Abstract
Apple parallelepipeds were dewatered by osmosis in sucrose solution at 30°C for 3 h, and at 70°C for 1 h. Dewatered material was air-frozen at −12, −20, and −35°C and stored for 1, 3, and 6 months. After a prescribed time of storage, cryoscopic temperature, amount of non-frozen water, and effective diffusion coefficients were calculated based on sucrose concentration profiles in the frozen material. In frozen, osmotically dewatered apple, mass transfer was very slow. After 6 months of storage, large concentration gradients still occurred in the frozen material. Effective diffusion coefficients, based on sucrose concentration expressed as percent of the total mass of the sample, were statistically not dependent on storage temperature. They were dependent on sucrose concentration and varied from 2·10−14 m2/s, at high sucrose concentration, to 5·10−11 m2/s, at low sucrose concentration. On the other hand, effective diffusion coefficients, based on the sucrose concentration in non-frozen water, were dependent on storage temperature and sucrose concentration as well. They were from 1·10−15 m2/s to 2·10−12 m2/s.
INTRODUCTION
Osmotic dehydration is a mild processing method of fruits and vegetables having many advantages in comparison to other dehydration methods.[Citation1] Processing proceeds without a change in phase, and is done at room or moderate temperature. Osmotic dewatering reduces water content in the material by 40 to 70% and increases dry matter content by 5 to 25%. Reduced water activity of dewatered material is not sufficient to assure stability of the product. The product is perishable and needs further processing. One of the alternative methods of preservation of osmotically dewatered material is its freezing. The osmotic dehydrofreezing process is composed of two processes applied one after another.
Osmotic dewatering causes injury to tissue structure. Cells undergo plasmolysis, and their shape and size change during osmotic treatment.[Citation2,Citation3] Degradation of the middle lamella and detachment of cells occurs during processing. Changes in number, size, and shape of intercellular spaces accompany osmotic dewatering. Osmotically dewatered samples of apple showed significant damage to cell membrane measured by conductivity of rehydrating solution.[Citation4] Osmotic dewatering decreases water content in the treated material, and mobility of water molecules as well. Cornillon[Citation5] showed that T1 and T2 are, respectively, seven and four times larger for fresh apple than those for apple dewatered by osmosis. The decrease of water molecule mobility was strongly dependent on sucrose gradients. The tissue structure hinders diffusion of hypertonic solute, which is mostly sugar; hence, its penetration is restricted to the surface layers of the material only. Sugars penetrate osmotically treated tissue no deeper than 2–3 mm from the mass exchange surface.[Citation6,Citation7] Concentration gradients occurring in the material decrease during storage. It has been shown that, at temperatures above zero, the equilibration of sugar concentration in the osmotically dewatered apple is fast, and at 9°C it takes about 144 h.[Citation8]
Freezing of plant tissue causes physical and chemical changes due to the formation of ice crystals.[Citation9] Physical changes involve mechanical stresses resulting in cell damage and freeze cracking, alterations in the tissue structure and dislocation of water, and cryconcentration of solubles.
Apple samples frozen at −20°C showed large changes in the tissue structure. In comparison with untreated tissue, cell walls looked collapsed and more and larger intercellular spaces occurred. Intact vacuole were absent in the frozen material. Hence, destruction of tonoplast took place during freezing. Ice crystals formed during freezing of the apple tissue at −20°C were irregular and measured from 10 to 30 μm.[Citation10] Due to the mechanical stress and cryoconcentration, slow freezing at −20°C promoted membrane denaturation and cell wall degradation. Intercellular spaces were also deformed.[Citation11] Freezing of apple at −35°C caused loss of smoothness of the middle lamella indicating its damage. Ice crystals formed inside as well as outside the cell.[Citation4] Diffusion of small molecules from frozen and thawed material to surrounding water was faster than that from unfrozen material.[Citation12] Extensive research considering the effect of freezing on fruit quality have shown that changes in texture and solubles content are the most appropriate quality parameters to discriminate frozen/thawed material from fresh material.[Citation13]
Osmotic dewatering and subsequent freezing both affect structure of the plant tissue. However, the tissue already affected by osmosis is frozen; hence, freezing injury should be dependent on the extent of structure alterations due to osmosis. Osmotically treated material contains more sugar in the surface layers than in the center of the sample. These can mitigate adverse effects of freezing. It has been shown that pretreatment with sugars or calcium reduces tissue injury due to freezing.[Citation14−Citation17] In osmo-dehydrofrozen apple, structure alterations due to freezing were reduced by osmotic pretreatment.[Citation4] Apples dehydrated by osmosis and frozen in an air-blast tunnel at −40°C showed much smaller drip loss during thawing in comparison with the frozen fresh material.[Citation15] Research conducted by Delemos and Singh[Citation18] on dehydrofreezing of papaya cubes showed that the process minimized injury to the tissue structure yielding product with better texture than that obtained only by freezing.
Diffusion of solutes in a frozen system was not a subject of thorough investigations. In a review on heat and mass transfer in frozen foods,[Citation19] the problem of solutes migration is not considered. Discussed is the problem of weight loss due to water sublimation and surface desiccation in dense and porous materials. During freezing of vegetables in brine, pick-up of the salt was negligible.[Citation20] Water diffusion in frozen cod measured with the use of the heavy water showed that diffusion coefficients are much larger than those for pure ice. Hence, diffusion must proceed mainly through the unfrozen phase.[Citation21] Temperature gradients accelerated the diffusion of water in the frozen tissue. Freezing and formation of ice crystals decreased diffusion of volatile compounds in sucrose and maltodextrin solutions. Tortuosity and entanglement extended the path of molecules in the unfrozen phase.[Citation22] Hagiwara et al.[Citation23] investigated self-diffusion of water in sugar solutions. Recrystallization rate and diffusion of water were related directly, but there was no clear influence of temperature on both investigated processes. Observation of fluoresceine diffusion in sucrose solutions at −10°C showed that the presence of ice crystals at concentrations even higher that 65% does not influence the movement of the molecule. Hence, only the viscosity of the freeze-concentrated solution controls translational diffusion of fluoresceine molecules.[Citation24] Numerous publications showed that many reactions proceed in frozen material. Oxidation of ascorbic acid,[Citation25,Citation26] oxidation catalyzed by polyphenoleoxidase and peroxidase,[Citation27] and activity of alkaline phosphatase[Citation28] are just examples showing that, in freeze-concentrated solution, mobility of molecules is sufficiently fast to observe their changes in reasonable time.
An extensive tissue injury can be expected in the material osmotically pretreated followed by freezing. Character of the injury due to osmotic treatment is substantially different from that caused by freezing. Tissue injury caused by osmosis arises from the concentration gradients, while freezing injures material mechanically. Both types of injury are anticipated in the osmo-dehydrofrozen tissue, although presence of sugars modifies freezing influence on the tissue structure. Research of osmo-dehydrofreezing showed that, with injury incurred to tissue by osmosis, freezing modifies it a little. Therefore, freezing following osmotic dewatering should affect the mobility of sugar molecules mostly due to cryoconcentration, and not due to tissue injury. However, this supposition arising from the published data needs experimental verification.
The aim of this work was to investigate sucrose diffusion in osmo-dehydrofrozen apple during frozen storage. Different pretreatments preceding freezing were applied to follow the effect of tissue structure alterations on mobility of sucrose molecules. Samples with tissue structure injured by osmosis and osmosis combined with heating were prepared. The material dewatered by osmosis was frozen and stored at different temperatures. It was expected that an experiment designed that way would show which of the processes, osmotic dewatering or freezing, affect the mobility of sucrose molecules more in apple tissue.
MATERIALS AND METHODS
Materials
The Faculty of Horticulture and Landscape Architecture of the Warsaw University of Life Sciences supplied apples (cultivar Idared). Apples were stored at 4°C for no longer than a week before use. Slices (40 × 40 mm and 20 mm thick) cut out from the flesh part of fruits were used in the experiment.
Osmotic Dehydration
Osmotic dehydration was conducted in 61.5% sucrose solution in two variants: variant I—3 h at 30°C and variant II—1 h at 70°C. Osmotic solution concentration and time/temperature variables were chosen to obtain similar sucrose concentration profiles in the dewatered apple.[Citation29−Citation31] Osmotic dewatering was done in beakers placed in a water bath (ELPAN-357, Lubawa, Poland) set at a prescribed temperature. Eccentric rotation at 100 rpm of beakers assured circulation of osmotic solution. Mass ratio of the osmotic solution to the dehydrated material was 4:1 w/w. Apple slices were immersed in the preheated, to a prescribed temperature, solution to a depth of 18 mm, leaving the upper surface in contact with the air. The plastic sieve mounted on the top of the beakers assured the immersion depth of the apple slice. One sample was placed in the beaker, and nine beakers were used at a time. This arrangement of osmotic dewatering assured unidirectional mass transfer. After the specified dewatering time, the samples taken from the osmotic solution were rinsed with a spray of cold water and dried on a filter paper. For each storage temperature, osmotic dehydration was performed separately.
Freezing and Storage of Dewatered Samples
After osmotic dehydration, samples were single packed into aluminum foil. Freezing and storage was done in a Whirlpool freezer (model AFE 512, Croydon, UK) with forced air circulation, set to a prescribed temperature. Samples obtained in both variants were stored for 1 month at −12, −20, and −35°C. Samples of variant were also stored for 3 and 6 months at a temperature of −35°C. A thermocouple was inserted in the center of the sample, which was frozen at −35°C. Cryoscopic temperature, time of phase change, and total time of freezing was read from the freezing curve. The extrapolation method was used to analyze the freezing curve.[Citation32]
Determination of the Spatial Distribution of the Dry Matter Content
Cylinders, 20 mm in diameter, were cut out from the central part of the osmo-dehydrofrozen apple slices. Then, the cylinders were sliced in 0.5 mm thick samples, using a device equipped with a micrometer screw. The slicing began from the surface, which was in direct contact with the osmotic solution. The dry matter content was determined in slices cut at the following distance from the surface: 0–0.5; 0.5–1.0; 2.5–3.0; 4.5–5.0; 6.5–7.0; and 9.5–10.0 mm. Dry matter content in those slices was determined by a drying method according to Polish Standard; that is, at 98°C until constant weight was reached. Calculation of dry matter content was based on the loss of mass by the sample during drying. Triplicate measurements were used to calculate the average dry matter content.
Determination of the Spatial Distribution of Sugar Concentration
Sugar content in the 0.5-mm-thick slices was measured by colorimetric method using 3.5-dinitrosalicylic acid (DNS reagent).[Citation33] Simple sugars reduce the nitro group of the 3.5-dinitrosalicylic acid to the amine group. The intensity of orange color given by amine groups depends on the amount of sugar in the sample. Each slice was mixed with 20 ml of water. This mixture was heated at 90°C for 30 min and then filtered. Then, 2 ml of the DNS reagent was added to 2 ml of filtered liquid and heated at 90°C for 10 min. After cooling to room temperature in a cold-water bath, the absorbance was recorded with a spectrophotometer at 550 nm (Spekol 11, Analytik Jena AG, Jena, Germany). To determine the amount of nonreducing sugars, the filtrate was hydrolyzed. To 5 ml of filtered solution, 1 ml of 6-molar hydrochloric acid was added. The hydrolysis done was at the temperature of 90°C for 30 min. After cooling to room temperature, 1 ml of 6-molar sodium hydroxide was added. The mixture was filled up with water to 10 ml. Then 2 ml of the DNS reagent was added to 2 ml of hydrolyzed mixture and heated at 90°C for 10 min. Absorbance was recorded as described above. The amount of sucrose was calculated from the difference between the total and reducing sugars concentration. Measurement was done in triplicate. The reference relationship between color intensity and sugar concentration was prepared using glucose of analytic grade.
Modeling Mass Transfer
For all samples, the sucrose concentration profile at a given time of storage was described by the following equation:
where c is sucrose concentration, (%); x is distance, m; a, b, f, g, and h are constants; subscripts x and τ are distance and time, respectively. When x = 0, then cx,τ = a + b + g, that is the sugar content at the surface of the sample dewatered by osmosis. This equation, for each variant of storage parameters, was selected using the Table Curve 2D software (Systat Software Inc., San Jose, CA, USA). Since the process of sucrose diffusion was very slow, the difference between spatial distributions before and after frozen storage were too small to apply the analytical solution presented in a previous publication.[Citation8] Because of the very slow migration of sucrose molecules in the osmo-dehydrofrozen apple tissue, the steady state diffusion was assumed. The first Fick’s Law was applied to calculate effective diffusion coefficients.
Differentiation of Eq. (1) yields concentration gradients in a chosen slice of the apple before and after frozen storage at a given temperature.
The average (subscript av) concentration gradient was calculated as arithmetic mean:
The effective diffusion coefficient was calculated from the following equation:
Statistical Analysis
Data obtained in this work was analyzed using the t-test and analysis of variance at α = 0.05. Absolute differences in sucrose concentration profiles of osmotically dewatered apple and those of osmo-dehydrofrozen samples were analyzed using the t-test. Natural logarithms of effective diffusion coefficients were used for the ANOVA analysis. Microsoft Excel 2007 software (Redmond, WA, USA) processed all calculations.
RESULTS
Freezing Process
Osmotically pretreated samples prepared for freezing had an initial temperature of 20°C. Fresh apple frozen at −35°C showed a cryoscopic temperature of −1.9°C and that temperature was reached after 12 min of cooling. The cryoscopic temperature of the apple dewatered by osmosis at 30°C for 3 h was −5.2°C, and for that dewatered at 70°C for 1 h, it was −6.7°C. The time to reach that temperature was 12 and 11 min, respectively. The time of phase change arbitrarily taken was that between cryoscopic temperature and −10°C. For the raw apple, it was 46 min, and for apple dewatered by osmosis it was 32 and 30 min for the variants I and II, respectively. Calculated freezing velocity (about 0.5 cm/h) was lower than 1 cm/h. It means that all samples dewatered by osmosis were slowly frozen.
Sucrose Concentration Profile in the Apple Dewatered by Osmosis at 30°C for 3 h, Frozen and Stored at Different Freezing Temperatures
Differences in sucrose concentration set by osmotic dewatering caused mass transfer during frozen storage mostly in surface layers. This is in agreement with data published in the literature.[Citation6,Citation7,Citation34] In layers close to the surface, sucrose concentration after osmotic dewatering was 22.79 ± 1.36%. During 1 month of storage at −12°C it decreased to 19.58 ± 1.05%. At a distance of 10 mm from the surface, the increase of sucrose concentration during frozen storage was observed. In the dewatered apple, sucrose concentration at that distance was 1.60 ± 0.75% and increased during frozen storage at −12°C for 1 month to 1.77 ± 0.15% (). One month of storage at −35°C caused a decrease of sucrose concentration in the surface layers to 19.86 ± 0.58% and an increase at the distance of 10 mm to 1.85%. It means that substantial mass transfer took place only in layers close to the surface of the osmotically dewatered sample.
FIGURE 1 Sucrose concentration profiles for apple dewatered by osmosis at 30°C for 3 h, frozen, and stored at different temperatures for 1 month.
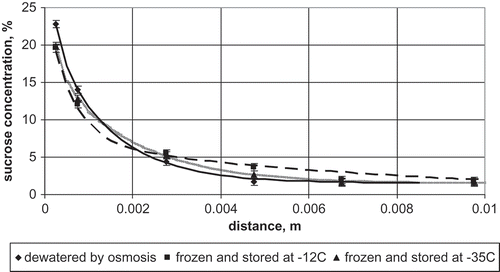
The difference in sucrose concentration profile of the apple dewatered at 30°C for 3 h and the profiles of samples frozen and stored for 1 month were statistically significant (the t test varied between 2.81 and 3.36). However, comparison of the sucrose concentration profiles in samples frozen at different temperatures and stored for 1 month showed no statistically significant differences (the t test varied between 0.44 and 1.10).
Storage of the dewatered apple at −35°C for 3 and 6 months caused further migration of sucrose from the surface layers to the interior of the sample. After 6 months of storage at −35°C, concentration of sucrose in the surface layers decreased to 17.18 ± 0.57%. At the distance of 10 mm from the mass transfer surface, the sucrose concentration increased to 4.30 ± 0.84%. Sucrose concentration profile obtained after 3 months of storage at −35°C statistically was not different from that observed after 1 month of storage (t = 2.08). Storage for 6 months resulted in statistical difference in sucrose concentration profile in comparison to that obtained after 1 month of storage (t = 3.63). The above presented data shows that sucrose migration takes place in osmotically dewatered and frozen samples. It is statistically dependent on time, but not affected by temperature of frozen storage.
Sucrose Concentration Profile in the Apple Dewatered at 70°C for 1 h, Frozen and Stored at Different Freezing Temperatures
A profile of sucrose concentration in apple osmotically dewatered at 70°C for 1 h and frozen was like that obtained for samples processed at 30°C for 3 h. Concentration of sucrose in the surface layers after osmosis was 20.18 ± 1.02%, and at the distance of 10 mm it was 1.50 ± 0.28%. At storage temperatures of −12, −20, and −35°C for 1 month, sucrose concentration in the surface layers was 15.72 ± 0.96, 16.09 ± 1.00, and 19.90 ± 0.89%, respectively. At the distance of 10 mm from the surface, respective concentrations were 2.78 ± 0.61, 1.77 ± 0.43, and 1.59 ± 0.51%. The effect of freezing and frozen storage on the sucrose migration in osmotically dewatered apple is evident as in the material dewatered at 30°C for 3 h. The effect of temperature on sucrose migration in the osmotically treated apple is more evident the higher the storage temperature. At -12°C the t-test is 5.06, at −20°C it is 3.32, and at −35°C it is 2.22. The last figure shows that sucrose concentration profile after 1 month of storage is not statistically different from that observed after osmotic dehydration.
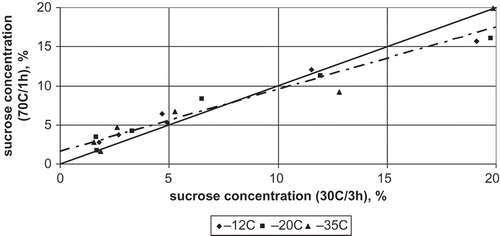
Comparison of sucrose concentration profiles in both variants, observed in the osmo-dehydrofrozen and stored for 1 month apple, showed that the way the material was dewatered affected the mass transfer process. In the apple dewatered by osmosis at 30°C for 3 h, mass transfer during frozen storage was slower than that in the tissue dewatered at 70°C for 1 h ().
Diffusion of Sucrose in Apple Dewatered by Osmosis at 30°C for 3 h and Frozen
Effective diffusion coefficient of sucrose in the frozen samples stored for 1 month at different temperatures was statistically dependent on the distance (F = 19.23) from the surface of the apple slice (). The coefficient was not dependent on the temperature of storage of the frozen samples (F = 1.89). In layers close to the surface, the average effective diffusion coefficient during 1 month of storage was (3.54 ± 1.67)·10−14 m2/s and, at the distance of 10 mm, it was (1.98 ± 0.54)·10−11 m2/s. The relationship between effective diffusion coefficient and the distance is linear in semi-log coordinates. On the distance of 10 mm, the effective diffusion coefficient increased by three orders of magnitude.
FIGURE 3 Relationship between effective diffusion coefficient and distance from the mass transfer surface for apple dewatered by osmosis at 30°C for 3 h, frozen, and stored at different temperatures for 1 month.
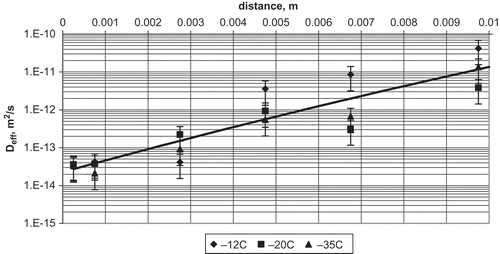
The time of frozen storage of osmotically dewatered apples stored at −35°C for 1, 3, and 6 months showed no statistically significant influence on the effective diffusion coefficient. The average effective diffusion coefficient in surface layers was (1.95 ± 1.05)·10−14 m2/s and at a distance of 10 mm it was (9.70 ± 1.23)·10−10 m2/s. The relationship between effective diffusion coefficient and distance was also linear in semi-log coordinates. The effect of storage time was not statistically significant, although a decreasing tendency was observed. After 1 month of storage, effective diffusion coefficient in surface layers was (3.32 ± 1.85)·10−14, while after 6 months of storage it was (7.78 ± 5.45)·10−15 m2/s. Since concentration of sucrose was dependent on the distance from the mass exchange surface, the relationship between effective diffusion coefficient and sucrose concentration was expected.
Effective diffusion coefficient of sucrose in the layers close to the surface of an apple slice was (3.36 ± 1.23)·10−14 m2/s, and was independent on sucrose concentration in the range of 10–22% (). At lower sucrose concentrations, the effective diffusion coefficient became strongly dependent on concentration and at 2% of sucrose it was (2.36 ± 1.88)·10−12 m2/s. The difference of two orders of magnitude occurred between sucrose concentrations of 10 and 2%. Moreover, at low sucrose concentration, the spread of data was large. It can suggest that the movement of sucrose molecules in the layers injured during osmotic processing and freezing is not as tortuous as that in layers, in which structure has been mostly changed by freezing.
FIGURE 4 Relationship between effective diffusion coefficient and sucrose concentration for apple dewatered by osmosis at 30°C for 3 h, frozen, and stored at different temperatures for 1 month.
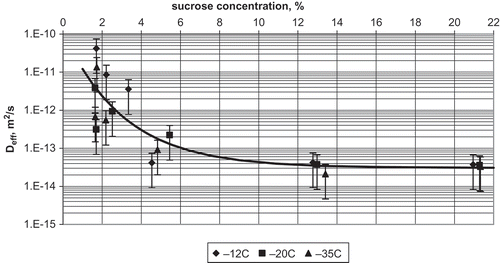
Storage time of samples frozen at −35°C had no effect on sucrose diffusion. The relationship between the effective diffusion coefficient and sucrose concentration at −35°C was like that at higher storage temperatures. In the sucrose concentration range from 10 to 22%, effective diffusion coefficient was (1.97 ± 0.56)·10−14 m2/s, and it was independent on sucrose concentration. However, observed was a tendency of lower diffusion coefficients at longer storage times, but the differences were too small to be statistically significant. At sucrose concentrations lower than 10%, the effective diffusion coefficient was strongly dependent on sugar concentration, and at 2% it was (1.67 ± 1.33)·10−11 m2/s. Again, a large spread of data was observed at low sucrose concentrations.
Diffusion of Sucrose in Frozen Samples of Apple Dewatered by Osmosis at 70°C for 1 h
The relationship between the sucrose effective diffusion coefficient and distance from the mass exchange surface is like that observed for apple osmotically treated at 30°C for 3 h. The effect of the distance on the movement of sucrose molecules is evident (F = 10.01). The effective diffusion coefficient increased on the distance of 10 mm from (1.97 ± 0.79)·10−14 m2/s to (1.04 ± 0.42)·10−11 m2/s, which is by three orders of magnitude. However, the influence of storage temperature in a frozen state was statistically insignificant.
Concentration of sucrose affects its diffusion in a manner similar to that observed for the apple osmotically dewatered at 30°C for 3 h (). At the sucrose concentration of 20%, the effective diffusion coefficient was (2.38 ± 0.95)·10−14 m2/s, and at the concentration of 2% it was (5.19 ± 2.08)·10−11 m2/s. The effect of storage temperature was statistically insignificant.
FIGURE 5 Relationship between effective diffusion coefficient and sucrose concentration for apple dewatered by osmosis at 70°C for 1 h, frozen, and stored at different temperatures for 1 month.
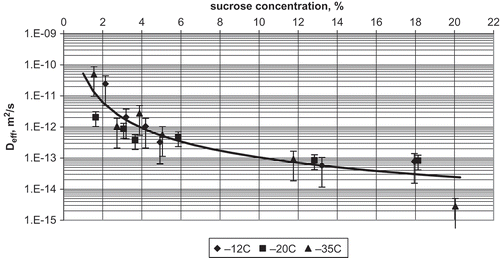
Comparison of the effective diffusion coefficients calculated for variants I and II, frozen and stored for 1 month, showed no statistically significant difference (F = 0.16). Correlation between the analyzed effective diffusion coefficients for variants I and II has a determination coefficient of 0.7364. It shows there is a tendency that the diffusion of sucrose in the frozen apple dewatered osmotically at 70°C for 1 h is easier than that in the apple dewatered at 30°C for 3 h.
DISCUSSION
Data presented above shows that effective sucrose diffusion coefficients in a frozen, osmotically dewatered apple are of the order from 10−14 to 10−11 m2/s, and are strongly dependent on the distance from the mass exchange surface and sugar concentration. The coefficients are not dependent on storage temperature, and the effect of storage time is small and statistically evident only at times as long as 6 months. These results are rather unexpected, because molecular diffusion in liquids and solids is dependent on temperature. Hence, expected effective diffusion coefficients should be lower, the lower the storage temperature.
The observed dependence of the effective diffusion coefficient on the distance from the mass exchange surface and sucrose concentration can arise from the tissue structure alterations and viscosity of the unfrozen part of the cell sap in apple. Sucrose concentration in the surface layers was high and influenced freezing temperature. Moreover, presence of sucrose in those layers could protect tissue against freezing injury. Hence, surface layers of the dewatered apple froze in different fashion than the interior of the sample. In the layers placed further from the mass exchange surface, concentration of solubles was low; therefore, the sample froze more like a native apple tissue.
Tissue injury caused by osmotic treatment and subsequent freezing changed along the distance from the mass exchange surface. Surface layers injured were mostly by osmosis, while in further placed layers, changes incurred were mostly by freezing. Osmotic process causes plasmolysis and changes structure of the tissue.[Citation3] However, cell walls are mostly intact, and the tissue structure, although loose, determines the main hindrance to movement of sucrose molecules. This explains the negligible influence of the sucrose concentration on the effective diffusion coefficient, at high sugar content in the osmo-dehydrofrozen apple. On the other hand, the effective diffusion coefficient was strongly dependent on the sucrose concentration at low sugar concentrations. The tissue placed further from the mass exchange surface was injured mostly by freezing. Destroyed integrity of the tissue and the viscosity of the unfrozen part of the cell sap affected movement of sucrose molecules. The effective diffusion coefficient became strongly dependent on the sucrose concentration.
Comparison of effective diffusion coefficients of sucrose in the osmo-dehydrofrozen apple dewatered at 30°C with those calculated for material osmotically treated at 70°C shows that tissue structure strongly influences molecular movement. At high sucrose concentration, the effect of osmosis temperature on sucrose diffusion, during frozen storage, was statistically insignificant. At 30°C Deff = (3.36 ± 1.23)·10−14, and at 70°C it was (2.38 ± 0.95)·10−14 m2/s. However, at low sucrose concentrations the difference was substantial. The respective values were (2.36 ± 1.88)·10−12 and (5.19 ± 2.08)·10−11 m2/s. The difference was larger than one order of magnitude.
The discussion presented above referred to effective diffusion coefficients based on the total mass of the sample. Hence, sucrose concentration profiles do not represent the real concentration gradients occurring in the osmotically treated apple. During freezing, a large part of the water, depending on the freezing temperature and solubles concentration, changes into ice crystals, and the remaining non-frozen solution undergoes concentration. In the remaining concentrated solution, gradients of sucrose concentration are much larger than the gradients based on the total mass of the sample.
Assuming that in an apple there is only free water, the real concentration of sucrose in frozen samples was calculated from the following equations:[Citation32,Citation35]
where W is water content, fraction; M is mass, fraction; t is temperature, °C; and subscripts are: dm, dry matter; cr, cryoscopic; fr, frozen; and s, sucrose. Data published by Lerici et al.[Citation36] was the basis for cryoscopic temperature calculation:
Calculated expected sucrose concentration in osmotically dewatered apple, frozen at different temperatures and stored for 1 month, are much larger than those calculated based on the sample mass. At -12°C, sucrose concentration in surface layers was 39 ± 3%, at −20°C it was 52 ± 3%, and at −35°C it amounted to 66 ± 3%. At the distance of 10 mm from the surface, the sucrose concentrations were 12.6 ± 2; 16.9 ± 2, and 25.0 ± 2%, respectively.
Applying calculated sucrose concentrations and the respective gradients, new effective diffusion coefficients were calculated. Influence of storage temperature in a frozen state on the diffusion of sucrose in osmo-dehydrofrozen apple is evident (). At sucrose concentrations in the range of 10–30%, the effect of temperature is small and diffusion coefficient changes by less than one order of magnitude, from 2·10−12 to 5·10−13 m2/s. At higher sucrose concentrations, the lines depart from each other. The lower the storage temperature is, the diffusion takes place at the higher sucrose concentrations.
FIGURE 6 Influence of sucrose concentration and frozen storage temperature on the effective diffusion coefficient in apple dewatered by osmosis at 30°C for 3 h and stored for 1 month.
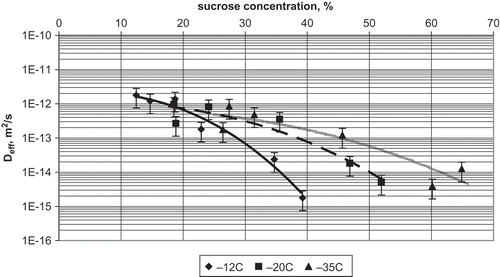
Analysis of the data presented on shows that there is a strict relationship between diffusion coefficient, sucrose concentration, and temperature of freezing and frozen storage. Diffusion coefficient 10−14 m2/s, taken as an example, occurs at concentrations dependent on temperature of storage in a frozen state. At temperatures of −12, −20, and −35°C, it occurs at the sucrose concentration close to 35, 50, and 62%, respectively. These data are surprising because diffusion in liquids and solids is slower the higher the concentration is and the lower the temperature is. From the presented data, it is evident that in addition to temperature and concentration, factors must affect diffusion of sucrose in the frozen osmotically dewatered apple.
Osmotic pretreatment caused some changes in apple structure, in which advancement was dependent on the distance from the mass transfer surface. Due to osmotic shock, cells in surface layers were highly plasmolyzed and mostly killed. Membranes lost their semi-permeability and compartmentation of the cell was lost. Cells placed further from the mass transfer surface affected are less by osmotic treatment and their integrity is preserved. From the structure point of view, ice crystallization should be easier in injured tissue in comparison to the native one. However, concentration of sucrose in surface layers was much higher than that in the central tissue. Hence, freezing temperature of surface layers was lower than that of tissue in the center of the sample.
The size of ice crystals is dependent on freezing temperature and concentration of the solution. Lower freezing temperature and higher sucrose concentration favor formation of small ice crystals. Hence, surface layers should contain mostly small ice crystals, while interior of the sample should contain large ice crystals. The tissue alterations caused by large ice crystals are little dependent on freezing temperature. Hence, sucrose diffusion coefficient, at low sucrose concentrations, should be little dependent on freezing temperature, which is evident on . In the range of 10–30% of sucrose concentration, effective diffusion coefficient was between 2·10−12 and 5·10−13 m2/s. On the other hand, the smaller the ice crystals, which occur, the lower the freezing temperature and the higher the sucrose concentration. Under this situation, porosity (inter-crystal spaces filled with concentrated solution) and tortuosity of diffusion path becomes dependent on sucrose concentration and freezing temperature. The porosity should be the larger and the tortuosity should be the smaller, the smaller the ice crystals are. According to the equation:
The effective diffusion coefficient increases with increasing porosity (ϵ) and decreasing tortuosity (E). Some other effects of freezing and frozen storage may affect transport of sucrose in apple tissue dewatered by osmosis. Growing ice crystals may redistribute concentrated solution. Since crystallization begins in the surface layers, the ice front can push concentrated solution into center layers of the sample. Recrystallization taking place during frozen storage may also affect sucrose concentration profile in the tissue. Hence, observed mass transfer can be a result of many processing variables in the osmo-dehydrofreezing process.
CONCLUSIONS
Diffusion of sucrose in osmo-dehydrofrozen apple can be analyzed in two ways. One approach is based on the total mass of the sample. Calculated that way, effective diffusion coefficients should be treated as practical ones, not representing real movement of the sucrose molecules in the stored osmo-dehydrofrozen apple. The other method is based on the sucrose concentration gradients in the non-frozen water in the sample. Effective diffusion coefficients based on actual sucrose concentration gradients represent intrinsic molecular mobility. The practical effective diffusion coefficients were not statistically dependent on the storage temperature in the frozen state. However, the observed tendency was that the lower the temperature was, the lower the effective diffusion coefficient was. The effective diffusion coefficients were dependent on the distance from the mass exchange surface. Along the distance of 10 mm, the coefficients increased by three orders of magnitude. The influence of sucrose concentration on the practical coefficients was more complicated. The relationship between effective diffusion coefficients and sucrose concentration was statistically significant at concentrations lower than 10%. At higher sucrose concentrations, the diffusion was not dependent on sugar concentration. Results of that part of the work suggested that mobility of sucrose molecules in osmo-dehydrofrozen apple was dependent on the structure alterations caused by osmosis and freezing, and the viscosity of concentrated cell sap. This explanation of observed relationships was supported by comparison of data of the variants I and II of the osmotic process. A tendency was observed that osmosis done at a higher temperature and shorter time resulted in higher effective diffusion coefficients of sucrose in the frozen state.
Intrinsic effective diffusion coefficients, based on the real sugar concentration gradients, were dependent on the temperature of freezing and frozen storage, and sucrose concentration. The result suggested that the tissue injury caused by osmosis and freezing affects diffusion of sucrose in osmotically dewatered and frozen apple. The extent of tissue injury, ice crystals shape and size, influenced by solubles concentration, and porosity affected by ice crystals size, all may influence mobility of sucrose molecules in osmotically dewatered and frozen material. In consequence, the temperature of frozen storage and the sucrose concentration are not the sole parameters affecting intrinsic effective diffusion coefficients in the osmo-dehydrofrozen apple.
REFERENCES
- Lewicki, P.P.; Lenart, A. Osmotic dehydration of fruits and vegetables. In: Handbook of Industrial Drying, 3rd Ed.; Mujumdar, A.S.; Ed.; Taylor & Francis: Boca Raton, FL, 2007; 665–687.
- Ferrando, M.; Spiess, W.E.L. Cellular response of plant tissue during the osmotic treatment with sucrose, maltose, and trehalose solutions. Journal of Food Engineering 2001, 49, 115–127.
- Lewicki, P.P.; Porzecka-Pawlak, R. Effect of osmotic dewatering on apple tissue structure. Journal of Food Engineering 2005, 66, 43–50.
- Tregunno, N.B.; Goff, H.D. Osmodehydrofreezing of apples: Structural and textural effects. Food Research International 1996, 29, 471–479.
- Cornillon, P. Characterization of osmotic dehydrated apple by NMR and DSC. LWT–Food Science & Technology 2000, 33, 261–267.
- Lenart, A.; Lewicki, P.P. Water diffusion in apple tissue subjected to osmotic dehydration. Zeszyty Naukowe SGGW-AR. Technologia Rolno-Spożywcza 1981, 14, 33–47 (in Polish).
- Salvatori, D.; Andres, A.; Chiralt, A.; Fito, P. Osmotic dehydration progression in apple tissue. I. Spatial distribution of solutes and moisture content. Journal of Food Engineering 1999, 42, 124–132.
- Kamińska, A.; Lewicki, P.P.; Malczyk, P. Mass transfer in osmotically dehydrated apple stored at temperatures above zero. Journal of Food Engineering 2008, 86, 140–149.
- Reid, D.S. Fruit freezing. In: Processing Fruits: Science and Technology, Vol. 1; Samogyi, L.P.; Ramaswamy, H.S.; Hui, Y.H.; Eds.; Technomic Publ. Comp.: New York, 1996; 275–286.
- Chassagne-Berces, S.; Poirier, C.; Devaux, M.-F.; Fonseca, F.; Lahaye, M.; Pigorini, G.; Girault, Ch.; Marin, M.; Guillon, F. Changes in texture, cellular structure and cell wall composition in apple tissue as a result of freezing. Food Research International 2009, 42, 788–797.
- Acevedo, N.C.; Briones, V.; Buera, P.; Aguilera, J.M. Microstructure affects the rate of chemical, physical and color changes during storage of dried apple slices. Journal of Food Engineering 2008, 85, 222–231.
- Garrote, R.L.; Silva, E.R.; Bertone, R.A. Effect of freezing on diffusion of ascorbic acid during water heating of potato tissue. Journal of Food Science 1988, 53, 473–474, 487.
- Chassagne-Berces, S.; Fonseca, F.; Citeau, M.; Martin, M. Freezing protocol effect on quality properties of fruit tissue according to the fruit, the variety and stage of maturity. LWT–Food Science & Technology 2010, 43, 1441–1449.
- Chirault, A.; Martinez-Navarrete, N.; Martinez-Monzo, J.; Talens, P.; Moraga, G.; Ayala, A.; Fito, P. Changes in mechanical properties throughaut osmotic processing: Cryoprotectant effect. Journal of Food Engineering 2001, 49, 129–135.
- Marani, C.M.; Agnelli, M.E.; Mascheroni, R.H. Osmo-frozen fruits: Mass transfer and quality evaluation. Journal of Food Engineering 2007, 79, 1122–1130.
- Buggenhout, S.V.; Lille, M.; Messagie, I.; Van Loey, A.; Autio, K.; Hendrickx, M. Impact of pretreatment and freezing conditions on the microstructure of frozen carrots: Quantification and relation to texture loss. European Food Research and Technology 2006, 222, 543–553.
- Sousa, M.B.; Canet, W.; Alvarez, M.D.; Fernandez, C. Effect of processing on the texture and sensory attributes of strawberry (cv. Heritage) and blackberry (cv. Thornfree). Journal of Food Engineering 2007, 78, 9–21.
- Delemos, B.R.; Singh, R.P. Texture of dehydrofrozen papaya. In: IFT Annual Meeting Book of Abstracts; Institute of Food Technologists: Chicago, IL, 1996.
- Pham, Q.T. Modeling heat and mass transfer in frozen foods: A review. International Journal of Refrigeration 2006, 29, 876–888.
- Goldberg, L.D.; Chumak, I.G.; Churkin, A.A. Penetration of salt into vegetable products during freezing in brine; Kholodilnaya Tekhnika Publishing House; Moscow, Russia, 1976, (in Russian).
- Storey, R.M. The diffusion of water in frozen cod. Bulletin of the International Institute of Refrigeration 1970, 3, 235–238.
- Covarrubias-Cervantes, M.; Champion, D.; Debeaufort, F.; Voilley, A. Translational diffusion coefficients of volatile compounds in various aqueous solutions at low and subzero temperatures. Journal of Agricultural and Food Chemistry 2005, 53, 6771–6776.
- Hagiwara, T.; Hartel, R.W.; Matsukawa, S. Relationship between recrystallization rate of ice crystals in sugar solutions and water mobility in freeze-concentrated matrix. Food Biophysics 2006, 1, 74–82.
- Champion, D.; Lopez, E.C.; Blond, G.; Simatos, D.; Le Meste, M. Small-molecule translational diffusion in frozen products. Proceedings of the Second IDF International Symposium on Ice Cream; Thessaloniki, Greece, May 14–16, 2003; p. 409.
- Biliaderis, C.G.; Swan, R.S.; Arvanitoyannis, I. Physicochemical properties of commercial starch hydrolizates in the frozen state. Food Chemistry 1999, 64, 537–546.
- Champion, D.; Simatos, D.; Le Meste, D. Diffusion-controlled reactions in frozen products. How state diagrams may be used for the prediction of storage stability. Proceedings of the Second IDF International Symposium on Ice Cream; Thessaloniki, Greece, May 14–16, 2003; pp. 264–275.
- Manzocco, L.; Nicoli, M.C.; Anese, M.; Pitotti, A.; Maltini, E. Polyphenoleoxidase and peroxidase activity in partially frozen systems with different physical properties. Food Research International 1999, 31, 363–370.
- Champion, D.; Blond, G.; Le Meste, M.; Simatos, D. Reaction rate modeling in cryconcentrated solutions: Alkaline phosphatase catalyzed DNPP hydrolysis. Journal of Agricultural and Food Chemistry 2000, 48, 4942–4947.
- Kaymak-Ertekin, F.; Sultanoglu, M. Modeling of mass transfer during osmotic dehydration of apples. Journal of Food Engineering 2000, 46, 243–250.
- Panagiotou, N.M.; Karathanos, V.T.; Maroulis, Z.B. Effect of osmotic agent on osmotic dehydration of fruits. Drying Technology 1999, 17, 175–189.
- Koocheki, A.; Azarpazhooh, E. Evaluation of mass exchange during osmotic dehydration of plum using response surface methodology. International Journal of Food Properties 2010, 13, 155–166.
- Rahman, M.S.; Machado-Velasco, K.M.; Sosa-Morales, M.E.; Velez-Ruiz, J.F. Freezing point: Measurement, data and prediction. In: Food Properties Handbook, 2nd Ed.; Rahman, M.S.; Ed.; CRC Press: Boca Raton, FL, 2009; 153–192.
- Toczko, M.; Grzelińska, A. Biochemistry Exercises. Wydawnictwo SGGW: Warszawa, Poland, 1997 (in Polish).
- Li, H.; Ramaswamy, H.S. Osmotic dehydration: Dynamics of equilibrium and pseudo-equilibrium kinetics. International Journal of Food Properties 2010, 13, 234–250.
- Rahman, M.S. Prediction of ice content in frozen foods. In: Food Properties Handbook; 2nd Ed.; Rahman, M.S.; Ed.; CRC Press: Boca Raton, FL, 2009; 193–206.
- Lerici, C.R.; Piva, M.; Dalla Rosa, M. Water activity and freezing point depression of aqueous solutions and liquid foods. Journal of Food Science 1983, 48, 1667–1669.