Abstract
The present study was evaluated using the following in vitro antioxidant methods: 2,2′-azinobis (3-ethyl-benzothiozoline)-6-sulfonic acid disodium salt, phosphomolybdenum, ferric reducing antioxidant power, metal ion chelating activity, super oxide anion radical scavenging, hydrogen peroxide, and hydroxyl radical. Among these assays, acetone extract showed maximum free radical scavenging activity in 2,2′-azinobis (3-ethyl-benzothiozoline)-6-sulfonic acid disodium salt, phosphomolybdenum, metal ion chelating, superoxide anion, hydrogen peroxide, and hydroxyl radical assays. Moreover, the physiochemical, nutritional, and anti-nutritional parameters were analyzed. Its qualitative and quantitative composition was studied by gas chromatography-mass spectroscopy and out of 27 peaks, 27 compounds were identified. These compounds have the property of antioxidant, antimicrobial, and anti-inflammatory activity.
INTRODUCTION
The adverse effects of oxidative stress on human health have become a serious issue. Under stress, our bodies produce more reactive oxygen species (ROS) (e.g., superoxide anion radicals, hydroxyl radicals, and hydrogen peroxide) than enzymatic antioxidants (e.g., superoxide dismutase [SOD], glutathione peroxidase [GPx], and catalase) and non-enzymatic antioxidants (e.g., ascorbic acid [vitamin C], α-tocopherol [vitamin E], glutathione, carotenoids, and flavonoids). This imbalance leads to cell damage [ Citation1] and health problems.[Citation2] A lack of antioxidants, which can quench the reactive free radicals, facilitates the development of degenerative diseases,[Citation3] including cardiovascular diseases, cancers,[Citation4] neurodegenerative diseases, Alzheimer's disease, and inflammatory diseases.[Citation5] One solution to this problem is to supplement the diet with antioxidant compounds that are contained in natural plant sources.[Citation6] These natural plant antioxidants can, therefore, serve as a type of preventive medicine. Recent reports indicate that there is an inverse relationship between the dietary intake of antioxidant-rich foods and the incidence of human disease.[Citation7] However, synthetic antioxidants, such as butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA), have been widely used as antioxidants in the food industry and may be responsible for liver damage and carcinogenesis.[Citation8] For this reason, interest in the use of natural antioxidants has increased.
Plants constitute an important source of active natural products, which differ widely in terms of structure, biological properties, and mechanisms of actions. Various phytochemical components are known to be responsible for antioxidant, antimicrobial, antilarvicidal, and anti-inflammatory activities of plants. The antioxidant vitamin content of fruits has attributed them to the protective role before. However, recent interest in food phenolics has increased greatly because of their antioxidant and free radical scavenging abilities.[Citation9] All of the most commonly sold fruit juices contain phenolic compounds showing a wide range of antioxidant activities in vitro.[Citation10,Citation11] Individual antioxidant compounds do not act alone.[Citation12]
The ethnobotanical views on Ficus amplissima (common name in Tamil Kal Ittchi) suggest that fruits are edible and chewed for mouth ulcers,[Citation13] and also the fruit is used to cure the mentally deranged.[Citation14] Mitra and Kapoor[Citation15] reported the pharmacognostical studies of Ficus tsiela Roxb. in 1972. But fruit of F. amplissima still remains unexplored for their pharmacological properties. There are no previous reports relating the antioxidant and chemical composition of F. amplissima. Hence, the present investigation on F. amplissima fruit was undertaken.
MATERIALS AND METHODS
Collection and Identification of Plant Material
The fresh fruits were collected during the month of October 2009 from Sathyamangalam, district of Erode, Tamil Nadu, India. The taxonomic identity of the plant was confirmed by Dr. A. Rajendran, and voucher specimen (No: 006147) was deposited at Botany Department Herbarium, Coimbatore, Tamil Nadu. The plant materials were washed under running tap water to remove the surface pollutants and the different parts of bark were separated mechanically. The separated parts were air dried under shade. The dried samples were powdered and used for further studies.
Chemicals
Sodium nitroprusside, potassium persulfate, 2,2′-azinobis (3-ethyl-benzothiozoline)-6-sulfonic acid disodium salt (ABTS), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), ferrous chloride, ferric chloride, ferric cyanide, hydrogen peroxide, ferrous ammonium sulfate, ethylene diamine tetra-acetic acid (EDTA), disodium salt, N-(1-naphthyl) ethylene diamine dihydrochloride, and riboflavin were obtained from Himedia, Merck, and Sigma. All other chemicals and solvents used were of analytical grade.
Extraction of Plant Material
The powdered fruit were packed in small thimbles separately and extracted successively with organic solvents, such as petroleum ether, chloroform, acetone, methanol, and hot water, in the increasing order of polarity using Soxhlet apparatus. Each time, before extracting with the next solvent, the thimble was air dried. Finally, the material was macerated using hot water with constant stirring for 24 h and the water extract was filtered. The different solvent extracts were concentrated by a rotary vacuum evaporator (Yamato RE300, Japan) and then air dried. The dried extract obtained with each solvent was weighed. The percentage of yield was calculated in terms of the air dried weight plant material (1 mg/mL of respective organic solvents), and the extract obtained was used for the assessment of various antioxidant assays and for further analysis.
In Vitro Antioxidant Assays
ABTS radical scavenging activity
The total antioxidant activity of the samples was measured by ABTS (2,2′-azinobis (3-ethylbenzothiozoline)-6-sulfonic acid) radical cation decolourization assay according to the method of Re et al.[Citation16] ABTS•+ was produced by reacting 7 mM ABTS aqueous solution with 2.4 mM potassium persulfate in the dark for 12–16 h at room temperature. Prior to assay, this solution was diluted in ethanol (about 1:89 v/v) and equilibrated at 30°C to give an absorbance of 0.700 ± 0.02 at 734 nm. The stock solution of the sample extracts were diluted such that after introduction of 10 μL aliquots into the assay, they produced between 20 and 80% inhibition of the blank absorbance. After the addition of 1 mL of diluted ABTS solution to 10 μL of sample or Trolox (final concentration 0–15 μM) in ethanol, absorbance was measured at 30°C exactly 30 min after the initial mixing. Triplicate determinations were made at each dilution of the standard, and the percentage inhibition was calculated against the blank (ethanol) absorbance at 734 nm and then was plotted as a function of Trolox concentration. The unit of total antioxidant activity (TAA) is defined as the concentration of Trolox having equivalent antioxidant activity expressed as μmoles/g sample extracts.
Phosphomolybdenum assay
The antioxidant activity of samples was evaluated by the green phosphomolybdenum complex formation according to the method of Prieto et al.[Citation17] An aliquot of 10–40 μL of sample or ascorbic acid in 1 mM dimethyl sulphoxide (standard) or distilled water (blank) was added with 1 mL of reagent solution (0.6 M sulphuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate) in a test tube. The test tubes were covered with foil and incubated in a water bath at 95°C for 90 min. After the samples had cooled to room temperature, the absorbance of the mixture was measured at 695 nm against the reagent blank. The results reported (total antioxidant capacity) are mean values expressed as milligrams of ascorbic acid equivalents per gram extract.
Ferric reducing antioxidant power (FRAP) assay
The antioxidant capacities of different extracts of samples were estimated according to the procedure described by Pulido et al.[Citation18] FRAP reagent (900 μL), prepared freshly and incubated at 37°C, was mixed with 90 μL of distilled water and 30 μL of test sample or methanol (for the reagent blank). The test samples and reagent blank were incubated at 37°C for 30 min in a water bath. The final dilution of the test sample in the reaction mixture was 1/34. The FRAP reagent was prepared by mixing 2.5 mL of 20 mM TPTZ (2,4,6-tripyridyl-s-triazine) in 40 mM HCl (hydrochloric acid), 2.5 mL of 20 mM FeCl3.6H2O, and 25 mL of 0.3 M acetate buffer (pH 3.6). At the end of incubation, the absorbance readings were taken immediately at 593 nm against the reagent blank using a spectrophotometer. Methanolic solutions of known Fe (II) concentration, ranging from 100 to 2000 μM (FeSO4.7H2O), were used for the preparation of the calibration curve. The parameter equivalent concentration was defined as the concentration of antioxidant having a ferric-TPTZ reducing ability equivalent to that of 1 mM FeSO4.7H2O. Equivalent concentration was calculated as the concentration of antioxidant giving an absorbance increase in the FRAP assay equivalent to the theoretical absorbance value of a 1 mM concentration of Fe (II) solution.
Metal chelating activity. The chelating of ferrous ions by various extracts of Ficus amplisssima fruit was estimated by the method of Dinis et al.[Citation19] Briefly, 400 μL of samples and BHT (standard) were added to a 50 μL solution of 2 mM FeCl2. The reaction was initiated by the addition of 200 μL of 5 mM ferrozine, and the mixture was shaken vigorously and left standing at room temperature for 10 min. Absorbance of the solution was then measured spectrophotometrically at 562 nm against the blank (deionized water). The metal chelating capacities of the extracts were evaluated using the following equation:
Superoxide radical scavenging activity
The assay was based on the capacity of various extracts to inhibit formazan formation by scavenging the superoxide radicals generated in riboflavin-light-NBT system.[Citation20] Each 3-mL reaction mixture contained 50 mM sodium phosphate buffer (pH 7.6), 20 μg riboflavin, 12 mM EDTA (ethylenediamine tetracetic acid disodium salt), 0.1 mg NBT (nitro blue tetrazolium), and 10–40 μL of aliquot of sample solution or BHA and BHT (standard). Reaction was started by illuminating the reaction mixture with sample extract for 90 s. Immediately after illumination, the absorbance was measured at 590 nm against the reagent blank (reaction mixture without plant sample). Identical tubes with reaction mixture kept in the dark served as negative control. The scavenging activity on superoxide anion generation was calculated as:
Hydrogen peroxide scavenging activity
The ability of the extracts to scavenge hydrogen peroxide was determined according to the method of Ruch et al.[Citation21] A solution of hydrogen peroxide (2 mM) was prepared in phosphate buffer (0.2 M, pH 7.4) and its concentration was determined spectrophotometrically from absorption at 230 nm with molar absorbtivity 81 M− Citation1/cm. The plant extracts (10 μg/mL), BHT, and vitamin C (10 μg/mL) were added to 3.4 mL of phosphate buffer together with hydrogen peroxide solution (0.6 mL). The identical reaction mixture without the sample was taken as negative control. Absorbance of hydrogen peroxide at 230 nm was determined after 10 min against the blank (phosphate buffer). The scavenging activity (%) was calculated as:
Hydroxyl radical scavenging activity
The scavenging activity of different solvent extracts of F. amplissima fruits on hydroxyl radical was measured according to the method of Klenin et al.[Citation22] An aliquot of 10–40 μg of different solvent extracts was added with 1 mL of iron-EDTA solution (0.13% ferrous ammonium sulphate and 0.26% EDTA), 0.5 mL of EDTA solution (0.018%), and 1 mL of DMSO (dimethyl sulphoxide) (0.85% v/v) in 0.1 M phosphate buffer, pH 7.4. The reaction was initiated by adding 0.5 mL of ascorbic acid (0.22%) and incubated at 80–90°C for 15 min in a water bath. After incubation, the reaction was terminated by the addition of 1 mL of ice cold TCA (17.5 % W/V). Then, 3 mL of Nash reagent (75 g of ammonium acetate, 3 mL of glacial acetic acid, and 2 mL of acetyl acetone were mixed and raised to 1 L with distilled water) was added and left at room temperature for 15 min. The reaction mixture without sample was used as control. The intensity of color formed was measured spectrophotometrically at 412 nm against reagent blank. The percentage of hydroxyl scavenging activity was calculated by the following formula:
Physical parameters
The F. amplissima fruits were analysed for physical parameters, such as shape, colour (through visual perception), weight (100 fruit weight), size (length and diameter of the fruit was measured with the help of Vernier caliper), and specific gravity (weight/volume ratio).
Chemical and nutritional parameters
The edible part of F. amplissima fruits were analyzed for moisture, ash, fat, and fibre as per methods reported in AOAC.[Citation23] Total nitrogen was analyzed by the micro-Kjeldahl method and for crude protein the value was multiplied by 6.25. The other parameter analysed was pH. Using a digital portable pH meter, TSS (hand refractometer), titratable acidity,[Citation23] and NPN (non protein nitrogen) by Pelletier,[Citation24] true protein was calculated by difference in values of total crude nitrogen and NPN and then multiplying by the factor of 6.25. Sugars, which include total and reducing sugars, were measured by the method reported in Rangana.[Citation25] Total carbohydrates were obtained by subtracting the values of moisture, crude fibre, crude protein, crude fat, and ash from 100. Energy was estimated by the method of O'Shea and Maguire.[Citation26] The minerals analysed were sodium, potassium, and iron using an atomic absorption spectrophotometer, calcium by flame photometer (Mediflame, 127), and phosphorus by following the method of Chen et al.[Citation27] Ascorbic acid in fresh fruit was estimated titrimatically using 2,6-dichlorophenol indophenols dye, β-carotene was determined as per method the method of Roy,[Citation28] and anthocyanin in fruit juice was estimated following the method reported in Rangana.[Citation25] The dietary fibre constituents, viz. NDF, ADF, lignin, cellulose, and hemicelluloses were analyzed according to Van Soest and Wine.[Citation29]
Antinutritional parameters and organoleptic evaluation
Various antinutritional parameters analysed were tannins,[Citation23] phytic acid,[Citation30] phytate phosphorus, and oxalates.[Citation31] Sensory evaluation depends upon the responses by different sense organs, such as eyes, taste buds of the tongue, and factory lobes of the nostril, as per the method suggested by Gould.[Citation32]
Gas Chromatography-Mass Spectroscopy
Preparation of extract
An amount of 1 μl of the acetone extract of F. amplissima fruit was employed for GC-MS analysis.
Instruments and chromatographic conditions
GC-MS analysis was carried out on a GC Clarus 500 Perkin Elmer system comprising a AOC-20i auto sampler and gas chromatograph interfaced to a mass spectrometer (GC-MS) instrument employing the following conditions: column Elite-1 fused silica capillary column (30 × 0.25 mm ID ×1 EM df, composed of 100% dimethyl poly siloxane), operating in electron impact mode at 70 eV; helium (99.999%) was used as the carrier gas at a constant flow of 1 ml/min and an injection volume of 0.5 EI was employed (split ratio of 10:1) injector temperature 250°C; ion-source temperature 280°C. The oven temperature was programmed from 110°C (isothermal for 2 min), with an increase of 10 to 200°C/min, then 5 to 280°C/min, ending with a 9 min isothermal at 280°C. Mass spectra were taken at 70 eV, a scan interval of 0.5 s, and fragments from 40 to 550 Da.
Statistical Analysis
The results were statistically analysed and expressed as mean (n = 3) ± standard deviation.
RESULTS AND DISCUSSION
In Vitro Antioxidant Assays
ABTS radical scavenging activity
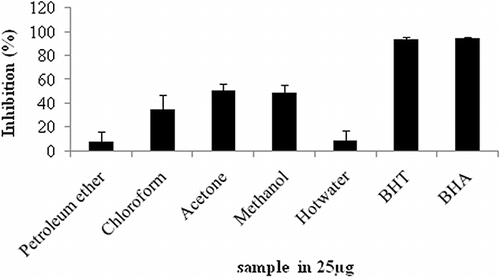
The TEAC (Trolox equivalents antioxidant capacity) was measured using the improved ABTS ·+ radical decolourisation assay, one of the most commonly employed methods for antioxidant capacity, which measures the ability of a compound to scavenge ABTS .+ radical.[Citation33] The results were expressed as μmol Trolox/g dry weight of plant material. The results of ABTS·+ cation radical scavenging activities of different solvent extracts of F. amplissima fruit were shown in . The higher scavenging activity of fruit was observed in its acetone extract (6587.96 μmoles TE/g extract).
Table 1 ABTS·+, phosphomolybdenum, FRAP, metal ion radical scavenging activities of Ficus amplissima fruit
Hagerman et al.[Citation34] have reported that the high molecular weight phenolics (tannins) have more ability to quench free radicals (ABTS·+) and their effectiveness depends on the molecular weight, the number of aromatic rings, and nature of hydroxyl group's substitution than the specific functional groups. Similarly, Rajesh et al.[Citation35] have reported that free radical scavenging activity of F. racemosa samples might be due to the presence of high phenolics. From the result, we conclude that F. amplissima could act as a high ABTS·+ radical scavenger.
Phosphomolybdenum assay
The phosphomolybdneum method is based in the reduction of Mo (VI) and to Mo (V) by the antioxidant compound and the formation of green phosphate/Mo (V) complex with the maximum absorption at 695 nm. The total antioxidant capacity of different solvent extracts of fruit of F. amplissima were analyzed and shown in . The better antioxidant capacity was shown by acetone extract of fruit (277.15 mg AAE/g extract). Among the different solvents used, the acetone extract showed better antioxidant capacity as compared to other solvent extracts. Thus, the antioxidant capacity observed from the extracts of F. amplissima can be correlated with its free radical scavenging activity equivalent to that of natural antioxidant ascorbic acid.
Ferric reducing antioxidant power (FRAP) assay
The simple and reliable test was adopted, which measures the reducing potential of an antioxidant reacting with a ferric 2,4,6-tripyridyl-S-triazine (Fe (III)-TPTZ) complex and produces a coloured ferrous 2,4,6-tripyridyl-S-triazine (Fe (II))-TPTZ) complex by a reductant at low pH. This complex can be monitored at 593 nm.[Citation36] Higher absorbance power indicates a higher ferric reducing power. The result shows () that the ferric reducing capacity was much higher (864.44 mM/g) in acetone extract.
Metal ion chelating activity
The method of metal chelating activity is based on chelating of FeCitation2 + ions by the reagent ferrozine, which is a quantitative formation of a complex with FeCitation2 + ions.[Citation19] The formation of a complex is probably disturbed by the other chelating reagents, which would result in the reduction of the formation of red-colored complex. Measurement of the rate of reduction of the color, therefore, allows estimation of the chelating activity of the coexisting factor.[Citation37] In this assay, F. amplissima and standard antioxidant compounds interfered with the formation of ferrous and ferrozine complex, suggesting that they have chelating activity and capture ferrous ion before ferrozine. Iron can stimulate lipid peroxidation by the Fenton reaction, and also accelerates peroxidation by decomposing lipid hydroperoxides into peroxyl and alkoxyl radicals that can themselves abstract hydrogen and perpetuate the chain reaction of lipid peroxidation.[Citation38]
The metal chelating capacity for methanol extract of fruit was found to be 38.74 mg EDTA/g (). From these results, the extracts may be able to play a protective role against oxidative damage by sequestering iron (II) ions that may otherwise catalyze Fenton-type reactions or participate in metal-catalyzed hydroperoxide decomposition reactions.[Citation39] Chelating agents may serve as secondary antioxidants because they reduce the redox potential thereby stabilizing the oxidized form of the metal ions.[Citation35] The present study reveals that the acetone extract of F. amplissima has a marked capacity for iron binding, suggesting their action as peroxidation protector.
Superoxide radical scavenging activity
Superoxide radical is known to be a very harmful species to cellular components as a precursor of more reactive oxygen species.[Citation40] The superoxide radical is known to be produced in vivo and can result in the formation of H2O2 via dismutation reaction. Moreover, the conversion of superoxide and H2O2 into more reactive species, e.g., the hydroxyl radical, has been thought to be one of the unfavorable effects caused by superoxide radicals.[Citation41] The extracts are found to be an efficient scavenger of superoxide radical generated in riboflavin-NBT-light system in vitro and their activities are comparable to that of ascorbic acid. Photochemical reduction of flavins generates 02ċ−, which reduces NBT, resulting in the formation of blue formazan.[Citation20]
The results of superoxide anion scavenging activities of fruit of F. amplissima are shown in . The extracts were found to be an efficient scavenger of superoxide radical generated in riboflavin-NBT-light system in vitro. The scavenging activity of acetone extract of fruit was found to be 50.81%, whereas methanol extract was also found to be better activity (49.35%). Superoxide radical is one of the most effective free radicals, implicated in cell damage as the precursor of important reactive oxygen species, like hydroxyl radical and peroxynitrite, contributing to the pathological process of many diseases. Similarly, Andreia et al.[Citation42] have also reported on Ficus carica latex protective effect against superoxide radical. From this assay, using different extracts of fruit, it is noted that the inhibition of the formation of blue formazan and also the percentage inhibition are directly proportional to the concentration of the plant extract.
Hydrogen peroxide scavenging activity
Hydrogen peroxide itself is not very reactive but sometimes it is toxic to cells because it may give rise to hydroxyl radicals in the cells.[Citation41] Therefore, removing of H2O2 is very important for antioxidant defence in cell or food systems. Dietary polyphenols have been shown to protect mammalian and bacterial cells from cytotoxicity induced by hydrogen peroxide, especially compounds with the orthodihydroxy phenolic structure, quercetin, catechin, gallic acid ester, and caffeic acid ester.[Citation43]
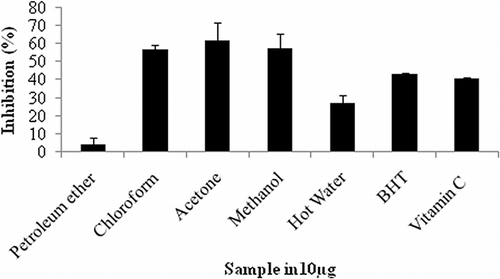
The scavenging ability of various extracts of F. amplissima on hydrogen peroxide is shown in and compared with the standard BHT and vitamin C. The higher percentages of scavenging activity were found in acetone and methanol extracts. Therefore, the acetone extract F. amplissima may probably be involved in removing the H2O2. Kumaran and Karunakaran[Citation37] reported that all the extracts of Phyllanthus possess higher hydrogen peroxide radical scavenging activity at a concentration 10 μg/mL. Similarly, in F. amplissima, acetone extract of fruit (62%), methanol (57.2%), and chloroform (56.9%) extract of fruit possesses higher radical scavenging activity at a concentration of 10 μg/mL.
Hydroxyl radical scavenging activity
The hydroxyl radical is an extremely reactive free radical formed in biological systems and has been implicated as a highly damaging species in free radical pathology, capable of damaging almost every molecule found in living cells.[Citation44] This radical has the capacity to join in nucleotides of DNA and cause strand breakage, which contributes to carcinogenesis, mutagenesis, and cytotoxicity. In addition, plant species are considered to be one of the quick initiators of the lipid peroxidation process, abstracting hydrogen atoms from unsaturated fatty acids. The activity is expressed as % hydroxyl radical scavenging.[Citation45]
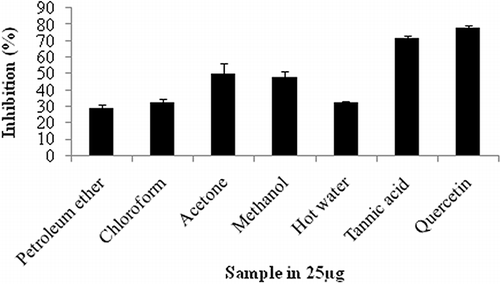
The hydroxyl scavenging activities of all the samples were investigated at the concentration of 250 μg in the reaction mixture (). The acetone extract of the fruit of F. amplissima showed higher scavenging activity (49.9%). Rajesh et al.[Citation35] have also recently reported the ability of F. racemosa to quench hydroxyl radicals, which seems to be directly related to the prevention of propagation of the process of lipid per oxidation and seems to be a good scavenger of active oxygen species, thus reducing the rate of chain reaction.
Chemical and nutritional parameters
The physicochemical parameters of the fruit are given in . Ripe F. amplissima fruits are bluish purple and thin and oblong in shape. The difference might have been due to species differences and varied agro-climatic conditions. The weight of 100 fruit was low and similarly the length, diameter, and specific gravity of the fruit were less, which might be due to its small size and light weight. shows that the moisture, ash, crude protein, crude fat, and crude fibre content are 83.29, 0.82, 1.81, 0.63, and 0.81%. The slight variation in the result might be due to varied agro-climatic conditions, species, and time of maturity. The energy content in fruit is 52.09 kcal/100 g.
Table 2 Physicochemical parameters and organoleptic evaluation of F. amplissima fruit
Table 3 Nutritional parameters of F. amplissima fruit
The organoleptic scores of the fruit are displayed in . The scores were 7, 17, 8, and 63 for colour, texture, taste, and overall acceptability. The scores are in the good range; however, a slightly lower value might be due to small size, multiple seeds, and slightly astringent flavor. Various nutritional parameters of fruit are given in . The pH of the fruit is acidic; however, the TSS of the fruit is 17.28°Brix and acidity is low (1.22%), TSS:acid ratio was 12.85%. NPN (a desirable trait) content (nitrates and nitrites) of the fruit was low and true protein content was also low; this might be due to a lower amount of crude protein present in the fruit. This fruit contains 14.45% total sugar, 8.61% reducing sugars, and 4.33% non-reducing sugar (). However, Kharitonova[Citation46] reported that the total sugars content in Berberis species varies from 1.08–1.17%. Total carbohydrate in the fruit was 11.64%, whereas Goel and Kumar[Citation47] reported the total carbohydrate in B. asiatica DC. as 9.2%, which is slightly lower than the result of the present investigation.
The various dietary fibre constituents analyzed were NDF, ADF, lignin, cellulose, and hemicelluloses and are given in . The higher NDF content might have been due to the higher amount of cell wall constituents, i.e., hemicelluloses, cellulose, heat damaged protein, keratins, and silica. The higher amount of ADF in this fruit might be due to the presence of the high amount of fibrous protein, leading to an increase in ADF content. The higher amount of lignin could be related to constituents of ADF, and the higher amount of cellulose in fruit is also perhaps due to a higher amount of ADF. The lower amount of hemicelluloses is related to less difference between the NDF and ADF values. Hemicellulose is found to be low in this fruit. Vitamins an pigments present in fruit are given in . The ascorbic acid content was 9.83 mg/100 g whereas other workers have reported higher value of ascorbic acid in other species of the genus. Novruzov[Citation48] reported vitamin C content in B. orientalis to be 135 mg/100 g, whereas the vitamin C content in B. asiatica is 46. 24 mg/100 g. The β-carotene and calculated value of vitamin A of fruits are 342.0 μg/100 g and 84.65 μg/100 g, whereas β-carotene is reported to be 453 μg/100 g in B. asiatica.[Citation47] Ficus fruit contained a higher amount of anthocyanin in juice (81.47 mg/100 g). Srivastava and Kumar[Citation49] reported that the higher the content of anthocyanins, the more publishes or bluish would be the colour of the fruit. Further, the pigment is an indication of maturity as when green unripe fruits turn to bluish purple in colour. The high value of anthocyanin in Ficus fruits strongly support the need for its exploitation and increased utilization.
Table 4 Vitamins, pigments, minerals, and anti-nutritional constituents of F. amplissima fruit
The mineral content of the fruit is in appreciable amounts of calcium (25.97 mg/100 g), phosphorus (37.0 mg/100 g), sodium (13.5 mg/100 g), potassium (160.42 mg/100 g), and iron (2.60 mg/100 g) (). The slight variation in the result of the present investigation might be due to the difference in topography of soil and climatic condition coupled with varietal differences. shows the presence of various antinutritional factors present in this fruit. F. amplissima fruits contained 8.8 mg/100 g tannins, which forms complexes with dietary protein and digestive enzymes, thereby reducing the digestibility proteins.[Citation50] Kharitonova, while studying some berberis species, concluded that B. Canadensis Mill. contained 7% tannins[Citation46] whereas Ficus fruit contains a low concentration of phytic acid and phytate phosphorus. The oxalates in F. amplissima fruit are not present.
Table 5 Chemical compounds of acetone extract of F. amplissima fruit
Identification of bioactive compounds
Interpretation of mass spectrum GC-MS was conducted using the database of National Institute Standard and Technique (NIST08) and WILEY08 having more patterns. The spectrum of the unknown component was compared with the spectrum of the known components stored in the NIST08s and WILEY08 library. The name, molecular weight, molecular formula, and structure of the component of the test material were ascertained.
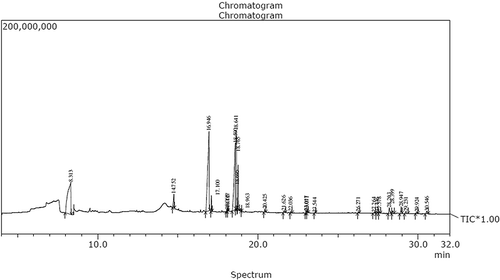
There are several bioactive compounds in plants that are responsible for antioxidant and anti-inflammatory activity. Based upon this, the chemical constituents of F. amplissima fruit was done using GC-MS. The acetone extract of fruit were taken for this study because it shows higher radical scavenging activity. GC-MS chromatogram of acetone extract showed 27 peaks indicating the presence of 27 chemical constituents (). On comparison of the mass spectra of the constituents with the NIST8 and WILEY8 library, 27 chemical constituents were characterized and identified ().
The prevailing compounds were n-Hexadecanoic acid (26.84%), 5-Hydrxoymethylfurfural (22.27%), cis-9,cis-12-Octadecadienoic acid (22.21%), and Octadeca-9,12,15-trien-1-ol (13.22%). n-Hexadecanoic acid (26.84%) has the property of larvicidal effect[Citation51] and lupeol (0.46%) as a therapeutic and chemopreventive agent for the treatment of inflammation and cancer.[Citation52] These results indicate that F. amplissima also contains nonpolar compounds like fatty acids, sterols, and triterpenes. Thus, the compounds present in the active fraction are responsible for the activity of the rind extract.
CONCLUSION
F. amplissima exhibited a strong antioxidant activity in the following assays studied, including ABTS .+ , phosphomolybdenum, FRAP, metal ion chelating, superoxide, hydrogen peroxide, and hydroxyl in activity. The antioxidant activity of F. amplissima might be attributed to effective hydrogen donating ability and metal ion chelating capability. Moreover, the physiochemical, nutritional, and anti-nutritional parameters results were expressed. The F. amplissima fruit may be used as a natural dietary food supplement. Phytochemical analysis showed that F. amplissima also contains non-polar compounds like fatty acids (Myristic acid, n-Hexadecanoic acid), sterols (Ergost-5-en-3-ol, (3.beta.)-, Stigmasterol), and triterpenes (Lupenone, Lupeol). A more detailed investigation is under way to determine the exact phytoconstituents, which are responsible for antioxidant, anti-inflammatory, antimicrobial, and antilarvicidal activity.
REFERENCES
- Peuchant , E. , Brun , J. , Rigalleau , V. , Dubourg , L. , Thomas , M. and Daniel , J. 2004 . Oxidative and antioxidative status in pregnant women with either gestational or type 1 diabetes . Clinical Biochemistry , 37 : 293 – 298 . –
- Steer , P. , Milligard , J. , Sarabi , D.M. , Wessby , B. and Kahan , T. 2002 . Cardiac and vascular structure and function are related to lipid peroxidation and metabolism . Lipids , 37 : 231 – 236 .
- Shahidi , F. , Janitha , P.K. and Wanasundara , P.D. 1992 . Phenolic antioxidants. Critical Reviews in Food Science and Nutrition , 32 : 67 – 103 .
- Gerber , M. , Boutron-Ruault , M.C. , Hercberg , S. , Riboli , E. , Scalbert , A. and Siess , M.H. 2002 . Bull Cancer . Food and cancer: State of the art about the protective effect of fruits and vegetables , 89 : 293 – 312 . –
- Sreejayan , N. and Rao , M. 1996 . Free radical scavenging activity of Curcuminoids . Drug Research , 46 : 169 – 171 .
- Knekt , P. , Jarvinen , R. , Reunanen , A. and Maatela , J. 1996 . Flavonoid intake and coronary mortality in Finland: A cohort study . British Medical Journal , 312 : 478 – 481 .
- Sies , H. 1993 . Strategies of antioxidant defense . European Journal of Biochemistry , 215 : 213 – 219 .
- Grice , H.P. 1988 . Food Chemistry and Toxicology . Enhanced tumour development by butylated hydroxyanisole (BHA) from the prospective of effect on forestomach and oesophageal squamous epithelium , 26 : 717 – 723 .
- Emine , N.H. and Salih , G. 2010 . Total antioxidant capacity and total phenol contents of selected commercial fruit juices in turkey . International Journal of Food Properties , 13 : 1373 – 1379 .
- Rice-Evans , C.A. , Miller , N.J. and Paganga , G. 1996 . Structure-antioxidant activity relationships of flavonoids and phenolic acids . Free Radical Biology and Medicine , 20 : 933 – 956 .
- Wang , H. , Cao , G. and Prior , R.L. 1996 . Total antioxidant capacity of fruits . Journal of Agriculture and Food Chemistry , 44 : 701 – 705 .
- Strazzulo , G. , De Giulio , A. , Tommonaro , G. , La Pastina , C. , Poli , A. , Nicolaus , B. , De Prisco , R. and Saturnino , C. 2007 . Antioxidative activity and lycopene and β-carotene contents in different cultivars of tomato (Lycopersicon esculentum) . International Journal of Food Properties , 10 : 321 – 329 .
- Pulliah , T. 2002 . Biodiversity in India , P. 114 ( 1; ) Vol.Daya Publishing House, Delhi, India
- Singh , M.P. , F.M.A.; and Panda , H. 2005 . Medicinal Herbs with Their Formulations, Vol. 2; Daya Publishing House, Delhi, India Vol. 405 ,
- Mitra , R. and Kapoor , L.D. 1972 . Pharmacognostical studies of Ficus tsiela Roxb. Indian Journal of Pharmacy , 34, 171
- Re , R. , Pellegrini , N. , Proteggente , A. , Pannala , A. , Yang , M. and Rice Evans , C. 1999 . Antioxidant activity applying an improved ABTS radical cation decolourization assay . Free Radical Biology and Medicine , 26 : 1231 – 1237 .
- Prieto , P. , Pineda , M. and Aguilar , M. 1999 . Spectrophotometric quantity of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of Vitamin E . Analytical Biochemistry , 269 : 337 – 341 .
- Pulido , R. , Bravo , L and Sauro-Calixto , F. 2000 . Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay . Journal of Agriculture and Food Chemistry , 48 : 3396 – 3402 .
- Dinis , T.C.P. , Madeira , V.M.C. and Almeida , L.M. 1994 . Action of phenolic derivatives (acetoaminophen, salycilate and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers . Archives of Biochemistry and Biophysics , 315 : 161 – 169 .
- Beauchamp , C. and Fridovich . 1971 . I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels . Analytical Biochemistry , 44 : 276 – 277 .
- Ruch , R.J. , Cheng , S.J. and Klaunig . 1989 . J.E. Prevention of cytotoxicity and inhibition of intracellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis , 10 : 1003 – 1008 .
- Klenin , S.M. , Cohen , G. and Cederbaum , A.I. 1991 . Production of formaldehyde during metabolism of dimethyl sulphxide by hydroxyl radical generating system . Biochemistry , 20 : 6006 – 6012 .
- AOAC . 1970 . Approved Method of Analysis; Association of Official Analytical Chemists: Washington, DC
- Pelletier , D.L. 1994 . The potentiating effects of malnutrition on child mortality: Epidemiologic evidence and policy implications . Natural Review , 52 : 409 – 415 .
- Rangana , S. 1995 . Handbook of Analysis and Quality Control for Fruits and Vegetable Products , 3rd , Tata McGraw-Hill Publishing Company Limited: New Delhi .
- O'Shea; Maguire , M.F. , Determination and of . 1962 . calorific value of food stuff by chromic acid oxidation . Journal of the Science of Food and Agriculture , 13 : 530 – 534 .
- Chen , P.S. , Tosibora , T.Y. and Warner , H. 1956 . Micro determination of phosphorus . Analyst Chemistry , 28 : 1756 – 1759 .
- Roy , S.K. 1973 . A simple and rapid method for estimation of total carotenoid pigment in mango . Journal of Food Science and Technology , 10 : 45 – 46 .
- Van Soest , P.J. and Wine , R.H. 1967 . Use of detergent in the analysis of fibrous foods: Determination of plant cell wall constituents . Journal of AOAC , 50 : 50 – 55 .
- Haugh , W. and Lantzch , H.J. 1983 . Sensitive method for the rapid determination of phytates in cereals and cereal products . Journal of the Science of Food and Agriculture , 34 : 1423 – 1427 .
- Abaza , R.H. , Blake , J.T. and Fisher , E.J. 1968 . Oxalate determination In: analytical problems encountered with certain plant species . Journal of AOAC , 51 : 963 – 964 .
- Gould , W.A. 1978 . “ Quality Assurance; The AVI Publishing Company Inc.: Westport, Connecticut ” . In Food
- Awika , J.M. , Rooney , L.W. , Wu , X.L. , Prior , R.L. and Cisneroszevallos , J. 2003 . Screening methods to measure antioxidant activity of Sorghum (Sorghum bicolor) and Sorghum products . Journal of Agriculture and Food Chemistry , 51 : 6657 – 6662 .
- Hagerman , A.E. , Riedl , K.M. , Jones , G.A. , Sovik , K.N. , Ritchard , N.T. , Hartzfeld , P.W. and Riechel , T.L. 1998 . High molecular weight plant polyphenolics (tannins) as biological antioxidants . Journal of Agriculture and Food Chemistry , 40 : 801 – 805 .
- Rajesh , M. , Anusuya , N. , Siddhuraj , P. and Manian , S. 2008 . The antioxidant activity and free radical scavenging potential of two different solvent extracts of Cammelia sinensis (L.) O. Kuntz, F. bengalensis L. and F. racemosa L. Food Chemistry , 107 : 1000 – 1007 .
- Loo , A.Y. , Jain , K. and Darah , I. 2008 . Antioxidant activity of compounds isolated from the pyroligneous acid, Rhizophora apiculata . Food Chemistry , 107 : 1151 – 1160 .
- Kumaran , A.R. and Karunakaran , J. 2007 . In vitro antioxidant activities of methanol extract of five Phyllanthus . species from India. Food Science and Technology , 40 : 344 – 352 .
- Chang , L.W. , Yen , W.J. , Huang , S.C. and Duh , P.D. 2002 . Antioxidant activity of sesame coat . Food Chemistry , 78 : 347 – 354 .
- Dorman , H.J.D. , Kosar , M. , Kahlos , K. , Holm , Y. and Hilturien , R. 2003 . Antioxidant properties and composition of aqueous extracts from Mentha species, hybrids, varieties and cultivars . Journal of Agriculture and Food Chemistry , 51 : 4563 – 4569 .
- Halliwell , B. and Gutteridge . 1985 . J.M.C. Free radical, aging and disease. Free Radicals in Biology and Medicine , 2nd Ed 279 – 315 . Clarendon Press, Oxford
- Halliwell , B. 1991 . Reactive oxygen species in living system: Source, biochemistry, and role in human disease . American Journal of Medicine , 91 : 14 – 22 .
- Andreia , P. , Oliveira , Luis, R , Guedes , de Pinho, S.P. , Gil-Izquierdo , A , Valentao , P , Branca, , M , Silva , J.A , Pereira , P and Andrade , B . 2010 . Volatile profiling of Ficus carica varieties by HS-SPME and GC–IT-MS . Food Chemistry , 123 : 548 – 557 .
- Nakayama , T. 1994 . Suppression of active hydroperoxide-induced cytotoxicity by polyphenols . Cancer Research , 54 1991s–1993s
- Hochestein , P. and Atallah , A.S. 1988 . The nature of oxidant and antioxidant systems in the inhibition of mutation and cancer . Mutation Research , 202 : 363 – 375 .
- Babu , B.H. , Shylesh , B.S. and Padikkala , J. 2001 . Antioxidant and hepatoproductive effects of Alanthus icicifocus , 72 : 272 – 277 . Fitoterapia
- Kharitonova , L.A. 1986 . Biochemical characteristics of fruits of some Berberis species introduced in the Kaliningrad region . Rastitel'nye Resursy , 22 : 510 – 513 .
- Goel , B. and Kumar , A. 1989 . Composition of uncommon foods . Journal of Food Science and Technology , 26 : 44 – 45 .
- Novruzov , E.N. 1988 . Chemical composition of fruits and berries of plants growing wild in Azerbayan . Rastitel'ney Resursy , 24 : 48 – 51 .
- Srivastava , R.P. and Kumar , S. 2003 . “ Fruit and Vegetable Preservation—Principles and Practices ” . In International Book Distributing Company: Lucknow, India
- Akapanyung , E.Q. , Udoh , A.P. and Eteng , M.U. 2001 . Anti-nutrient profile and chemical composition of custard powder produced in Nigeria. Journal of Food Science and Technology , 38 : 120 – 123 .
- Falodun , A. , Siraj , R. and Choudhary , M.I. 2009 . GC–MS analysis of insecticidal leaf essential oil of Pyrenacantha staudtii Hutch and Dalz (Icacinaceae) . Tropical Journal of Pharmacolgical Research , 8 : 139 – 143 .
- Saleem , M. Lupeol . 2009 . a novel anti-inflammatory and anti-cancer dietary triterpene . Cancer Letters , 285 : 109 – 115 .