, which induce oxidative damage in lipids, proteins, and DNA. In cellular oxidation reactions, superoxide radicals normally are formed first and their effects can be magnified because they produce other kinds of free radicals and oxidizing agents.[Citation27]
The effect of ME and its fractions in scavenging hydroxyl radicals to prevent the oxidative degradation of deoxyribose substrate was determined and compared with that of catechin (10.26 μg/ml). The EA fraction of MPI extract exhibiting the highest hydroxyl radical scavenging activity with an IC50 value of 19.71 μg/ml is significantly higher than ME (39.70 μg/ml), PE (85.80 μg/ml), BU (63.50 μg/ml), and WA (75.40 μg/ml) (). Among the free radicals, hydroxyl radicals are most reactive and capable of inducing severe damages to cells. The hydroxyl radical scavenging activity of EA fraction may be due to the active hydrogen-donating ability of hydroxyl substitutions of phenolic compounds present in MPI. Phenolic compounds are good electron donors and they may accelerate the conversion of H2O2 to H2O. Hydroxyl radicals are very strong reactive oxygen species and there is no specific enzyme to defend against them in humans. Therefore, it is important to discover chemicals with good scavenging capacity for these reactive oxygen species. The hydroxyl radical scavenging capacity of an extract is directly related to its antioxidant activity.[Citation28]
Nitric oxide was generated from sodium nitroprusside and measured by the Greiss reaction. Sodium nitroprusside in aqueous solution at a physiological pH spontaneously generates nitric oxide, which interacts with oxygen to produce nitrite ions that can be estimated by use of Greiss reagent. Scavengers of nitric oxide compete with oxygen leading to reduced production of nitric oxide.[Citation29] Nitric oxide radical generated from sodium nitroprusside at physiological pH was found to be inhibited by MPI extracts. The IC50 values of ME (43.00 μg/ml), PE (113.00 μg/ml), EA (25.73 μg/ml), BU (80.00 μg/ml), and WA (103.00 μg/ml) were compared with the standard curcumin (IC50 17.00 μg/ml) (). Among the extracts, EA fraction showed superior nitric oxide (NO) radical scavenging activity (IC50 25.73 μg/ml). NO has an important role in various inflammatory processes. Production of nitric oxide radicals are toxic to tissues, when it reacts with superoxide radical forms highly reactive peroxy nitrite anion (ONOO−).[Citation30]
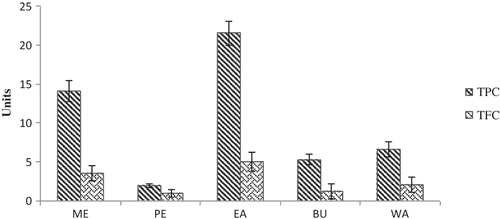
Figure 1 TPC and TFC of the ME and its different fractions of Musa paradisiaca inflorescence. Units: total phenolic content expressed as mg GAE/g dry weight; total flavonoid content expressed as mg CE/g dry weight. ME: methanolic extract; PE: petroleum ether fraction; EA: ethyl acetate fraction; BU: butanol fraction; WA: water fraction.
Total Phenolic and Flavonoid Content
Total phenolic content was estimated by the Folin–Ciocalteu colorimetric method, using gallic acid as the standard. From a linear calibration curve of gallic acid, in the range of 20–100 μg/ml, total phenolic content was obtained and expressed as gallic acid equivalents in mg/g dry material (mg GAE/g dry weight). Results showed that total phenolic content in ME, PE, EA, BU, and WA were 14.13, 2.30, 21.52, 5.33, and 6.60 mg GAE/g, respectively. There was a positive linear correlation between antioxidant activity and total phenolic content. These results suggested that the phenolic compounds contributed significantly to the antioxidant capacity of MPI. Previous studies on fruits and vegetables also show a correlation between phenolic content and antioxidant activities.[Citation31] Total flavonoid content (TFC) of MPI was evaluated by aluminum trichloride-sodium nitrite colorimetric assay. TFC in ME, PE, EA, BU, and WA were 3.59, 1.80, 5.02, 1.22, and 2.10 mg CE/g, respectively. Among the ME and its fractions, EA fraction contained the highest amount of phenolics and flavonoids (Fig. 1). Plant foods contain different classes and types of antioxidants; knowledge of their total antioxidant capacity, which is the cumulative capacity of food components to scavenge free radicals, would be useful for epidemiologic purposes.[Citation32] Phenolics and flavonoids have been reported to be the main phytochemicals responsible for the antioxidant capacity of fruits and vegetables. It was suggested that polyphenolic compounds have inhibitory effects on mutagenesis and carcinogenesis in humans, when ingested up to 1 g daily from a diet rich in fruits and vegetables.[Citation33] Most of the beneficial effects of flavonoids are attributed to their antioxidant and chelating abilities.[Citation34] Studies have also shown that certain flavonoids exhibit hypoglycemic effects.[Citation35]
Total Reducing Power
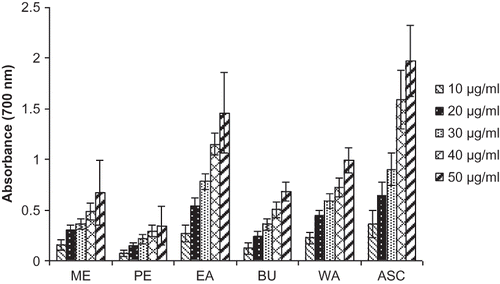
Reducing power is one mechanism of action of antioxidants and may serve as a significant indicator of potential antioxidant activity. In the present study, reducing activity was determined based on the ability of extracts to reduce a Fe3+/ferricyanide complex to form Fe2+/ferrous complex and was compared to ascorbic acid. The amount of Fe2+ was monitored by measuring the formation of Perl's Prussian blue at 700 nm. Reducing power of ME and its different fractions of MPI using the potassium ferricyanide reduction method and the results were depicted in . The reducing power of the MPI extracts and the reference compound ascorbic acid increased linearly with increasing concentrations. The total reducing power (TRP) of the ME, PE, EA, BU, WA, and the ASC were 0.672, 0.346, 1.46, 0.681, 0.995, and 1.97 nm, respectively, at a dose of 50 μg concentration. The antioxidant activity of phenolic compounds is mainly due to their redox properties, which can play an important role in absorbing and neutralizing free radicals, quenching singlet and triplet oxygen, or decomposing peroxides.[Citation36] Tanaka et al.[Citation33] have observed a direct correlation between antioxidant activities and reducing power of certain plant extracts. The reducing properties are generally associated with the presence of reductones, which have been shown to exert antioxidant action by breaking the free radical chain by donating a hydrogen atom.[Citation37] The higher free radical scavenging activities of the EA fraction of MPI may be due to its higher phenolic and flavonoid content.
Antiglycation Activity
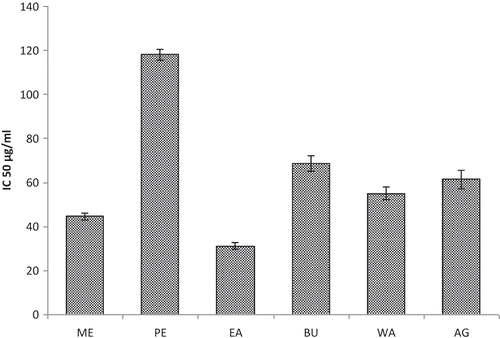
The protective effect of MPI on advanced glycation end products (AGEs) formation was studied. The results were shown in . To confirm the potent inhibitory effects of ME, different solvent fractions of MPI were evaluated for their ability to suppress AGE formation in a BSA/glucose system. The IC50 values of ME, PE, EA, BU, WA, and aminoguanidine were 44.00, 118.00, 31.00, 68.00, 55.00, and 61.00 μg/ml, respectively. EA fraction was able to achieve more inhibitory effect against AGE formation than ME and other fractions in vitro. Endogenous AGE formation is known to contribute to the progression of pathogenesis in conditions associated with diabetic complications, aging, and Alzheimer's disease, and free radicals have been shown to participate in AGE formation.[Citation38] The present study clearly indicates that phytoconstituents of MPI can afford protection against the formation of advanced glycation end products. So dietary supplementation of inflorescence will be beneficial in modulating complications associated with diabetes mellitus and it can be included as functional food for ameliorating diabetic complications. A representative drug used is aminoguanidine (AG), a hydrazine compound, which prevents AGE formation by trapping intermediates at the initial glycation stages.[Citation39] Recent attention has focused on the benefits of medicinal plants with both antiglycation and antioxidant properties.[Citation40]
CONCLUSION
On the basis of results of different in vitro antioxidant models, it is evident that MPI has effective antioxidant and antiglycation potential. This study also indicates that EA fraction of MPI has the highest antioxidant and antiglycation effects. The presence of different phytoconstituents in MPI may be responsible for the antioxidant and antiglycation potential. It is therefore concluded that Musa paradisiaca inflorescence is a good source of natural antioxidants. The results of this study support the use of this plant in the Indian system of medicine and as a functional food. Based on the antioxidant and antiglycation properties, further studies are required for the isolation and identification of antioxidant compounds from EA fraction of MPI, and in vivo antioxidant and hypoglycemic activities of this extract need to be assessed.
REFERENCES
- Halliwell , B. , Gutteridge , J.M.C. , Halliwell , B. and Gutteridge , J.M.C. 1999 . “ Free radicals, other reactive species and disease ” . In Free Radicals in Biology and Medicine , 3rd , 617 – 783 . Oxford : Clarendon Press . InEd.Eds.
- Senevirathne , M. , Kim , S. , Siriwardhana , N. , Ha , J. , Lee , K. and Jeon , Y. 2006 . Antioxidant potential of Ecklonia cava on reactive oxygen species scavenging metal chelating, reducing power and lipid peroxidation inhibition . Food Science Technology International , 12 ( 1 ) : 27 – 38 .
- Rababah , T.M. , Ereifej , K.I. , Al-Mahasneh , M.A. , Ismaeal , K. , Hidar , A. and Yang , W. 2008 . Total phenolics, antioxidant activities, and anthocyanins of different grape seed cultivars grown in Jordan . International Journal of Food Properties , 11 : 472 – 479 .
- Larson , R.A. 1988 . The antioxidants of higher plants . Phytochemistry , 27 : 969 – 978 .
- Pereira , J.A. , Oliveira , I. , Sousa , A. , Valentao , P. , Andrade , B.P. and Ferreira , C.F.R.I. 2007 . Walnut (Juglans regia L.) leaves: Phenolic compounds, antibacterial activity and antioxidant potential of different cultivars. Food and Chemical Toxicology Vol. 45 , 2287 – 2295 .
- Bonnefont , R.D. 2002 . Glucose and reactive oxygen species . Current Opinion in Clinical Nutrition and Metabolic Care , 5 ( 5 ) : 561 – 568 .
- Wolff , S.P. and Dean , R.T. 1987 . Glucose autoxidation and protein modification. The potential role of ‘autoxidative glycosylation’ in diabetes . The Biochemical Journal , 245 ( 1 ) : 243 – 250 .
- Chan , E. 1998 . Tropical Plants of Southeast Asia , 12 – 13 . Singapore : Periplus Editions .
- Mohammad , Z.I. and Saleha , A. 2011 . Musa paradisiaca L. and Musa sapientum L.: A phytochemical and pharmacological review . Journal of Applied Pharmaceutical Science , 1 ( 5 ) : 14 – 20 .
- Swathi , D. , Jyothi , B. and Sravanthi , C. 2011 . A review: Pharmacognostic studies and pharmacological actions of Musa paradisiaca . International Journal of Innovative Pharmaceutical Research , 2 ( 2 ) : 122 – 125 .
- Pari , L. and Maheshwari , U.J. 2000 . Antihyperglycemic activity of Musa sapientum flowers: Effect on lipid peroxidation in alloxan diabetic rats. Phytotherapy Research Vol. 14 , 136 – 138 .
- Gomathy , R. , Vijayalekshmi , N.R. and Kurup , P.A. 1989 . Hypolipidemic principle of the inflorescence stalk of plantain (Musa sapientum) . Journal of Biosciences , 14 ( 3 ) : 301 – 309 .
- Kirtikar , K.R. and Basu , B.D. 1991 . “ Eugenia Jambolana L., Musa paradisiaca L. ” . In Indian Medicinal Plant , Edited by: Kirtikar , K.R. and Basu , B.D. Vol. IV , 2452 – 2456 . Delhi , India : Periodical Experts Book Agency . InVol.Eds.1048–1055
- Duan , W.W. , Zhang , X.M. , Li , X.M. and Wang , B.G. 2006 . Evaluation of antioxidant property of extract and fractions obtained from red alga, Polysiphonia urceolata . Food Chemistry , 95 : 37 – 43 .
- Ravishankara , M.N. , Neeta , S. , Harish , P. and Rajani , M. 2002 . Evaluation of antioxidant properties of root bark of Hemidesmus indicus R. Br. (Anantmul) . Phytomedicine , 9 : 153 – 160 .
- Shimada , K.K. , Fujikawa , K. , Yahara , K. and Nakamura , T. 1992 . Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion . Journal of Agricultural and Food Chemistry , 40 : 945 – 948 .
- Re , R. , Pellegrini , N. , Proteggente , A. , Pannala , A. , Yang , M. and Rice , E.C. 1999 . Antioxidant activity applying an improved ABTS radical cation decolorisation assay . Free Radical Biology and Medicine , 26 ( 9/10 ) : 1231 – 1237 .
- Robak , J. and Gryglewski , R.J. 1998 . Flavonoids are scavengers of superoxide anions . Biochemical Pharmacology , 37 : 837 – 841 .
- Halliwell , B. , Gutteridge , J.M.C. and Aruoma , O.I. 1987 . The deoxyribose method: A simple test tube assay for determination of rate constant for reactions of hydroxyl radicals . Analytical Biochemistry , 165 : 215 – 219 .
- Green , L.C. , Wagner , D.A. and Glogowski , J. 1982 . Analysis of nitrate, nitrite and (15N) nitrate in biological fluids . Analytical Biochemistry , 126 ( 1 ) : 131 – 138 .
- Singleton , V.L. and Rossi , J.A. 1965 . Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents . American Journal of Enology and Viticulture , 16 : 144 – 158 .
- Jia , Z. , Mengcheng , T. and Wu , J. 1999 . The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals . Food Chemistry , 64 : 555 – 559 .
- Oyaizu , M. 1986 . Studies on products of browning reaction: Antioxidant activities of products of browning reaction prepared from glucoseamine . Japanese Journal of Nutrition , 44 : 307 – 315 .
- Matsuura , N. , Aradate , T. , Sasaki , C. , Kojima , H. , Ohara , M. and Hasegawa , J. 2002 . Screening system for the Maillard reaction inhibitor from natural product extracts . Journal of Health Science , 48 : 520 – 526 .
- Scalbert , A. , Manach , C. , Morand , C. and Remesy , C. 2005 . Dietary polyphenols and the prevention of diseases . Critical Reviews in Food Science and Nutrition , 45 : 287 – 306 .
- Meyer , A.S. , Frankel , E.N. and Lester , P. 2001 . Antioxidant activity of hydroxycinnamic acids on human low-density lipoprotein oxidation . Methods in Enzymology , 335 : 256 – 265 .
- Chin , Y.H. 2006 . Antioxidant activity of extract from Polygonum aviculare L . Biological Research , 39 : 281 – 288 .
- Chance , B. , Sies , H. and Boveris , A. 1979 . Hydroperoxide metabolism in mammalian organs . Physiological Reviews , 59 : 33 – 140 .
- Smith , C. , Halliwell , B. and Arouma , O.I. 1992 . Protection by albumin against the prooxidant actions of phenolic dietary components . Food and Chemical Toxicology , 30 : 483 – 489 .
- Huie , R.E. and Padmaja , S. 1993 . The reaction of NO with superoxide . Free Radical Research Communication , 18 : 195 – 199 .
- Omken , B. , Sigva , H.O. , Mutlu , S. , Doganlar , S. , Yemenicioglu , A. and Frary , A. 2009 . Total antioxidant activity and total phenolic contents in different Turkish eggplant (Solanum melongena L.) cultivars . International Journal of Food Properties , 12 : 616 – 624 .
- Pellegrini , N. , Serafini , M. , Colombi , B. , Del Rio , D. , Salvatore , S. , Bianchi , M. and Brighenti , F. 2003 . Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays . The Journal of Nutrition , 33 : 2812 – 2819 .
- Tanaka , M. , Kuie , C.W. , Nagashima , Y. and Taguchi , T. 1988 . Applications of antioxidative Maillard reaction products from histidine and glucose to sardine products . Nippon Suisan Gakkaishi , 54 : 1409 – 1414 .
- Hassig , A. , Liang , W.X. , Shwable , K. and Stampfl , K. 1999 . Flavonoids and tannins: Plant based antioxidants with vitamin character . Medical Hypotheses , 52 : 471 – 481 .
- Ahmad , M. , Akthar , M.S. , Malik , T. and Gilani , A.H. 2000 . Hypoglycemic action of the flavonoid fraction of Cuminum nigrum seed . Phytotherapy Research , 14 : 103 – 104 .
- Osawa , T. and Mendoza , E.M. 1994 . “ Novel natural antioxidants for utilization in food and biological systems ” . In Post Harvest Biochemistry of Plant Food Materials in the Tropics , Edited by: Uritani , I. and Garcia , V.V. 241 – 251 . Tokyo, Japan : Japan Scientific Societies Press . InEds.
- Gordon , M.H. 1990 . “ The mechanism of antioxidant action in vitro ” . In Food Antioxidants , Edited by: Hudson , B.J.F. 1 – 18 . London : Elsevier Applied Science . InEd.
- Chase , K.V. , Carubelli , R. and Nordquist , R.E. 1991 . The role of nonenzymatic glycosylation, transition metals and free radicals in the formation of collagen aggragates . Archives of Biochemistry and Biophysics , 288 : 473 – 480 .
- Brownlee , M. , Vlassara , H. , Kooney , A. , Ulrich , P. and Cerami , A. 1986 . Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking . Science , 232 : 1629 – 1632 .
- Booth , A.A. , Khalifah , R.G. and Hudson , B.G. 1996 . Thiamine pyrophosphate and pyridoxamine inhibit the formation of antigenic advanced glycation endproducts: Comparison with aminoguanidine. Biochemical and Biophysical Research Communications , 220 : 113 – 119 .