Abstract
The starch and protein content of substituted and complete triticales were quantitated and evaluated for their contribution to the functional properties of flours by means of the amylograph, farinograph, and the breadmaking test. Although no clear differences were observed in the starch and protein contents of the two triticale genotypes, a detailed analysis of their components revealed compositional differences between them. The amylose content was lower in the substituted triticales Duron S and Alamos 83 than in the complete types Tarasca 87 and Brumby II (P ≤ 0.05). Among the protein fractions, only the albumin and the total polymeric protein, although with an opposite effect, significantly contributed to the rheological properties of triticale doughs evaluated with the farinograph and in the breadmaking test. The SDS-PAGE test showed that the substituted triticales contained an additional HMW-GS band that was not observed in the complete triticales. Amylograph peak temperatures and maximum viscosities, farinograph development times, and loaf volumes of triticale doughs were higher in substituted triticale flours than those of the complete ones. This study demonstrated that the triticale genotypes can be differentiated not only by quantitating their starch and protein composition, but also by testing their functional dough properties.
INTRODUCTION
Triticale (X Triticosecale Wittmack) is the product of an artificial cross between tetraploid wheat (Triticum aestivum) and rye (Secale cereale) genomes resulting in hexaploid (AABBRR) or octaploid (AABBDDRR) triticales.[Citation1, Citation2] Since triticales have not undergone natural selection, it has been necessary to create populations as genetically diverse as possible. The search for triticales with good agronomic traits has led breeders to explore its chromosomic manipulation.[Citation3] The substitution of the rye chromosome 2R by the wheat chromosome 2D from bread wheat produces “substituted” triticales (AABBDR),[Citation4] which improves the bread-making quality of triticale, although not comparable to that of bread wheat.[Citation5 − Citation7] Some studies have reported that triticales are better suited for cracker production[Citation7, Citation8] and other unleavened products[Citation9, Citation10] than for breadmaking.
There is another type of triticales having all R chromosomes from rye and are called “complete” triticales (AABBRR). Generally these types retain much of the adaptation characteristics from rye for an appropriate plant growth in marginal agricultural areas where wheat does not perform well.[Citation3, Citation11] In the complete triticales, the R genome from rye substitutes the D genome from wheat causing the gluten content to be low with a concomitant low bread-making quality.[Citation11, Citation12] According to reports derived from CIMMYT, complete triticales perform agronomically much better than the substituted ones. Therefore, a balance has to be made between the breadmaking quality of substituted triticales and the high yield potential and good grain quality of wheat with disease and environmental tolerance of the complete triticales.[Citation13] Reports have suggested that the incorporation of triticale into the breadmaking industry can be met with the incorporation of triticale flour into wheat at levels up to 50% with no detrimental effect on baking performance.[Citation14, Citation15]
For many years, the agricultural area of northwest Mexico has been a major wheat producer. However, much production acreage is now dedicated toward cultivation of other crops due to adverse environmental factors. This situation has led the search for alternative crops, such as triticale, that can resist drought or arid soil conditions.[Citation11] However, not all types of triticales are suitable to fulfill the needs of the grain market for either food or feed.[Citation4, Citation16] Aside from their agronomic adaptability, evaluation of their chemical and physical characteristics will be useful to discriminate the most appropriate one(s) for commercial breadmaking purposes. The present work was carried out to evaluate and compare certain physicochemical properties of some substituted and complete triticales.
MATERIALS AND METHODS
Materials
For this study, the breadmaking wheat cultivar Bacanora, the rye cultivar Webber, and four triticales cultivars were employed. Two of them were complete triticales (Brumby II and Tarasca 87) and two were substituted triticales (Duron S and Alamos 83). Seeds of triticales were from the Wellhausen-Anderson Plant Genetic Resource Center located at the International Maize and Wheat Improvement Center (CIMMYT; El Batán, México) and were kindly donated to be used in this study. The triticales were cultivated at the experimental field of the University of Sonora, Mexico. Once harvested, samples of 1 kg of triticales were tempered to 15% moisture overnight, milled in a Brabender Quadrumat Senior mill (Duisburg, Germany), and stored at −20°C until analysis.
Chemical Analyses
The protein contents of the triticales (14% moisture basis) were determined according to the procedures of the AOAC.[Citation17] The protein fractions were extracted following the method of Khan et al.[Citation18] Briefly, albumins, globulins, gliadins, and glutenins were sequentially extracted with deionized water, 0.5 M NaCl, 70% ethanol, and 0.05 N acetic acid, respectively. First, 5 g of flour was combined with 50 mL of deionized water (1:10, w/v) to remove albumins. The mixture was stirred for 2 h at ambient temperature and centrifuged at 10,000× g for 1 h. The supernatant was saved and the residue was resuspended in 50 mL of 0.5 M NaCl for globulins extraction. The rest of the protein fractions were extracted following the same procedure. The supernatants were analyzed in protein content by the micro Kjeldahl method,[Citation17] whereas the residues were saved for extraction and analysis of the unextractable polymeric protein by SDS-electrophoresis.
The starch and amylose content of the flours was determined following the procedure of Kaldy et al.[Citation19] Briefly, flour samples (50 mg) were dispersed in 5 mL of deionized water, and vigorously mixed in a vortex mixer to distribute it uniformly to avoid sedimentation of the flour. For starch determination, 0.25 mL aliquots of flour dispersions were rapidly taken after mixing, combined with 2.25 mL of dimethyl sulfoxide (DMSO), and incubated overnight at 50°C. One milliliter of the resulting mixtures was used for starch analysis following the phenol-sulfuric acid method of Dubois et al.[20] The amount of starch was calculated from a standard curve prepared with pure starch and expressed as a percentage of the amount of flour used for extraction. For apparent amylose determination, 100 μL aliquots of flour dispersions were combined with 900 μL DMSO containing 6.7 × 10−3 M I2 and incubated overnight at 50°C. After dilution with 8 mL of water, the color intensity was measured at 600 nm. Quantitation of apparent amylose does not make a distinction between the iodine-staining capacity of the linear amylose molecule and the long branches of amylopectin. Therefore, apparent amylose values were first calculated from a standard curve and corrected for amylopectin according to the procedure of Knutson.[Citation21] The resulting values were expressed as the percentage of amylose in total starch.
SDS-Electrophoresis of Unextractable Polymeric Protein
The unextractable polymeric protein remaining in the flour residues, free of soluble protein fractions, was extracted following the procedure of Gupta and MacRitchie.[Citation22] Briefly, residue samples (10 mg) were dispersed in 165 μL of 70% ethanol, mixed with 5 μL 2-mercaptoethanol, and vortexed for 30 min at room temperature. After centrifugation, the supernatants containing unextractable polymeric proteins (glutenins) were mixed with 5 μL of 4-vinylpyridine for protein alkylation in the dark for 2 h. Aliquots of 50 μL of protein extracts were diluted 1:4 with reducing sample buffer (0.0625 M Tris-HCl, pH 6.8, containing 10% (v/v) glycerol, 2% (w/v) SDS, and 5% (v/v) β-mercaptoethanol). From the resulting solutions, aliquots of 20 μL were used for electrophoresis in a 10% acrylamide separating gel and a 3.83% acrylamide stacking gel, both containing 1.35% bisacrylamide according to the procedure of Laemmli.[Citation23] The protein separation was carried out at a constant voltage of 200 V for 45 min in a Mini-Protean II Cell (Bio-Rad, Hercules, CA, USA). Detection of bands was carried out using the silver method of Blum et al.[Citation24]
Rheological Tests of Flours
Rheological tests of flours were carried out following recommended methods of the AACC:[Citation25] farinograph analysis (method 54-21), breadmaking test (method 10-10), falling number test (method 56-81), and amylograph test (method 22-10).
Statistical Analysis
All analyses were run in triplicate. Results were expressed as mean values ± standard deviation. Data was subjected to an analysis of variance following general model procedures.[Citation26] The resulting mean values were compared using Tukey's multiple range test with significance defined at P ≤ 0.05.
RESULTS AND DISCUSSION
Starch and Amylose Content
The results of the starch determination of wheat and rye, and the two types of triticales are shown in . In general, all triticales showed similar values in starch content with those previously reported.[Citation7, Citation27] No clear statistical relationship between starch content and type of triticale was observed. The triticales Duron S (substituted) and Brumby II (complete) showed the highest and similar starch content, whereas Alamos 83 (substituted) and Tarasca 87 (complete) showed the lowest (P ≤ 0.05). Overall, these results were similar to those from previous studies,[Citation28, Citation29] although under our experimental growing conditions the values tended to be a little bit higher. Burešová et al.[Citation29] reported that starch and amylose content in triticales were influenced by weather during the growing season; however, no statistical difference was observed among the different triticales used in this study, perhaps because they were cultivated under the same environmental conditions. The values of amylose content for wheat, rye, and triticale flours tested are shown in . The highest value of amylose content was observed in durum wheat, which is in agreement with results previously reported.[Citation30, Citation31] High amylose content in durum wheat has been attributed to the low activity of the enzymes involved in amylopectin synthesis leading to the increase of amylose content.[Citation32] Some of the triticale flours showed similar amylose values to rye but were lower than wheat (P ≤ 0.05) and comparable to those previously published.[Citation26, Citation28, Citation33, Citation34] However, in our study the substituted triticales, Duron S and Alamos 83, contained lower amounts of amylose than the complete triticales. The amounts of amylose have been related to the starch granule size, being the A-type are the ones containing higher amylose content than the B-type.[Citation33, Citation35]
Table 1 Starch and amylose content in wheat, rye, and substituted and complete triticale flours.Footnote 1, Footnote 2
Quantitation of Protein Fractions
The total protein content values of the flours used in this study, as well as those of their corresponding protein fractions, are shown in . It was observed that wheat, rye, and triticale flours contained similar total protein contents (P ≤ 0.05). However, quantitation of the different protein fractions showed that all triticales contained, on average, higher values of albumins and globulins (42.7 and 24.2%) than those of wheat (30.1 and 16.2%) and rye (11.3 and 8.2%), respectively (P ≤ 0.05). These results are similar to those reported by Bushuk and Larter[Citation36] and Varughese et al.,[Citation37] which was attributed to an additive effect of the albumin and globulin protein fractions from wheat and rye inherited in all triticales. However, there were two protein fractions that significantly differentiated the triticale genotypes. One was the albumin fraction, which was present in lower amounts in the substituted triticales Duron S and Alamos 83 (42.6 and 38.0%, respectively) and higher in the complete genotypes Tarasca 87 and Brumby II (44.8 and 45.4%, respectively) (P ≤ 0.05). The other protein fraction was the acetic acid soluble glutenins, which was higher in the substituted triticales (average 9.35%) and lower in the complete genotypes (average 7.1%). Moreover, a comparative analysis of the amounts of the total polymeric protein (soluble glutenin plus residue protein) determined in all triticales showed that the substituted genotypes contained, on average, more total polymeric protein (15.9%) than the complete ones (13.5%) (P ≤ 0.05). An analysis of the glutenin subunit composition by SDS-PAGE showed that the substituted triticales Duron S and Alamos 83 contained an additional HMW-GS band that was not observed in the complete triticales Tarasca 87 and Brumby II (). In contrast, the latter triticales contained a protein band with intermediate mobility between the HMW- and LMW-GS, which can be a secalin[Citation1] and that was not observed in the substituted triticales.
Figure 1 SDS-PAGE of the polymeric protein of triticale flours Duron S (substituted), Alamos 83 (substituted), Tarasca (complete) and Brumby II (complete). An additional HMW-GS band is indicated in substituted triticales (arrows), whereas an additional protein band was only observed in complete triticales (arrows). (Color figure available online.)
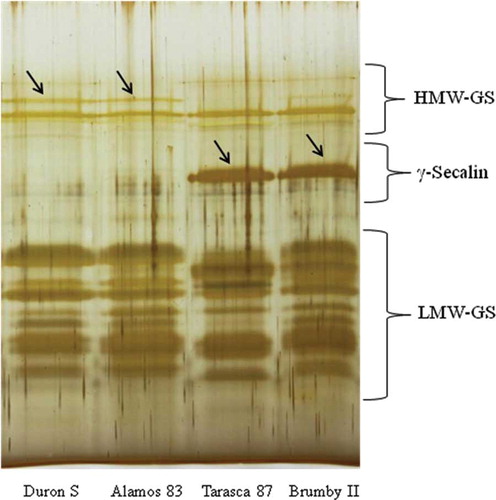
Table 2 Total protein content and relative concentrations of the different protein fractions of wheat, rye, and triticale flours.Footnote 1
Amylograms
The main amylographic parameters of the flours used in this study are shown in . Peak temperatures of wheat and rye were higher than those of triticales (P ≤ 0.05). However, among triticales, the substituted ones showed an average peak temperature higher than that of complete triticales. High peak temperatures have been related to low amylose contents of the small B-granule starch,[Citation33, Citation38, Citation39] as well as to greater loaf bread volumes.[Citation40]
Table 3 Values of amylographic parameters and falling number of wheat, rye, and triticale flours.Footnote 1
The substituted triticales showed, on the average, maximum viscosity values around 38% more than those observed in complete triticales. A moderate linear inverse relationship was observed between starch content and peak temperatures (r = −0.60) and starch content and falling number values (r = −0.78). However, a high positive linear correlation value (r = 0.93) was obtained when the peak viscosity temperatures and falling number values were related, indicating that both parameters are strongly influenced by the amylase activity of the flours. Durum wheat and rye flours showed the highest values of maximum viscosity and falling number values, which together indicated low α-amylase enzyme activity.[Citation41, Citation42] In contrast, all triticale flours showed low falling number values evidencing their high amylase activity.[Citation43] Furthermore, values of maximum viscosity and falling number were low for all triticales as compared to those observed in wheat and rye flours (P ≤ 0.05), although those of complete triticales were slightly lower than the substituted types. Viscosity of flours, as determined by the falling number method, is greatly influenced by their α-amylase activity.[Citation2, Citation42] This was demonstrated by the high negative linear correlation (r = -0.91) observed between the amounts of albumin content and the falling number of the triticale flours used in this study. Klassen and Hill[Citation44] reported that the low amylograph viscosity of triticales was due to their α-amylase activity, mainly derived from rye. However, the rye flour used in our study showed a significant high falling number value than those determined in all triticales, indicating that the latter did not inherit all the α-amylase activity from rye.
Table 4 Values of the main farinographic parameters of doughs prepared from wheat and triticale flours.Footnote 1
Farinograms
The values of the main farinographic parameters are shown in Of all those parameters, only stability time and dough development time were able to differentiate the triticale types. In general, all triticales showed lower stability times than that of the wheat flour. However, the substituted types showed longer stability times than the complete ones (P ≤ 0.05). A similar behavior was observed with the measurement of the dough development time. The substituted triticales showed larger development values than those observed in complete types. The substituted triticales used in this study contained an additional HMW-GS (), which could be the reason for higher stability mixing times (from 8.6 to 25%) and dough development times (from 30 to 35%) than those observed in the complete triticales. It is known that the mixing properties of triticale are highly influenced by their HMW-GS composition,[Citation1, Citation4] as it is in wheat dough.[Citation18, Citation45]
Table 5 Breadmaking parameters of loaves made from wheat, rye, and triticale flours.Footnote 1
Breadmaking Test
The values of the main breadmaking parameters for the different flours are shown in . It was observed that the substituted triticales performed better than the complete ones in bread volume. A parameter that also helped to differentiate the breadmaking quality of triticales was the loaf specific volume. Higher loaf specific volume values were obtained in loaves made from substituted triticale flours than those from the complete ones (P ≤ 0.05). This result can be attributed to the higher content of polymeric protein (soluble glutenin plus residue protein) determined in substituted triticales because it was highly related to loaf volume (r = 0.91) and specific loaf volume (r = 0.97) of triticales flours. The positive role of the wheat polymeric protein and its glutenin subunit composition in breadmaking has been well demonstrated in wheat[Citation5, Citation18, Citation45] and triticale,[Citation2, Citation3, Citation15] and its contribution to the functional properties of triticale flours allowed us to differentiate the breadmaking quality of substituted and complete triticales. The significance of these results is in accordance with those of Singh et al.[Citation5] and Cinco-Moroyoqui and MacRitchie.[Citation46] These researchers have indicated that the total polymeric protein and glutenin subunit composition are determinant factors in the breadmaking quality of wheat flours. Therefore, the additional HMW-GS detected in the substituted triticales may be the reason why these genotypes perform better in the breadmaking test, although they were unable to perform wheat-like breadmaking properties.[Citation2] In contrast, among the rest of the protein fractions isolated from triticale flours, only the albumin fraction was inversely related to bread volume (r = −0.88). That result can be attributed to the variable α-amylase activity of the triticales, which is found in the albumin fraction[Citation2] and determines the falling number test. However, the albumin fraction comprises a heterogeneous group of proteins whose influence in breadmaking has not been studied in detail and assessed their functional role in the breadmaking process. Nevertheless, some studies have reported that wheat albumins coagulate during the thermal process contributing to create the structure and texture of wheat bread loaves,[Citation47] whereas Oszvald et al.[Citation48] found that amaranth albumins improve the rheological properties of wheat dough. According to those results and ours, it appears that the albumin fraction plays an important role in breadmaking and must be present in low amounts to favorably influence the rheological properties of triticale flours. Hence, it seems that a suitable balance must exist between the total polymeric protein and the water soluble albumins to obtain an optimum bread volume. Although some studies have shown that triticales are not appropriate for breadmaking,[Citation5, Citation7] others have demonstrated that they can be used in wheat-triticales blends without negatively affecting the resulting bread volume.[Citation10, Citation15, Citation16] The contradictory results allowed speculation that the triticales flours used in those studies differed in total polymeric protein and albumin contents.
CONCLUSIONS
Quantitation of starch and protein content of the triticales used in this study did not reveal differences in their chemical composition. However, an analysis of their components made it possible to establish differences between the two triticale genotypes used in this study. The amylose content in the substituted triticales was lower than the complete ones. The triticale flours showed very low viscosity and falling number values in comparison to those of the wheat and rye. All triticales possessed higher contents of albumins and globulins than those observed in durum wheat and rye. The functional properties measured in the farinograph showed that the triticale flours possessed very weak gluten as demonstrated by their low tolerance values to the mixing action. However, substituted triticales behaved better than the complete ones. The breadmaking test showed that the substituted triticales performed better than the complete triticales as demonstrated by their high bread volumes and loaf specific volume values. That result was attributed to the high content of total polymeric protein (soluble glutenin plus residual unextractable protein) and their concomitant lower albumin content, which was not observed in the complete triticales.
Although production of triticales with better breadmaking properties has not been intended to replace hexaploid wheat in the breadmaking industry, results derived from the present study suggest that the substituted triticales can be successfully used for bread production in those areas where the hexaploid wheat does not perform well due to adverse climatic conditions or to wheat shortages. Additionally, the tolerance of triticale to grow in a variety of environmental conditions, gives the opportunity to conveniently obtain polymeric (glutenin) protein from substituted triticales that can be fractionated and used as additive in wheat flours to improve their breadmaking properties. This practice would result in an increase in the proportion of the polymeric protein in the flour, whereas that corresponding to the albumin fraction would decrease, which supposedly would favorably affect the breadmaking properties of the resulting flours as it was observed in the present study with the substituted triticale flours. Finally, considering the adverse role of the albumin fraction on bread volume observed in the present work, it would be of interest to study the influence of the different albumin subunits on the improvement of the functional properties of triticale and wheat flours. Results of the present work lead to the conclusion that the starch and protein components are what really makes it possible to differentiate triticale flours in their functional properties.
ACKNOWLEDGMENTS
The authors wish to thank the Wheat Collection Wellhausen-Anderson Plant Genetic Resource Center of the International Maize and Wheat Improvement Center, CIMMYT-Mexico for kindly donating the triticale seeds used in this study. Authors A.L. Navarro-Contreras and C.F. Chaires-González acknowledge financial support from the Consejo Nacional de Ciencia y Tecnología (CONACyT, México).
REFERENCES
- Amiour , N. , Bouguennec , A. , Marcoz , C. , Sourdille , P. , Bourgoin , M. , Khelifi , D. and Branlard , G. 2002 . Diversity of seven glutenin and secalin loci within triticale cultivars grown in Europe . Euphytica , 123 : 295 – 305 .
- Martinek , P. , Vinterová , M. , Burešova , I. and Vyhnánek , T. 2008 . Agronomic and quality characteristics of triticale (X Triticosecale Wittmack) with HMW glutenin subunits 5+10 . Journal of Cereal Science , 47 : 68 – 78 .
- Tohver , M. , Kann , A. , Täht , R. , Mihhalevski , A. and Hakman , J. 2005 . Quality of triticale cultivars suitable for growing and bread-making in northern conditions . Food Chemistry , 89 : 125 – 132 .
- Lukaszewski , A.J. 2006 . Cytogenetically engineered rye chromosomes 1R to improve bread-making quality of hexaploid triticale . Crop Science , 46 : 2183 – 2194 .
- Singh , N.K. , Donovan , G.R. and MacRitchie , F. 1990 . Use of sonication and size-exclusion high performance liquid chromatography in the study of wheat flour proteins. II. Relative quantity of glutenin as a measure of breadmaking quality . Cereal Chemistry , 67 : 161 – 170 .
- Zeller , F.J. and Hsam , S.L.K. 1984 . “ Broadening the genetic variability of cultivated wheat by utilizing rye chromatin ” . In Proceedings of the Sixth International Wheat Genetics Symposium Edited by: Sakamoto , S. and Plant Germ-Plasm Institute, Kyoto University, Kyoto . 161 – 173 . Japan InEd.
- Pérez , G.T. , León , A.E. , Ribotta , P.D. , Aguirre , A. , Rubiolo , O.J. and Añón , M.C. 2003 . Use of triticale flours in cracker-making . European Food Research and Technology , 217 : 134 – 137 .
- Leon , A.E. , Rubiolo , A. and Añon , M.C. 1996 . Use of triticale flours in cookies: Quality factors . Cereal Chemistry , 73 : 779 – 784 .
- Skovmand , B. , Fox , P.N. and Villareal , R.L. 1984 . Triticale in commercial agriculture: Progress and promise . Advances in Agronomy , 37 : 1 – 45 .
- Serna-Saldívar , S.O. , Guajardo-Flores , S. and Viesca-Rios , R. 2004 . Potential of triticale as a substitute for wheat in flour tortilla production . Cereal Chemistry , 81 : 220 – 225 .
- Li , G. , He , Z. , Peña , R.J. , Xia , X. , Lillemo , M. and Sun , Q. 2006 . Identification of novel secaloindoline-a and secaloindoline-b alleles in CIMMYT hexaploid triticale lines . Journal of Cereal Science , 43 : 378 – 386 .
- Peña , R.J. 1996 . “ Factors affecting triticale as a food crop ” . In Triticale Today and Tomorrow , Edited by: Guedes-Pinto , H. , Darvey , N. and Carnide , V.P. 753 – 761 . Dordrecht , The Netherlands : Kluwer Academic Publishers . InEds.
- Mergoum , M. , Singh , P.K. , Peña , R.J. , Lozano-del Río , A.J. , Cooper , K.V. , Salmon , D.F. and Gómez Macpherson , H. 2009 . “ Triticale: A “new” crop with old challenges ” . In Cereals , Edited by: Carena , M.J. 267 – 287 . Germany : Springer Science . InEd.
- Peña , R.J. and Amaya , A. 1992 . Milling and breadmaking properties of wheat-triticale grain blends . Journal of the Science of Food and Agriculture , 60 : 483 – 487 .
- Naeem , H.A. , Darvey , N.L. , Gras , P.W. and MacRitchie , F. 2002 . Mixing properties, baking potential, and functionality changes in storage proteins during dough development of triticale-wheat flour blends . Cereal Chemistry , 79 : 332 – 339 .
- Arseniuk , E. , Oleksiak , T. and Arseniuk , E. 2002 . “ Production and breeding of cereals in Poland ” . In Proc. 5th Int. Triticale Symposium , 11 – 20 . Radzikow, Poland : IHAR . InEd.
- AOAC . 2000 . Official Methods of Analysis of AOAC International , Gaithersburg , MD : AOAC . 17 Ed.
- Khan , K. , Tamminga , G. and Lukow , O. 1989 . The effect of wheat flour proteins on mixing and baking correlations with protein fractions and high molecular weight glutenin subunit composition by gel electrophoresis . Cereal Chemistry , 66 : 391 – 396 .
- Kaldy , M.S. , Rubenthaler , G.I. , Kereliuk , G.R. , Berhow , M.A. and Vandercook , C.E. 1991 . Relationships of selected flour constituents to baking quality in soft white wheat . Cereal Chemistry , 68 : 508 – 512 .
- Dubois , M. , Gilles , K.A. , Hamilton , J.K. , Rebers , P.A. and Smith , F. 1956 . Colorimetric method for determination of sugars and related substances . Analytical Chemistry , 28 : 350 – 356 .
- Knutson , C.A. 1986 . A simplified colorimetric procedure for determination of amylose in maize starches . Cereal Chemistry , 63 : 89 – 92 .
- Gupta , R.B. and MacRitchie , F. 1991 . A rapid one-step one-dimensional SDS-PAGE procedure for analysis of subunit composition of glutenin in wheat . Journal of Cereal Science , 14 : 105 – 109 .
- Laemmli , U.K. 1970 . Cleavage of structural proteins during the assembly of the head bacteriophage T4 . Nature , 22 : 77 – 80 .
- Blum , H. , Beier , H. and Gross , H.J. 1987 . Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels . Electrophoresis , 8 : 93 – 99 .
- AACC . Approved Methods for Analysis , 2000 St. Paul, MN : American Association of Cereal Chemists .
- Institute. , SAS . 2005 . PROC User's Manual , 8th Version , Cary , NC : SAS Institute .
- Matsuki , J. , Yasui , T. , Kohyama , K. and Sasaki , T. 2003 . Effects of environmental temperature on structure and gelatinization properties of wheat starch . Cereal Chemistry , 80 : 476 – 480 .
- Fortuna , T. , Gambuś , H. , Nowotna , A. and Palasiński , M. 1985 . Studies on the suitability of triticale for starch production . Acta Alimentaria Polonica , 11 : 53 – 62 .
- Burešová , I. , Sedláčková , I. , Faměra , O. and Lipavský , J. 2010 . Effect of growing conditions on starch and protein content in triticale grain and amylose content in starch . Plant, Soil and Environment , 56 : 99 – 104 .
- De Angelis , M. , Minervini , F. , Caputo , L. , Cassone , A. , Coda , R. , Calasso , M.P. , Divella , F. , Divella , F. and Gobbetti , M. 2008 . Proteomic analysis by two-dimensional gel electrophoresis and starch characterization of Triticum turgidum L. var. durum cultivars for pasta making . Journal of Agricultural and Food Chemistry , 56 : 8619 – 8628 .
- Hansen , L.E. , Jackson , D.S. , Wehling , R.L. and Graybosch , R.A. 2010 . Functionality of chemically modified wild-type, partial waxy and waxy starches from tetraploid wheats . Journal of Cereal Science , 51 : 409 – 414 .
- Sestili , F. , Janni , M. , Doherty , A. , Botticella , E. , D'Ovidio , R. , Masci , S. , Jones , H.D. and Lafiandra , D. 2010 . Increasing the amylose content of durum wheat through silencing of the SBEIIa genes . BMC Plant Biology , 10 : 144 – 155 .
- Ao , Z. and Jane , J.L. 2007 . Characterization and modeling of the A- and B-granule starches of wheat, triticale, and barley . Carbohydrate Polymers , 67 : 46 – 55 .
- Dennett , A.L. , Schofield , P.R. , Roake , J.E. , Howes , N.K. and Chin , J. 2009 . Starch swelling power and amylose content of triticale and Triticum timopheevii germplasm . Journal of Cereal Science , 49 : 393 – 397 .
- Zhang , T. , Wang , Z. , Yin , Y. , Cai , R. , Yan , S. and Li , W. 2010 . Starch content and granule size distribution in grains of wheat in relation to post-anthesis water deficits . Journal of Agronomy and Crop Science , 196 : 1 – 8 .
- Bushuk , W. and Larter , E.N. Triticale: . “ Production, chemistry and technology ” . In Advances in Cereal Science and Technology, Vol. III;1980 , Ed. , Edited by: Pomeranz , Y. St. Paul , MN : American Association of Cereal Chemists . In
- Varughese , G. , Barker , T. and Saari , E. 1987 . Triticale; , Mexico , D.F. : CIMMyT .
- Kumari , S.K. and Thayumanavan , B. 1998 . Characterization of starches of proso, foxtail, barnyard, kodo, and little millets . Plant Foods for Human Nutrition , 53 : 47 – 56 .
- Gupta , M. , Bawa , A.S. and Semwal , A.D. 2009 . Morphological, thermal, pasting, and rheological properties of barley starch and their blends . International Journal of Food Properties , 12 : 587 – 604 .
- Lee , M.R. , Swanson , B.G. and Baik , B.K. 2001 . Influence of amylose content on properties of wheat starch and breadmaking quality of starch and gluten blends . Cereal Chemistry , 78 : 701 – 706 .
- Ragaee , S.M. , Campbell , G.L. , Scoles , G.J. , McLeod , J.G. and Tyler , R.T. 2001 . Studies on rye (Secale cereale L.) lines exhibiting a range of extract viscosities. 2. Rheological and baking characteristics of rye and rye/wheat blends and feeding value for chicks of wholemeals and breads . Journal of Agricultural and Food Chemistry , 49 : 2446 – 2453 .
- Hansen , H.B. , Møller , B. , Andersen , S.B. , Jørgensen , J.R. and Hansen , Å. 2004 . Grain characteristics, chemical composition, and functional properties of rye (Secale cereale L.) as influenced by genotype and harvest year . Journal of Agricultural and Food Chemistry , 52 : 2282 – 2291 .
- Seguchi , M. , Ishihara , C. , Yoshino , Y. , Nakatsuka , K. and Yoshihira , T. 1999 . Breadmaking properties of triticale flour with wheat flour and relationship to amylase activity . Journal of Food Science , 64 : 565 – 752 .
- Klassen , A.J. and Hill , R.D. 1971 . Comparison of starch from triticale and its parental species . Cereal Chemistry , 48 : 647 – 654 .
- Gupta , R.B. , Khan , K. and MacRitchie , F. 1993 . Biochemical basis of flour properties in bread wheats. I. Effects of variation in the quantity and size distribution of polymeric protein . Journal of Cereal Science , 18 : 23 – 41 .
- Cinco-Moroyoqui , F.J. and MacRitchie , F. 2008 . Quantitation of LMW-GS to HMW-GS ratio in wheat flours . Cereal Chemistry , 85 : 824 – 829 .
- Shomer , I. , Lookhart , G. , Salomon , R. , Vasilier , R. and Bean , S. 1995 . Heat coagulation of wheat flour albumins and globulins, their structure and temperature fractionation . Journal of Cereal Science , 22 : 237 – 249 .
- Oszvald , M. , Tamás , C. , Rakszegi , M. , Tömösközi , S. , Békés , F. and Tamás , L. 2009 . Effects of incorporated amaranth albumins on the functional properties of wheat dough . Journal of the Science of Food and Agriculture , 89 : 882 – 889 .
