Abstract
Total soluble sugar content and composition was studied by high performance liquid chromatography in four high dry-matter sweet potato cultivars at 3, 4, and 5 months maturity. Total soluble sugar consisted of sucrose, glucose, and fructose, ranging from 4.10–10.82 g/100 g (dry-weight basis). At harvest, there were significant differences in total soluble sugar due to maturity (p < 0.001) and cultivar (p < 0.05). The highest total soluble sugar contents were in 5-month samples at harvest (7.36–10.34 g/100 g) and 4-month samples after short-term storage under tropical ambient conditions (8.66–10.82 g/100 g). Estimated amylase enzyme activity varied significantly with harvest age (p < 0.05). Although reducing sugar contents were low, fructose levels in 5-month samples increased considerably after storage.
INTRODUCTION
Sweet potato (Ipomoea batatas L.), a highly productive and nutritious root crop, is grown in more than 100 countries, ranks seventh among the world's food crops, and ranks fifth in developing countries where it has the potential to play key roles in food security.[Citation1] Few major food crops are so genetically diverse and underexploited as the sweet potato.[2] Although nutritionally superior to most starchy staples, being rich in vitamins, minerals, and dietary fiber; having a low glycemic index;[Citation3] and containing several bioactive compounds with anti-oxidant, radical-scavenging, and other therapeutic and immunity-boosting properties,[Citation4 − Citation6] the crop has experienced persistent low utilization for decades.[Citation7] Sugar in sweet potato is a fundamental aspect of its eating quality;[Citation8, Citation9] this has been directly linked with its characteristic flavor and there have been speculations by specialists in the field that reducing sweetness will lead to increased utilization of sweet potato as a staple.[Citation7, Citation10] Categorization of sweet potato into staple, supplemental staple, and luxury types is based on sugar and dry matter contents;[Citation11] for certain ethnic groups, the preferred types are non-sweet or only slightly sweet being regarded as a staple carbohydrate energy source (or supplemental staple type), while sweeter types are preferred in some Western countries as dessert or sweet vegetable (luxury type). Sweetness in sweet potato is due to the presence of endogenous sugars (sucrose, glucose, and fructose) present at harvest, and additional sugar (maltose) formed through starch hydrolysis by amylase enzymes during cooking. Breeding efforts targeted at obtaining low- or non-sugar sweet potato have, therefore, aimed at controlling genes linked with the formation of endogenous mono- and di-saccharides and those linked with amylase enzyme activity.[Citation10, Citation12] Different sugars at the same concentrations are known to have different perceivable sweetness levels. For example, glucose is twice as sweet as maltose, sucrose is three times sweeter than maltose, and fructose is five times as sweet as maltose.[Citation13] In addition to taste/flavor characteristics, sugars are known to also affect the processing behavior of starchy raw materials, for example, gelatinization temperature, degree of gelatinization, and retrogradation.[Citation14 − Citation16] The presence of high levels of reducing sugars may also significantly affect the appearance of some heat-processed products by causing excessive darkening.[Citation17] Sweet potato sugars have been the subject of much interest and several reports in this area have been documented.[Citation18 − Citation23] In the search for factors affecting variability of sugar content, various pieces of information have emerged. For example, high N fertilizer was found to increase free sugar content, and free sugar correlated positively with N, Ca, and Mg content.[Citation24] However, it appears that the influence of the age of the crop at the time of harvesting on sugar content and composition has not been studied much. Harvest age in sweet potato is not hard and fast and may vary at the discretion of the farmer depending on target markets, personal economic situation, type of cultivar under cultivation, etc. Early-maturing types may be harvested with good yields from 90 days after planting, although it is known that roots can be maintained in the field for extended periods up to 6 months or more. In many tropical regions, there are no long-term storage practices for sweet potato due to the warm climate and lack of sophisticated facilities, unlike some other countries where storage for up to 8 months or more is possible.[Citation8, Citation25] It is documented that under marketing conditions in tropical developing countries sweet potatoes have a shelf-life of only 1–2 weeks.[Citation26]
The objective of this study was to assess free sugar contents in the roots of staple-type high dry-matter sweet potato cultivars at different levels of maturity right after harvest and after 3 weeks storage under ambient conditions. If sugar contents and composition of sweet potato cultivars (and how these change during storage) are significantly affected by harvest age, then timing the harvest appropriately would be a simple and effective means of controlling the eating quality and processing characteristics of such cultivars. This could possibly be applied in targeting desired sugar levels for specific end-uses, for instance, low sugar for staple food uses and high sugar for industrial production of sweeteners from sweet potato.
MATERIALS AND METHODS
Sweet Potato Cultivars
Two relatively new cultivars, Hi-starch and CRI-Otoo, released in 2005 and two old cultivars, Faara and Sauti, all with high dry matter contents (30% or more), planted at the same location (with 30–34°C day temperatures, 22–25°C night temperatures), and given the same management practices were obtained from the Crops Research Institute, Ghana. They were harvested at 3, 4, and 5 months after planting and processed into flour. At each harvest, some roots were saved, stored at room temperature (25°C ± 4) for 3 weeks, and also processed into flour.
Flour Preparation
Representatively sized roots (i.e., small, medium, and large) were selected at each sampling time, peeled, washed, shredded with a hand grater, dried in an air oven at 60°C for 72 h, and milled (using a Cyclotec 1093 sample mill, Foss, Denmark) to pass through a 60–80 mesh screen.
Sugar Analysis
Extraction of alcohol-soluble sugars
Flour samples (100 mg) were accurately weighed into glass centrifuge tubes (16 × 120 mm; 17 ml capacity). Soluble sugars were extracted by adding 10 ml of 80% aqueous ethanol to the tubes and incubating at 80–85°C for 10 min, with intermittent mixing on a vortex stirrer. The tubes were centrifuged for 10 min at 1000× g (3000 rpm). The supernatants were carefully poured off into 50-ml beakers; the pellets were re-suspended in another 10 ml of 80% ethanol and the process was repeated. Supernatants were pooled to obtain the total extracts of soluble sugars, quantitatively transferred into 25-ml volumetric flasks, and made up to the volume with 80% ethanol. Duplicate extractions were carried out for all samples.
Preparation of standards for high performance liquid chromatography (HPLC)
| • | Internal standard solution: Cellobiose (40 g) was weighed into a 100-ml volumetric flask; 50 ml of distilled water was added and sonicated to dissolve. It was then made up to volume with more distilled water and mixed thoroughly. | ||||
| • | Standard solution: Into a 50-ml volumetric flask, 5 mg of glucose (Sigma, St. Louis, MO, USA), 15 mg of fructose (Fisher, Waltham, MA, USA), and 50 mg of sucrose (Fisher) were weighed; 30 ml of distilled water was added, the mixture was sonicated to dissolve, and the contents made up to the volume with distilled water. | ||||
| • | HPLC standard: Into a test tube, 9.5 ml of water was carefully measured with a pipette. Then, 250 μl each of internal standard solution and standard solution (described above) was added, vortexed to mix, and filtered through a Dionex OnGuard-H column (Dionex Corporation, Sunnyvale, CA, USA). into an HPLC auto sampler vial. This standard was run at least three times during the course of the analysis. | ||||
Analysis
Five ml of each extract was placed in a 50-ml beaker and the ethanol was evaporated by leaving uncovered beakers overnight in a working fume hood. Precisely 1 ml of internal standard was added to each beaker and the residue dissolved using swirling and sonication. The solutions were transferred into 2-ml screw-capped vials, capped and kept frozen where necessary till the time of analysis. For analysis, 50 μl of each solution was diluted with distilled water to 2 ml in a small test tube and poured into a syringe fitted with a Dionex OnGuard-H filter. The solution was passed through the filter, the first 1 ml discarded, the remaining 1 ml collected in an HPLC autosampler vial, and analyzed using a Dionex BioLC AD 50 HPLC system (Dionex Co., Sunnyvale, CA, USA). The sample was injected and eluted through a Carbo PAC PA-1 column (250 × 4.6 mm id) (Dionex Corporation) at 30°C with the mobile phase consisting of 200 mM sodium hydroxide at an isocratic flow rate of 1.0 ml/min. A Dionex PAD (pulse amperometric detector) was used for detection of peaks, and the sugars were identified based on retention times.
A = Peak height of sugar; | |||||
B = Peak height of internal standard; | |||||
C = Dilution factor;[Citation40] | |||||
D = Weight of sample in HPLC vial; | |||||
E = Concentration of sugar in the standard mix; | |||||
F = Standard ratio. | |||||
Flour Pasting Properties
Pasting properties were determined by a Rapid Visco-Analyzer (RVA) (Newport Scientific Pty. Ltd., Warriewood, NSW, Australia) using 12% (dry basis) flour slurries, both in distilled water and in 0.05 mM AgNO3, a potent amylase inhibitor.[Citation27] An estimated index of amylase activity was calculated as (PV2-PV1)/PV1, where PV1 was peak apparent viscosity in amylase-active samples (obtained using distilled water) and PV2 was that in amylase-deactivated samples (obtained using AgNO3).[Citation28] Tests were run in duplicate. Apparent viscosity was expressed in Rapid Visco Units (RVU, where 1 RVU = 12 Centipoise).
Statistical Analysis
All experiments were performed in duplicate. Statistical analysis was performed using GenStat Discovery statistical software (Edition 3, VSN International, Hemel Hempstead, UK). Data were analyzed by general linear model (GLM). Differences at p < 0.05, p < 0.01, and p < 0.001 were considered to be significant. Pair-wise comparison of all means was performed using Duncan's multiple comparison procedure.
RESULTS AND DISCUSSION
Total Soluble Sugar Contents
Total soluble sugar (TSS) was made up of sucrose, glucose, and fructose and ranged from 4.10–10.82 g/100 g on a dry-weight basis (). It has been suggested that sugar levels for staple and supplementary staple sweet potato cultivars should be up to 2 and 5%, respectively, while ‘luxury’ types could have variable sugars, generally higher.[Citation29, Citation30] Sucrose was the major component of TSS in the staple-type, high dry-matter cultivars studied () and this is in line with previous reports that cultivars with high dry weight tend to have high sucrose and low reducing sugar content (glucose and fructose).[Citation11, Citation25] Mcharo and LaBonte,[Citation31] working with 45 sweet potato clones, also confirmed that clones with high sucrose had low levels of glucose and fructose.
Figure 1 Individual components of soluble sugar (sucrose, glucose, and fructose) in sweet potato varieties at 3, 4, and 5 months maturity both (a) at the time of harvest and (b) after 3 weeks in storage. The error bar in each chart shows the least significant difference (LSD) for effect of harvest time (p < 0.05).
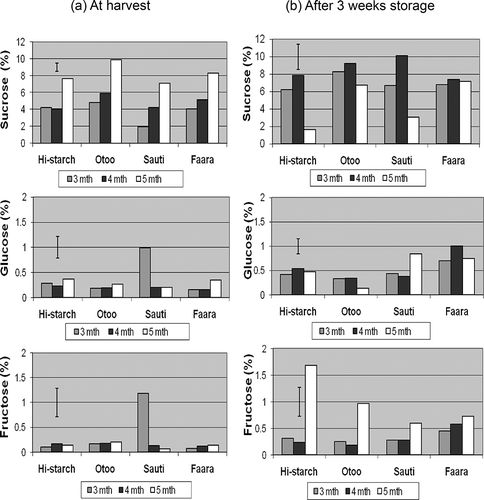
Table 1 Total soluble sugar contents (sucrose + glucose + fructose, g/100 g dry matter basis) of four sweet potato cultivars harvested at 3, 4, and 5 months maturity
Reducing Sugar Contents
In our cultivars, reducing sugar contents were generally low at all harvest times (glucose was 0.1331–1.006 g/100 g and fructose 0.0665–1.6838 g/100 g dry-weight basis). Wang and others[Citation32] found that starch and sugar (i.e., sucrose, glucose, and fructose) levels correlated with root weight/size during development, with glucose and fructose decreasing gradually, and sucrose and starch contents increasing with the expansion of tuberous roots. This to some extent appears to be in line with our findings, i.e., sucrose contents increasing with increasing maturity () although their studies were limited to root weights below or up to 200 g, and in our study the effect of root size within each cultivar would not come into play since representative sampling was done before processing.
Effects of Maturity and Storage on Sugars
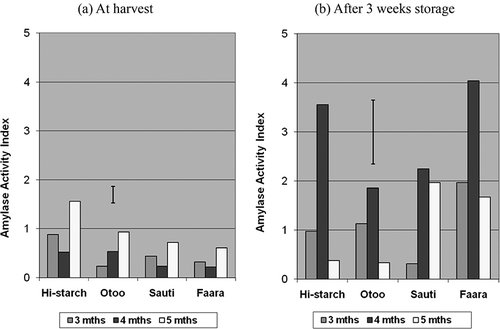
Fructose levels in samples harvested at 5 months increased considerably after storage (). These observed increases in fructose content may have implications in heat processing due to characteristic darkening of products (for example during frying) and harvest timing could be studied for other cultivars that tend to have high contents of endogenous reducing sugars. TSS contents on the day of harvest were highest at 5 months maturity for all the cultivars, ranging from 7.36 to 10.33 g/100 g (). After 3 weeks of storage there were large increases in TSS levels in samples harvested at 3 and 4 months, ranging from 50.5 to 137.7% of their original levels (). On the contrary, in samples harvested at 5 months, TSS contents dropped after storage () with the greatest declines occurring in Hi-starch and Sauti (−53.4 and −38.8%, respectively). Since sucrose was the predominant sugar, net changes in TSS showed virtually the same trends as for sucrose. Sucrose contents on the day of harvest were lower at earlier maturity (i.e., 3 and 4 months) and highest at 5 months (). After 3 weeks of storage under ambient tropical conditions, however, sucrose contents increased in 3- and 4-month samples but decreased in those harvested at 5 months, while glucose and fructose contents generally increased after storage. A similar observation has been reported[Citation22] in two white-fleshed cultivars during the early stages of a prolonged storage period, where sucrose concentration decreased and alcohol-insoluble solids increased. This was consistent with the belief that sucrose may be used up as a carbon source for postharvest starch synthesis in reserve tissue.[Citation33] Since sucrose is made up of glucose and fructose units, it is suggested that in our 5-month samples, the decrease in sucrose and increase in fructose levels after storage may reflect the uptake of the glucose fraction from sucrose for starch synthesis. Such reduction in sucrose content after storage was only observed in 5-month harvested samples for all four of our cultivars (). Other workers[Citation34, Citation35] had somewhat conflicting results in terms of changes in individual and total sugar concentrations in sweet potato at harvest and during storage and this could be due to the fact that maturity at the time of harvest was not taken into account. Lee and Chen,[Citation36] studying sweet potato in short-term storage (7 to 14 days), found TSS to either increase progressively or increase and then decrease depending on cultivar, even though age of the cultivars at the time of harvest was not factored in. The activity of sugar-related enzymes, such as acid invertase and sucrose synthase, in sweet potato have been studied by various workers,[Citation37, Citation38] and sucrose metabolism has been linked with the regulation of processes associated with sink strength and starch accumulation in sweet potato.[Citation39, Citation40] This has direct implications on economic factors, such as dry matter yield, and may explain why, in spite of the quest for low- or non-sugar types, the world's sweet potato clones were found to be predominantly either high or moderate in terms of sweetness, measured as sucrose equivalents.[Citation10] In freshly harvested roots, differences in TSS due to maturity were highly significant at both p < 0.01 and p < 0.05, while cultivar differences were only significant at p < 0.05 (). After storage, the influence of maturity was significant but cultivar differences were not (p < 0.05).
Table 2 ANOVA for cultivar differences and the effect of harvest age on total soluble sugars (TSS) (dry weight basis) and amylase activity index (AAI) of sweet potato flour prepared from roots at harvest and after 3 weeks storage
Flour Pasting Properties and Amylase Activity
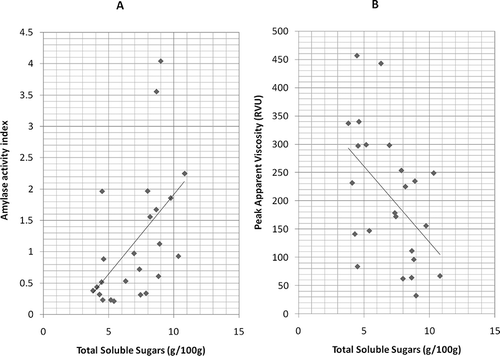
An index of amylase activity was calculated for each sample using RVA data obtained from both amylase-active and amylase-inhibited slurries. Trends in amylase activity were significantly affected by harvest timing (p < 0.05) but not by cultivar (). The estimated index of amylase enzyme activity right after harvest was highest in 5-month samples, and in 4-month samples after storage (). The relatively low levels of amylase activity in freshly harvested samples at 4 months, a maturity stage that coincides with the highest starch content in the cultivars studied (unpublished data), could be attributed to the action of endogenous inhibitors of amylase. The presence of amylase inhibitors in the development of starchy storage organs is essential for protecting starch granules from breakdown by enzymes, especially during phases of active starch accumulation.[Citation41] The high amylase activity of 5-month samples at harvest and 4-month samples after storage could be due to various regulatory mechanisms including a possible drop in amylase inhibitor action. In the same vein, the reduction in amylase activity after storage observed in Hi-starch and Otoo when harvested at 5 months could be due to increases in amylase inhibitor activity (). The possibility of such postharvest increase in inhibition of amylase enzyme may be supported by previously reported evidence of starch synthesis in some reserve tissues during the postharvest phase.[Citation33] Amylase activity in sweet potato storage roots during processing is of much importance since it results in the breakdown of starch through hydrolysis to form more sugar (maltose) during cooking,[Citation35, Citation36, Citation42] giving rise to particular product characteristics. The amylase activity index correlated positively with TSS (r = 0.60; ). This implies that in the cultivars studied those with relatively higher initial sugar content in the raw/unprocessed state would also have more potential for increases in total sugar levels during cooking based on the higher amylase enzyme activities. TSS correlated negatively with RVA peak apparent viscosity (r = −0.54, ), reflecting previous reports that higher sugar levels to some extent are linked with less thickening of starchy pastes during the cooking process.[Citation14 − Citation16]
CONCLUSION
Maturity was found to significantly affect total soluble sugar content and amylase activity of the cultivars studied, and how these changed during short-term post-harvest storage. This information can serve as a springboard to provide opportunities to possibly control the eating quality of sweet potato through the formulation of recommendations for harvest timing and fresh produce quality of specific target cultivars, hopefully to match specific needs.
ACKNOWLEDGMENT
The authors gratefully acknowledge the Agricultural Sub-Sector Improvement Project (AgSSIP), The World Bank, Ghana, for providing funding for this study.
REFERENCES
- Food and Agriculture Organization (FAO) . 2002 . FAO Production Year Book , Vol. 56 , Rome , Italy : Food and Agriculture Organization . Vol.
- International Potato Center (CIP). 1994 . Vines to roots: Sweet potato breeding for impact , Peru : Recommendations and abstracts from an international workshop held in Lima . June
- Kays , S.J. and Kays , S.E. 1998 . “ Sweet potato chemistry in relation to health ” . In Sweet potato Production Systems toward the 21st Century , 231 – 272 . Miyakonojo , Miyazaki : Kyushu National Agricultural Expt. Station . In
- Patil , K.S. , Chaudhari , V.V. and Sachin , M. 2007 . Effect of Ipomoea batatas Linn. (Lam) root extracts on phagocytosis by human neutrophils . Journal of Natural Remedies , 7 : 195 – 199 .
- Hung , M. 2004 . Sweet potato extract improves glycemic control in type 2 diabetes . Diabetes Care , 27 : 436 – 440 .
- Shih , M.-C. , Kuo , C.-C. and Wenchang , C. 2009 . Effects of drying and extrusion on colour, chemical composition, antioxidant activities and mitogenic response of spleen lymphocytes of sweet potatoes . Food Chemistry , 117 : 114 – 121 .
- Kays , S. 2005 . Flavour the key to sweet potato consumption , 14 – 17 . Malaysia : Proceedings of the Second International Symposium on Sweet potato and Cassava . June
- Lewthwaite , S.L. , Sutton , K.H. and Triggs , C.M. 1997 . Free sugar composition of sweet potato cultivars after storage . New Zealand Journal of Crop and Horticultural Science , 25 : 33 – 41 .
- Takahata , Y. , Noda , T. and Nagata , T. 1992 . Varietal diversity of free sugar composition in storage root of sweet potato . Japanese Journal of Breeding , 42 : 515 – 521 .
- Kays , S.J. , Wang , Y. and McLaurin , W.J. 2005 . Chemical and geographical assessment of the sweetness of the cultivated sweet potato clones of the world . Journal of the American Society for Horticultural Science , 130 ( 4 ) : 591 – 597 .
- Villareal , R.L. 1981 . Sweet potato. Proceedings of the First International Symposium of the Asian Vegetable Research and Development Center 3 – 16 . Tainan , Taiwan 23–27 March 1981,
- Kays , S.J. , McLaurin , W.J. and Wang , Y. 2001 . GA90-16: A nonsweet, staple-type sweet potato breeding line . Hortscience , 36 ( 1 ) : 175 – 177 .
- Biester , A.; Wood , M.W. and Wahlin , C.S. 1925 . Carbohydrate studies. 1. The relative sweetness of pure sugars . American Journal of Physiology , 73 : 387 – 396 .
- Kohayama , K. and Nishinari , K. 1991 . Effect of soluble sugars on gelatinization and retrogradation of sweet potato starch . Journal of Agricultural and Food Chemistry , 39 : 1408 – 1410 .
- Johnson , J.M. , Davis , E.A. and Gordon , J. 1990 . Interactions of starch and sugar water measured by electron spin resonance and differential scanning calorimetry . Cereal Chemistry , 67 ( 3 ) : 286 – 291 .
- Bean , M.M. and Yamazaki , W.T. 1978 . Wheat starch gelatinization in sugar solutions. I . Sucrose: Microscopy and viscosity effects. Cereal Chemistry , 55 ( 6 ) : 936 – 944 .
- Kays , S.J. 1985 . “ Formulated sweet potato products ” . In Sweet Potato Products: A Natural Resource for the Tropics , Edited by: J.C. , Bouwkamp . 205 – 218 . Boca Baton, Florida : CRC Press . InEd.
- Walter , M.W. 1992 . Use of refractive index to monitor changes in sugar content of stored sweet potatoes . Hortscience , 27 ( 4 ) : 333 – 335 .
- Truong , V.D. , Biermann , C.J. and Marlett , J.A. 1986 . Simple sugars, oligosaccharides, and starch concentrations in raw and cooked sweet potato . Journal of Agricultural and Food Chemistry , 34 : 421 – 425 .
- Picha , D.H. 1985 . HPLC determination of sugars in raw and baked sweet potatoes . Journal of Food Science , 50 ( 1210 ) : 1189 – 1190 .
- Picha , D.H. 1986 . Influence of storage duration and temperature on sweet potato sugar content and chip colour . Journal of Food Science , 51 : 239 – 240 .
- Picha , D.H. 1987 . Carbohydrate changes in sweet potatoes during curing and storage . Journal of the American Society Horticultural Science , 112 ( 1 ) : 89 – 92 .
- Picha , D.H. 1987 . Chilling injury, respiration, and sugar changes in sweet potatoes stored at low temperature . Journal of the American Society for Horticultural Science , 112 ( 3 ) : 497 – 502 .
- Inukai , Y. , Shibayama , H. and Matsubayashi , T. 2002 . Growth conditions affecting palatability, especially sweetness of sweet potato . Marine and Highland Bioscience Center Report , 14 : 1 – 7 .
- Zhang , Z. , Wheatley , C.C. and Corke , H. 2002 . Biochemical changes during storage of sweet potato roots differing in dry matter content . Postharvest Biology and Technology , 24 : 317 – 325 .
- Rees , D. , Oirschot , Q.E.Q. , Amour , R. , Rwiza. , E. , Kapinga , R. and Carey , T. 2003 . Cultivar variation in keeping quality of sweet potatoes . Postharvest Biology and Technology , 28 ( 2 ) : 313 – 325 .
- Dixon , M. and Webb , E. 1964 . “ Enzyme inhibitors ” . In Enzymes , 2nd , 344 – 347 . London : Longmans, Green and Co . InEd.
- Collado , L.S. and Corke , H. 1999 . Accurate estimation of sweet potato amylase activity by flour viscosity analysis . Journal of Agricultural and Food Chemistry , 47 : 832 – 835 .
- Collins , W.W. 1987 . “ Improvement of nutritional and edible qualities of sweet potato for human consumption ” . In Exploration, Maintenance and Utilization of Sweet Potato Genetic Resources: Report of the First Sweet Potato Planning Conference , 221 – 226 . Lima , Peru : International Potato Center . In
- Collins , W.W. 1993 . “ Root vegetables: New uses for old crops ” . In New Crops Edited by: J. , Janick and J.E. , Simon . 533 – 537 . Wiley: New York InEds.
- Mcharo , M. and LaBonte , D. 2007 . “ Genotypic variation among sweet potato clones for beta-carotene and sugar content ” . In Proceedings of the 13th Symposium of the International Society for Tropical Root Crops , 746 – 754 . ISTRC), Tanzania, November .
- Wang , S.J. , Chen , M.H. , Yeh , K.W. and Tsai , C.Y. 2006 . Changes in carbohydrate content and gene expression during tuberous root development of sweet potato . Journal of Plant Biochemistry and Biotechnology , 15 : 21 – 25 .
- Preiss , J. 1982 . Regulation of biosynthesis and degradation of starch . Annual Review of Plant Physiology , 33 : 431 – 454 .
- Deobald , H.J. , Hasling , V.C. and Catalano , E.A. 1970 . Variability of increases in α-amylase and sugars during storage of Goldrush and Centennial sweet potatoes . Journal of Food Science , 36 ( 3 ) : 413 – 415 .
- Morrison , T. , Pressey , R. and Kays , S.J. 1993 . Changes in alpha- and beta-amylase during storage of sweet potato lines with varying starch hydrolysis potential . Journal of the American Society for Horticultural Science , 118 : 236 – 242 .
- Lee , H.C. and Chen , Y.S. 2004 . Application of the beta-amylase activity rapid test method on processing character of sweet potato . Journal of Agricultural Research of China , 53 ( 1 ) : 18 – 26 .
- Takahata , Y. , Noda , T. and Sato , T. 1996 . Relationship between acid invertase activity and hexose content in sweet potato storage roots . Journal of Agricultural Food Chemistry , 44 : 2063 – 2066 .
- Saitou , K. , Araki , T. , Agata , W. , Kubota , F. and Nakayama , K. 1997 . Expression of sucrose synthase in sweet potato . Japanese Journal of Crop Science , 66 : 624 – 631 .
- Rolland , F. , Baena-Gonzalez , E. and Sheen , J. 2006 . Sugar sensing and signaling in plants: Conserved and novel mechanisms . Annual Review of Plant Biology , 57 : 675 – 709 .
- Li , X.-Q. and Zhang , D. 2003 . Gene expression activity and pathway selection for sucrose metabolism in developing storage root of sweet potato . Plant and Cell Physiology , 44 ( 6 ) : 630 – 636 .
- Sasikiran , K. , Rekha , M.R. and Padmaja , G. 2002 . Proteinase and alpha-amylase inhibitors of sweet potato: Changes during growth phase, sprouting, and wound induced alterations . Botanical Bulletin of Academia Sinica , 43 : 291 – 298. .
- Deobald , H.J. , McLemore , T.A. , Hasling , V.C. and Catalano , E.A. 1968 . Control of sweet potato alpha-amylase for producing optimum quality precooked, dehydrated flakes . Food Technology , 22 : 627 – 632 .