Abstract
The qualitative and quantitative analysis of phenolic compounds of the methanol extract of Tunisian thornless form Opuntia ficus indica flowers was described. Reversed phase high-performance liquid chromatography UV photodiode array analysis detected four peaks at 320 nm, attributed to phenolic acids; three of them were quantified to 141 mg.kg−1 of fresh plant material. Electrospray ionization mass spectrometer analysis detected 8 glycosylated flavonols at 350 nm, identified among 11 chromatographic peaks. The amount of flavonoids was 4980 mg.kg−1 of fresh plant material, with isorhamnetin glycosylated derivatives as the main flavonoid components (55.1%), quercetin derivatives as the second (23.7%), and kaempferol derivatives as the third (8.4%).
INTRODUCTION
Cactus pears are produced by a perennial cactus (i.e., Opuntia ficus indica) associated with the semiarid zones of the world. This cactus is widely distributed in Mexico and in all American hemispheres and grows in many other parts of the world, such as Africa, Australia, and the Mediterranean basin. It is one of the few crops that can be cultivated in areas that offer very little growth possibility for common fruits and vegetables.[Citation1] Cactus pears were largely ignored by the scientific world until the beginning of 1980. These years corresponded to an increase of research works and symposia resulting in a large number of publications. This increasing interest motivated the investigation on the chemical content of the flowers and other organs of the plant. Renewed interest is to be ascribed in part to the multi-functionality of cactus fruits, pads, and flowers. The great number of potentially active nutrients and their multifunctional properties make cactus pears perfect candidates for the production of health promoting food and food supplements. Both cactus fruit and cladode yield high values of important nutrients such as: minerals and vitamins, as well as further antioxidants.[Citation2 − Citation5] The main studies on Opuntia fruits were on the chemical analysis of pulp, skin, and seeds;[Citation6] other authors have studied the nutritional significance of both despined cladodes and fruit pulp from Opuntia spp.[Citation7] Several studies have reported the efficiency of fruits and cladodes in the treatment of several diseases. For example, cactus pear fruit and stem extract exhibited anti-tumoral,[Citation8] anti-viral,[Citation9] anti-inflammatory,[Citation10] and antioxidant effects.[Citation11,Citation12] The use of prickly pear fruits is recommended for their beneficial and therapeutic properties.[Citation13] Literature data report that cactus pears and the high fiber content of the cladodes mucilage and pectin are also used in folk medicine, such as emollient, moisturizing, cicatrizing, hypocholesterolemic, hypoglycemic agent, and also as effective in gastric mucosa diseases.[Citation14 − Citation19] In Sicilian folk medicine, cactus flower infusion is considered as depurative and particularly used for its diuretic and relaxant action on the renal excretory tract.[Citation20,Citation21] Therefore, it is stipulated that cactus flower infusion may help the expulsion of renal calculus. Galati et al.[Citation22] investigated the diuretic effect of a 15% extract from: flowers, fruits, and peeled cladodes from O. ficus indica. The presence of phenolics has been detected in cactus pulp fruits.[Citation5,Citation23,Citation24] Kuti[Citation25] reported an antioxidative effect due to the major flavonoids encountered in cactus fruits (quercetin, kaempferol, and isorhamnetin). The effects of solvent extracts from natural sources of antioxidants on appropriate cell lines revealed that phenolic compounds were able to delay pro-oxydative effects on proteins, DNA, and lipids while producing stable radicals.[Citation26] Furthermore, O. ficus indica polyphenolic compounds were shown to induce a hyperpolarization of the plasma, and to raise the intracellular pool of calcium in human Jurkat T-cell lines.[Citation27] Flavonol derivatives detected in Opuntia spp. have been recently compiled.[Citation4] When fruits are investigated, it must be taken into account that higher phenolic contents are expected in the peel, rather than the pulp. Compared to fruits and cladodes of O. ficus indica, studies that focus on the phenolic compounds in flowers remained scarce in literature. Some authors explained the anti-diuretic effect of flowers to secondary metabolites including phenolic compounds. Previous studies on the plant flowers reported the isolation of β-sitosterol, fatty acids, some of their esters from a polar extract,[Citation20] and unidentified isorhamnetin glycoside.[Citation28] On the same topic, recent studies on Egyptian cactus flowers enable isolation and identification of some secondary metabolites, such as penduletin, kaempferol, luteolin, quercetin, and rutin.[Citation29] In the methanol extract from O. ficus indica flowers collected in Monasterace (Italy), the isorhamnetin glycosylated was the major component (73%), and it was followed by quercetin derivatives (14%) and quercetin 3-O-rutinoside (8.8%).[Citation30] In this context, the aim of the present study was to provide new findings about the phenolic metabolites of the methanol extract of the flowers of the Tunisian thornless form of O. ficus indica. Identifications and quantifications were achieved thanks to chromatographic, UV-visible, and mass spectrometry data.
MATERIALS AND METHODS
Plant Material
The thornless form of O. ficus indica is from local pilot cultivar of Bou Argoub region (Nabeul-Cap Bon Region, northeast of Tunisia). The region of Bou Argoub is located at 36° 32′ N latitude, 10° 33′ E longitude, and at an altitude of 62 m. Flowers have been collected during the flowering period in the month of June 2008.
Solvents and Standard Phenolics
Methanol (CH4O), acetonitrile (CH3CN), formic acid (CH2O2), and acetic acid (CH3COOH) requested for chromatographic analysis were purchased from Merck (Darmstadt, Germany). Water was purified on a MilliQ system (Millipore S.A., Molsheim, France). The standards used in this work provided by Extrasynthèse S.A. (Lyon, France) and consisted of the following: rutin (quercetin 3-O-rutinoside), myricitrin (myricetin 3-O-rhamnoside), hyperoside (quercetin 3-O-galactoside), kaempferol 3-O-rutinoside, isorhamnetin 3-O-rutinoside, kaempferide (4′-methylkaempferol), rhamnetin (7-methylquercetin), isorhamnetin (3-methylquercetin), quercitrin (quercetin 3-O-rhamnoside), and myricetin.
Direct Solvent Extraction of Polyphenols
Flowers including all parts (petals, sepals, and reproductive organs) were first dried in the dark at room temperature for 48 h, freeze-dried, and reduced into flower powders. Weighted aliquots (30 mg) were placed in eppendrof vials, and 1% methanolic acid acetic (1200 μl) was added. Vials were closed and incubated at room temperature in an ultrasonic water bath for 15 min. The mixture was immediately filtered through a polytetrafluoroethylene (PTFE) membrane (0.45 μm) into insert vials. The samples were ready for analysis by reverse phase-high-performance liquid chromatography (RP-HPLC) coupled to UV-visible detection. The same method was adopted for the preparation of the samples injected on HPLC system equipped with an electrospray ionization mass spectrometer (HPLC-ESI-MS) with only 10 mg of plant material, the weight reduction was recommended to avoid concentrating the solution. We note that the second HPLC system was also equipped with a photodiode array (PDA) detector. RP-HPLC-UV-PDA is used for quantitative investigation and ESI-MS for qualitative analysis.
RP-HPLC-UV-Visible Analysis
The RP-HPLC-UV-visible analyses were performed using a Waters Alliance 2690 HPLC system (Waters, Milford, MA, USA), which included a quaternary gradient pumping system, a thermostated column oven, and a thermostated automatic injection module. The system was coupled to a PDA 996 detector used in the 240–600 nm range (Waters, Milford, MA, USA). The column was a reversed phase column (Merck, Purospher TM C18, 250 × 4 mm, 60 Ǻ, 5 μm) coupled to precolumn containing the same phase (Merck, Purospher TM C18, 4 × 10 mm). The injected volume was 10 μl, and the column was thermostated at 30°C. The elution solvent was a mixture of solvent A consisting of ultrapure water/glacial acetic acid (97.5:2.5, v/v) and solvent B consisting of pure acetonitrile. Helium was used for degassing solvents. The following gradient was applied: initial 3% B, 0–5 min, 9% B linear; 5–15 min, 16% B linear; and 15–45 min, 50% B linear; the gradient was followed by washing and reconditioning of the column. Three particular wavelengths were used for quantification of polyphenols: 280 nm for flavan-3-ols and dihydrochalcones, 320 nm for hydroxycinnamic acids, and 350 nm for flavonols. Acquisition and processing of the data were achieved using MillenniumCitation32 chemometric software (version 3.20). Quantitative determination was carried out using calibration curves of standards. Hyperoside (quercetin 3-O-galactoside) and chlorogenic acid were chosen as external standards for quantification of flavanols and hydroxycinnamic acids, respectively. HPLC peaks were identified on chromatograms according to their retention times (RTs) and their UV-V is spectra by comparison with available standard compounds. Quantification of the identified compounds was performed by correlating the measured peak area to the calibration curves obtained with reference compounds. Integration of HPLC peaks was performed at 350 nm for flavonols, using the extinction coefficient of hyperoside, and at 320 nm for phenolic acids, using the extinction coefficient of chlorogenic acid.
ESI-MS Analysis
The ESI-MS system consisted of a LCQ DECA ion trap mass spectrometer (Thermofinnigan, San Jose, CA, USA) equipped with an ESI source and run by Xcalibur (version 1.2) software. ESI-MS analysis were performed in the negative mode using the following parameters for the ESI source: ion spray voltage, 3,69 kV; capillary voltage, –70.78 V; capillary temperature, 240.4°C; sheath nitrogen gas flow rate, 66.65 arbitrary units; auxiliary gas flow rate, 3.81 arbitrary units; scan range of m/z 50–2000. Samples corresponding to collected HPLC peaks were directly introduced into the ESI source by a built-in the syringe pump at the flow rate of 3 μl.mn−1. For the generation of MSn data, the precursor ions were fragmented by helium gas collision in the ion trap by optimizing the collision energy in order to obtain the intensity of the precursor ion close to 10% of the relative scale spectrum.
RESULTS AND DISCUSSION
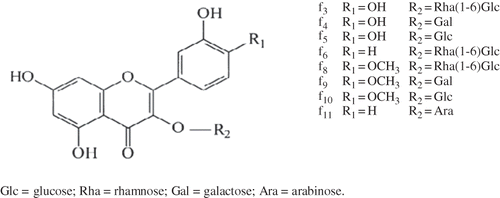
The purified methanol extract of O. ficus indica of thornless form flowers was analyzed by means of RP-HPLC coupled with UV-visible diode array detection and ESI-MS. To understanding the phenolic composition of grape vine leaves, chromatographic assays were applied by the HPLC-RP-PDA method to create a data base of the principal polyphenolic compounds.[Citation31] Two classes of phenolic compounds (i.e., phenolic acids and flavonols) were detected according to UV chromatograms at 320 nm () and 350 nm (). A total of four peaks numbered from a1 to a4 () and eleven peaks numbered from f1 to f11 () were considered for identification and quantification. Peaks numbered a1 to a4 showed typical UV spectra with maximum absorbance at the 320–330-nm region in accordance with hydroxycinnamic acid structures (), whereas peaks numbered f1 to f11 showed absorbance in the 255–263- and 350–355-nm regions, in accordance to flavonol structures (). De Leo et al.[Citation30] analyzed the methanolic extracts of O. ficus indica grown in Italy, the resulting chromatogram, showed nine peaks, with only seven identified as flavonol glycosylated derivatives. The results of this work provided further information on phenolic acids (four peaks: a1–a2) and revealed more peaks in flavonol glycoside region chromatogram (eleven peaks: f1–f11). The RP-HPLC-UV-PDA results displayed the presence of the secondary metabolites in O. ficus indica flowers belonging to the flavonol glycoside class indicated by absorbance at 255–263 nm and 350–355 nm were in accordance to Wollenweber's work.[Citation32] The chromatograms at 350 nm revealed eight peaks (f3, f4, f5, f6, f8, f9, f10, and f11) corresponding to flavanols available as standard compounds. Peaks were identified by comparison of their HPLC RTs, UV-visible spectrum with authentic standards compounds, and confirmation of their identity was given by their
Table 1 RT, spectral UV, and amount compounds (a1–a4) data from flowers' methanol extract of Tunisian O. ficus indica thornless form
Figure 1 UV spectra of phenolic acids (a) and flavonols (b), RP-HPLC of phenolic acids at 320 nm (c) and flavonols at 350 nm (d) of Tunisian thornless form of O. ficus indica flowers methanol extract. (Color figure available online.)
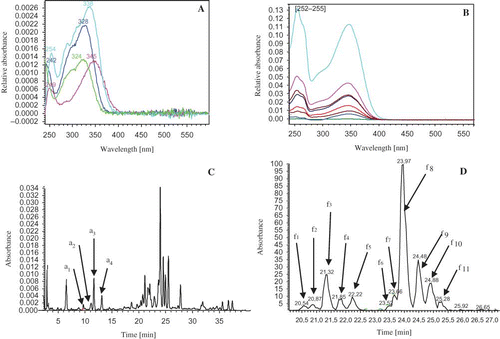
Table 2 RT, spectral UV, characteristic ions (MS and MS2), and amount compounds (f1–f11) data from flowers' methanol extract of Tunisian O. ficus indica thornless form
CONCLUSION
In this study, results indicate the presence of interesting phenolic compounds in the composition of Tunisian thornless form of O. ficus indica flowers. These components were characterized via chromatographic and spectral analysis by RP-HPLC-UV-PDA and ESI-MS. The main flowers polyphenols were flavonols—more precisely, isorhamnetin derivatives, quercetin derivatives, and kaempferol derivatives, as well as the presence of compounds from the phenolic acids family. These finding make flowers a promising source of biologically active polyphenolic mixtures. These investigations have to be completed by identification of these unknown peaks and compounds for which structures were not elucidated. Otherwise, polyphenols concentrations in Tunisian thornless form of O. ficus indica flowers could contribute to antioxidant status and would be interesting for further exploitation.
REFERENCES
- Han , H. and Felker , P. 1997 . Field validation of water-use efficiency of a CAM plant Opuntia ellisiana in south Texas . Journal of Arid Environments , 36 : 133 – 148 .
- Ramadan , F.M. and Mörsel , J.T. 2003 . Oil cactus pear (Opuntia ficus indica L.) . Food chemistry , 82 : 339 – 345 .
- Stintzing , F.C. , Herbach , K.M. , Mosshammer , M.R. , Carle , R. , Yi , W.G. , Sellappan , S. , Akoh , R. , Bunch , C.C. and Color , Felke, P. 2005 . betalain pattern, and antioxidant properties of cactus pear (Opuntia spp. ) clones . Journal of Agricultural and Food Chemistry , 53 : 442 – 451 .
- Stintzing , F.C. and Carle . 2005 . R. Cactus stems (Opuntia spp.) . A review on their chemistry, technology, and uses. Molecular Nutrition & Food Research , 49 : 175 – 194 .
- Tesoriere , L. , Fazzari , M. , Allegra , M. and Livrea , M.A. 2005 . Biothiols, taurine, and lipid-soluble antioxidants in the edible pulp of Sicilian cactus pear (Opuntia ficus-indica) fruits and changes of bioactive juice components upon industrial processing . Journal of Agricultural and Food Chemistry , 53 : 7851 – 7855 .
- El-Kossori , R.L. , Villaume , C. , El-Boustani , E. , Sauvaire , Y. and Mejean , L. 1998 . Composition of pulp, skin and seeds of prickly pears fruit (Opuntia ficus-indica sp.) . Plant Foods for Human Nutrition , 52 ( 3 ) : 263 – 270 .
- Stintzing , F.C. , Schieber , A. and Carle , R. 2001 . Phytochemical and nutritional significance of cactus pear . European Food Research Technology , 212 : 396 – 407 .
- Zou , D.M. , Brewer , M. , Garcia , F. , Feugang , J.M. , Wang , J. , Zang , R. , Liu , H. and Zou , C. 2005 . Cactus pear: a natural product in cancer chemoprevention . Nutrition Journal , 4 : 25 – 36 .
- Ahmad , A. , Davies , J. , Randall , S. and Skinner , G.R.B. 1996 . Antiviral properties of extract of Opuntia streptacantha . Antiviral Research , 30 : 75 – 85 .
- Park , E.H. , Kahng , J.H. , Lee , S.H. and Shin , K.H. 2001 . An anti-inflammatory principle from cactus . Fitoterapia , 72 : 288 – 290 .
- Tesoriere , L. , Butera , D. , Pintaudi , M. , Allegra , M. and Livrea , M.A. 2004 . Supplementation with cactus pear (O. ficus indica) fruit decreases oxidative stress in healthy humans: A comparative study with Vit. C . American Journal of Clinical Nutrition , 80 : 391 – 395 .
- Gentile , C. , Tesoriere , L. , Allegra , M. , Livrea , M.A. and D'Alessio , P. 2004 . Antioxidant betalains from catus pear (Opuntia ficus-indica) inhibit endothelial ICAM-1 expression . Annals of the New York Academy of Sciences , 1028 : 481 – 486 .
- Barbera , G. and Inglese , P. 1993 . “ La coltura del ficodindia ” . In Edagricole-Edizioni Agricole della Calderini s.r.l. , 174 – 176 . Bologna .
- Cruse , R.R. 1973 . Desert plant chemurgy: A current review . Economic Botany , 27 : 210 – 230 .
- Camacho-Ibanez , R. , Meckes-Lozoya , M. and Mellado-Campos , V. 1983 . The hypoglycemic effect of Opuntia streptacantha studied in different animal experimental models . Journal of Ethnopharmacol , 7 : 175 – 181 .
- Frati-Munari , A. , Jiménez , E. and Ariza , C.R. 1990 . Hypoglycemic effect of Opuntia ficus indica in non insulin-dependent diabetes mellitus patients . Phytotherapy Research , 40 ( 5 ) : 195 – 197 .
- Pimienta , B.E. 1990 . El Nopal Tunero , 1st , 246 Jalisco , Mexico : Universidad de Guadalajara .
- Fernandez , L.M. , Lin , E.C.K. , Trejo , A. and McNamara , D.J. 1994 . Prickly pear (Opuntia sp.) . pectin alters hepatic cholesterol metabolism without affecting cholesterol absorption in guinea pigs fed a hypercholesterolemic diet. Journal of Nutrition , 124 : 817 – 824 .
- Rosado , J.L. and Diaz , M. 1995 . Physicochemical properties related to gastrointestinal effects of six dietary fibers . Revista de Investigacion Clinica , 47 : 283 – 289 .
- Arcoleo , A. , Ruccia , M. and Natoli , M.C. 1966 . β-Sitosterol from flowers of Opuntia ficus-indica (Cactaceae) . Atti Accademici Scientifici. Lettere Arti Palermo , 25 : 323 – 332 .
- Sisini , A. 1969 . Sulla glucoso-6-fosfato isomerasi in Opuntia ficus indica . Bollettino Società Italiana di Biologica Sperimentale , 45 : 794 – 796 .
- Galati , E.M. , Tripodo , M.M. , Trovato , A. , Miceli , N. and Monforte , M.T. 2002 . Biological effect of Opuntia ficus indica (L.) . Mill. (Cactaceae) wast matter. Journal of Ethnopharmacology , 79 : 17 – 21 .
- Butera , D. , Tesoriere , L. , Di Gaudio , F. , Bongiorno , A. , Allegra , M. , Pintaudi , A.M. , Kohen , R. and Livrea , M.A. 2002 . Antioxidant activities of Sicilian prickly pear (Opuntia ficus indica) fruit extracts and reducing properties of its betalains: Betanin and indicaxanthin . Journal of Agricultural and Food Chemistry , 50 : 6895 – 6901 .
- Kuti , J.O. 2004 . Antioxidant compounds from four Opuntia cactus pear fruit varieties . Food Chemistry , 85 : 527 – 533 .
- Kuti , J.O. 1992 . Growth and compositional changes during the development of prickly pear fruit . Journal of Horticulture Science , 67 : 861 – 868 .
- Shahidi , F. , Janitha , P.K. and Wanasundara , P.D. 1992 . Phenolic antioxidanta, critical previous term reviews next term . Food Science Nutrition , 32 : 67 – 103 .
- Aires , V. , Adote , S. , Hichami , A. , Moutairou , K. , Boustani , E.S.E. and Khan , N.A. 2004 . Modulation of intracellular calcium concentrations and T cell activation by prickly pear polyphenols . Molecular and Cellular Biochemistry , 260 : 103 – 110 .
- Arcoleo , A. , Billino , A. and Ruccia , M. 1962 . Flavonoid pigments from family Opuntiae . The structure of a flavonoid glucoside from Opuntia ficus indica. Atti Accademici Scientifici, Lettere Arti Palermo , 22 : 115 – 118 .
- El-Moghazy , A.M. , El-Sayyad , S.M. , Abdel-Baky , A.M. and Bechait , E.Y. 1984 . A phytochemical study of Opuntia ficus-indica (L.) . Mill cultivated in Egypt. Egyptian Journal of Pharmaceutical Sciences , 23 : 247 – 254 .
- De Leo , M. , Bruzual , De Abreu , Pawlowska , M. , Cioni , A.M. and Barca , P.L. 2010 . A. Profiling the chemical content of Opuntia ficus-indica flowers by HPLC-PDA-ESI-MS and GC/EIMS analyses . Phytochemistry Letters , 3 : 48 – 52 .
- Katalinic , V. , Mozina , S.S. , Generalic , I. , Skroza , D. , Ljubenkov , I , Phenolic profile , Klancnik, A. and antioxidant , capacity . and antimicrobial activity of leaf extracts from six Vitis vinifera L. varieties , International Journal of Food Properties 2013, 16 (1), 45–60 .
- Wollenweber . 1982 . “ E. Flavones and flavonols ” . In The Flavonoids: Advances in Researches , Edited by: Harborne , J.B. and Mabry , T.J . 240 – 242 . London : Chapman and Hall .
- Kandaswami , C. and Middleton , E. 1994 . “ The impact of plant flavonoids on mammalian biology ” . In The Flavonoids Advances in Research Since 1986; , Edited by: Harborne , J.B. 619 – 652 . London : Chapman and Hall .
- Hadj Salem , J. 2009 . Extraction, identification, caractérisation des activités biologiques de flavonoïdes de Nitraria retusa et synthèse de dérivés acyles de ces molécules par voie enzymatique , 190 Nancy , France : Thèse de l'institut national polytechnique de lorraine .
- Halim , A.F. , Saad , H.E. and Hashish , N.E. 1995 . Flavonol glycosides from Nitraria retusa, Egypt . Phytochemistry , 40 : 349 – 351 .
- Arcoleo , A. , Ruccia , M. and Cusmando , S. 1961 . Flavonoid pigments from Opuntia . I. Isorhamnetin from flowers Opuntia ficus indica. Annal Chimica , 51 : 81
- Burret , F. , Lebreton , P. and Voirin , B. 1982 . Les aglycones flavoniques des Cactées: Distribution, signification . Journal Natural Products , 45 : 687 – 693 .
- Meyer , B.N. and McLaughlin , J.L. A note on the phytochemistry of Opuntia (Cactaceae) . Cactus & Succulent Journal (USA) 1982 , 54 226 – 228 .
- Miller , J.M. and Bohm , B.A. 1982 . Flavonol and dihydroflavonol glycosides of Echinocerus triglochidatus var . gurneyi. Phytochemistry , 21 : 951 – 952 .
- Rosler , H. , Rosler , U. , Mabry , T.J. and Kagan , J. 1966 . The flavonoid pigments of Opuntia lindheimeri . Phytochemistry , 5 : 189 – 192 .
- Clark , W.D. and Parfitt , B.D. 1980 . Flower flavonoids of Opuntia series Opuntiae . Phytochemistry , 19 : 1856 – 1857 .
- Shabbir , M. and Zaman , A. 1968 . Chemical investigation of the flowers of Opuntia elatior . Journal of Indian Chemical Society , 45 : 81
- Yang , S.K. , Yoneda , F. , Oide , H. , M , Sakae and Isorhamnetin . 1998 . 3-O-robinobioside, its manufacture and testosterone 5 α-reductase inhibitors and therapeutics containing the flavonoid derivative. Japanese Kokai Tokkyo Koho , 7 pp