Abstract
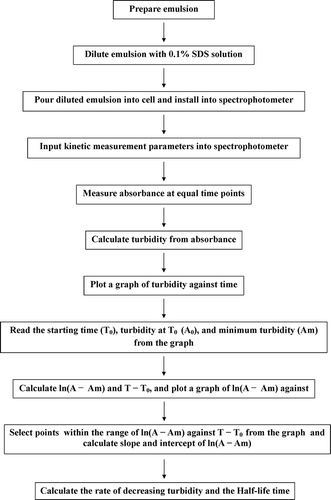
A novel pragmatic turbidimetric data analysis method for evaluating emulsion stability by using a spectrophotometer is proposed. An overview of this method is shown in a flowchart. The advantage of this method is that it uses change in turbidity against time, which allows it to be applied to all emulsions and food scientists to easily calculate half-life time. A high half-life time shows that turbidity is decreased slowly and that the emulsion state can be kept for a long time. Due to this simplicity, it is hoped that this will become a commonly used method.
INTRODUCTION
Information on the stability of emulsions, for example, how long the emulsion state can be kept, is key information for the quality control of the food emulsion products used in manufacturing and the development of new food emulsifiers. In food manufacturing, checking the stability of emulsions is routine work; in the development of new food emulsion products and new emulsifiers, developers begin by calculating the stabilities of their experimental products and emulsions prepared with their experimental emulsifiers. Economically simple techniques for evaluating the stability of emulsions—that is, methods that are cheap, quick, and accurate—are therefore in great demand.
Currently, the main methods for evaluating the stability of emulsions are visual inspection at constant time intervals,[Citation1 − Citation15] microscopic observation,[Citation5] the use of particle size analyzers to measure the size of the oil droplets in the emulsion,[Citation16 − Citation22] and devices to measure the absorption of light (e.g., spectrophotometer, turbidimeter, etc.).[Citation23 − Citation26]
Visual inspection at constant time intervals is a test to check whether the creaming layer of the emulsion remains or separates into two phases of oil and water. This is currently the standard method for evaluating emulsion stability and is cheap and simple; however, it is a qualitative, not a quantitative, analysis.[Citation1 − Citation15] Microscopic inspection and particle size analyzers to measure the size of the oil droplets in emulsions can provide a quantitative analysis. However, changes in particle size do not represent the stability of the emulsion because stability depends on a number of complex factors; rather, they represent the state of the emulsion. Moreover, particle size analyzers are unfit for installation within the flow of the manufacturing process because some time to determine the sizes of the oil droplets takes and the sizes of the oil droplets cannot be obtained immediately.
Devices to measure the absorption of light can be easily installed within the flow of the manufacturing process; moreover, spectrophotometers are already commonly available in research laboratories. Architects of manufacturing flow processes and developers of emulsifiers have, therefore, attempted to quantitatively evaluate emulsion stability by means of devices that measure absorption of light. Various indexes (emulsion activity index [EAI], emulsion stability [ES], stability index [SI; or turbidity ratio]) calculated from the absorption of light have been suggested.[Citation27−Citation37] The EAI is expressed as units of area of interface stabilized per unit weight of emulsifier (EAI = [2 × T] ÷ [φ × C], where T, φ, and C are turbidity calculated from absorption of light at 500 nm, volume fraction of the dispersed phase, and weight of emulsifier per unit volume of aqueous phase, respectively). For ES, as the value decreases, emulsion stability increases (ES = {[EAI(max) − EAI(at temperature)] ÷ EAI(max)} × 100). These two indexes are the most frequently used in food science.[Citation27 − Citation37] The stability index (SI = [absorption at 850 nm] ÷ [absorption at 450 nm]) has been suggested based on practical experience in cosmetics and toiletries science.[Citation30,Citation31]
These three indexes do not measure change in turbidity over time; however, since the state of emulsions changes over time, so turbidity must also change. We, therefore, propose that there is a need for a turbidimetric data analysis method for evaluating the stability of emulsions that uses change in turbidity over time. In this article, we present a novel, pragmatic, yet simple method for analyzing change in turbidity against time for evaluating the stability of emulsions.
MATERIALS AND METHODS
Preparation for Emulsification
Materials
Materials for buffer solutions
Disodium hydrogen phosphate (Na2HPO4•12H2O) and sodium dihydrogen phosphate (NaH2PO4•2H2O) were obtained from Wako Pure Chemical Industries (Osaka, Japan).
Oils
Corn oil, peanut oil, and soybean oil were obtained from Nacalai Tesque (Kyoto, Japan), and safflower oil was obtained from Sigma-Aldrich Japan Co. (Tokyo, Japan).
Emulsifiers
Bovine serum albumin (BSA) and casein sodium (Casein) were obtained from Wako Pure Chemical Industries, and sodium dodecyl sulfate (SDS) was obtained from Nacalai Tesque.
Stock solutions
Stock protein-emulsifier solutions
Stock BSA solution (pH 8.0, ionic strength 0.1) was prepared by dissolving BSA (1.5 g), Na2HPO4•12H2O (3.513 g), and NaH2PO4•2H2O (0.086 g) in 300 mL of water. Stock Casein solution (pH 8.0, ionic strength 0.1) was prepared by dissolving Casein (1.5 g), Na2HPO4•12H2O (3.513 g), and NaH2PO4•2H2O (0.086 g) in 300 mL of water. Stock 0.1% SDS solution was prepared by dissolving SDS (0.15 g) in 150 mL of water.
Emulsification and Dilution of Emulsions with Stock 0.1% SDS Solution
Pre-emulsion solutions were prepared by pipetting the oil and stock protein-emulsifier solution into a jar according to the compositions shown in . Air dissolved in the pre-emulsifying solutions was removed by aspiration for 20 min. The pre-emulsion solutions were emulsified by using a homogenizer (Ultra-disperser LK-21; Yamato Scientific Co. Ltd., Tokyo, Japan) for 5 min at 293 K. In accordance with the frequently used method of Pearce and Kinsella,[Citation33] 75 μL of each emulsion was gently dissolved into 15 mL 0.1% SDS solution to make a diluted emulsion capable of being analyzed with a spectrophotometer.
Table 1 Emulsion compositions
Measurement
Each diluted emulsion was gently poured into a 1-cm-path crystal cell. The cell was installed into a spectrophotometer (Shimadzu UV-1600; Shimadzu, Kyoto, Japan). Absorbance was measured at a wavelength of 500 nm at 30-s intervals for 1800 s. Absorbance at each interval was automatically recorded on a computer. Turbidity was then calculated from each absorbance value using the following equation:[27−37]
A graph of turbidity against time was then plotted.
RESULTS AND DISCUSSION
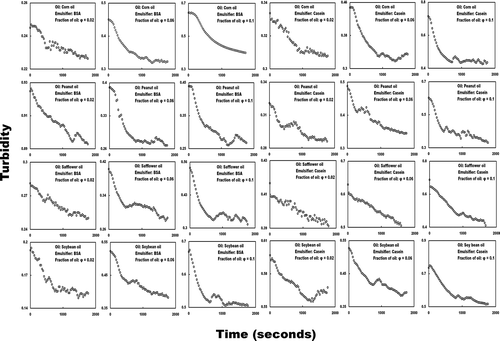
Light basically passes through an emulsion. However, if the light hits oil droplets at a high frequency and scatters, the emulsion can be considered to have a high turbidity. In contrast, if the light hits oil droplets at a low frequency, the emulsion can be considered to have a low turbidity. Thus, turbidity is related to the size and number of oil droplets present in the emulsion. The change in turbidity over time relates to the change in the state of the emulsion over time. Graphs of turbidity against time for all tested diluted emulsions are shown in . All of the graphs exhibit gradually decreasing curves for turbidity against time. According to the author's experience, this pattern approximates that of the curve of the following equation for a first-order reaction:[Citation38]
Figure 2 (a) Method for reading T 0 and A 0 from the graph. (b) Graph of turbidity against time for a diluted corn oil–BSA emulsion (Corn oil fraction, 0.1; emulsifier, BSA). The red line is a curve fitted by using Eq. (2) for first-order reactions.
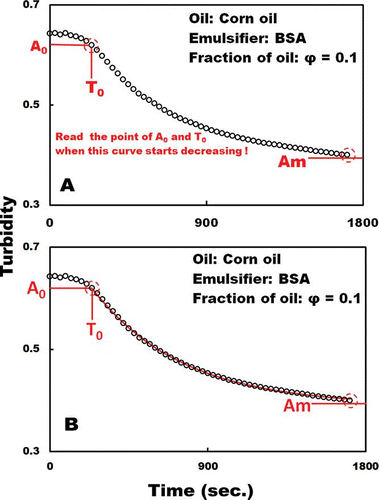
Table 2 Fraction of oil and turbidity decreasing parameters for the emulsions emulsified with BSA and coefficient of determination (R2) of the least square-fit calculation
Table 3 Fraction of oil and turbidity decreasing parameters for the emulsions emulsified with Casein and coefficient of determination (R2) of the least square-fit calculation
A high HLT means that turbidity is slowly decreasing and that the emulsion state can be kept for a long time. Because the graph of turbidity against time for the diluted corn oil–BSA emulsion (fraction of corn oil, 0.1; emulsifier, BSA) was a clear, gradually decreasing curve, the author attempted to fit Eq. (2) to the data by using a least square-fit calculation by means of a program that was custom-made by using Visual Basic 6.0 programming language.[Citation37 − Citation42] EquationEquation (2) provided the best fit for the data. The RDT, HLT, and coefficient of determination (R 2) of the least square-fit calculation were 1.96 ms−1, 354 s, and 0.98, respectively. The result, therefore, indicates that turbidity against time is a gradually decreasing curve according to the law of first-order reactions (). However, almost all of the graphs of turbidity against time did not exhibit a clear gradually decreasing curve; instead they exhibited an s-shaped decreasing curve () despite the author repeating all measurements. The author attempted to fit Eq. (2) to all tested data; however, it did not provide the best fit for the data over the whole range (results not shown). Almost all of the curves of turbidity against time did not exhibit a clear gradually decreasing curve according to the law of first-order reactions.
A further consideration is whether or not most food scientists are able to calculate the direct least squares fit from Eq. (2). Because the computer algorithm for the least square-fit calculation for Eq. (2) is not available in standard spreadsheet applications (e.g., MS-Excel), food scientists would have to make their own computer programs and we cannot expect the average scientist to be able to make their own program. Therefore, using the direct least square-fit calculation for Eq. (2) is not an adequate solution for most scientists. Nevertheless, it is necessary for food scientists to obtain the numerical values on the stability of emulsions from the s-shaped decreasing curves of turbidity against time to verify the stability of their emulsions and further their studies. A pragmatic method for analyzing the data is therefore in great demand. EquationEquation (2) can be converted to the following equation:
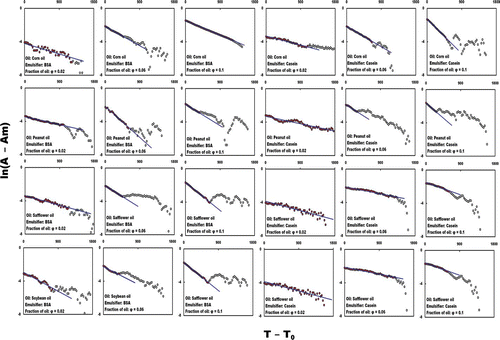
The graph of ln(A − Am) against T − T 0 should be a proportionately diagonally decreasing line. RDT can be calculated from the slope of this line, from which HLT can then be calculated. Graphs of ln(A − Am) against T − T 0 for all the diluted emulsions are shown in , along with the lines showing the least squares fit using Eq. (4). The fraction of oil and the turbidity decreasing parameters RDT and HLT of the emulsions, and the coefficient of determination (R 2) of the least square-fit calculation are listed in and , respectively. At some point within the range of ln(A − Am) against T − T 0, all of the graphs exhibited a diagonally decreasing line from which RDT could be calculated (). This method of calculating RDT was applied to all of the graphs from the diluted emulsions. Even if the graphs of turbidity against time did not exhibit a clear gradually decreasing curve, the turbidity decreasing parameters RDT and HLT were calculated by using this method and a standard spreadsheet application. This method, therefore, is a pragmatic and simple way of evaluating emulsion stability. Finally, an overview of this method is shown in the flowchart in . Because of the limited number of methods currently available for evaluating the stability of emulsions, the author hopes that this method will become accepted as a pragmatic and simple way of evaluating emulsion stability.
CONCLUSION
A pragmatic turbidimetric data analysis method for evaluating emulsion stability by using a spectrophotometer is proposed. An overview of this method is shown in the flowchart in . Absorbance of a diluted emulsion is measured at constant time intervals and turbidity is calculated. A graph of turbidity against time is plotted, and starting time (T 0), turbidity at T 0 (A 0) when the curve of turbidity against time in the graph starts decreasing, and minimum turbidity (Am) are read from the graph. T − T 0 and ln(A − Am) are calculated. A graph of ln(A − Am) against T − T 0 is plotted and the rate of decreasing turbidity (RDT) is determined from the slope of the curve. Finally, half-life time (HLT) is calculated. A high HLT shows that turbidity is slowly decreasing and that the emulsion state can be kept for a long time. This method will enable all food scientists and engineers to calculate the turbidity decreasing parameters RDT and HLT independently by means of standard spreadsheet applications. The author hopes that this method will become accepted as a pragmatic and simple way of evaluating emulsion stability.
ACKNOWLEDGMENT
The author wishes to thank Mariko Tada, a graduate of the Faculty of Pharmaceutical Sciences, Setsunan University, for performing the experiments.
Notes
Color versions of one or more of the figures in the article can be found online at www.tandfonline.com/ljfp.
REFERENCES
- Denka , Asahi and Kogyo , K.K. 2005 . β-glucan-protein complex composition. Japan Kokai Tokkyo Koho P2005 – 82517A .
- Corp , FujiFilm . 2008 . Emulsion-containing composition, foodstuff and preparation for external use. Japan Kokai Tokkyo Koho P2008 – 280256A .
- Corp , Kaneka . 2007 . Oil-in-water type emulsified oil and fat composition containing fat-soluble vitamins. Japan Kokai Tokkyo Koho P2007 – 275020A .
- Corp , Kaneka . 2007 . Food using oleo resin-containing emulsified product. Japan Kokai Tokkyo Koho P2007 – 202534A .
- Corp , Kaneka . 2009 . Purified cone oil, food processed with oil-and-fat utilizing purified cone oil and food. Japan Kokai Tokkyo Koho P2009 – 96936A .
- Corp , Kao . 2007 . Acidic oil-in-water type emulsion composition. Japan Tokkyo Koho P3961548
- Corp , Kao . 2009 . “ Water-in-oil emulsified composition ” . In Japan Tokkyo Koho P4381362
- Corp , Kikkoman . 2010 . “ Mayonnaise-like seasoning free from purified oil and fat and egg yolk ” . In Japan Tokkyo Koho P4646072
- Corp , Kikkoman . 2010 . “ Fermented soymilk containing homogeneous composition ” . In Japan Tokkyo Koho P4570097
- Oillio , Nisshin and Group , Ltd. 2009 . “ UV ray protective preparation and cosmetic containing UV ray protective preparation ” . In Japan Tokkyo Koho P4284147
- Oillio , Nisshin and Ltd , Group . 2011 . “ Oil and fat composition for oil-in-water emulsified product, and oil-in-water emulsified product containing the same ” . In Japan Tokkyo Koho P4657239
- Pharmaceutical , Rohto . 2009 . Co. Ltd. Method for preparing emulsion composition. Japan Kokai Tokkyo Koho P2009 – 256377A .
- FFI , Sanei Gen . 2011 . Inc. Emulsified composition containing modified gum arabic. Japan Kokai Tokkyo Koho P2011 – 41512A .
- Kagaku , Taiyo . 2005 . Co. Ltd. Method of evaluating physical property of fatty acid ester of polyhydric alcohol by measuring cloud point. Japan Kokai Tokkyo Koho P2005 – 134289A .
- Minagawa , M. , Fuji , T. and Ohya , M. 2003 . The Encyclopedia of Detergents and Washing (in Japanese); Asakura Publishing Co., Ltd: Tokyo, Japan 193
- Beckman Coulter Home Page(accessed October 17, 2011 https://www.beckmancoulter.com/wsrportal/wsr/index.htm (http://https://www.beckmancoulter.com/wsrportal/wsr/index.htm)
- Horiba Home Page(accessed October 17, 2011). http://www.horiba.com/us/en/ (http://www.horiba.com/us/en/)
- Malvern Instruments Home Page(accessed October 17, 2011). http://www.malvern.com/ (http://www.malvern.com/)
- Nikkiso Home Page(accessed October 17, 2011). http://www.nikkiso.com/index.html (http://www.nikkiso.com/index.html)
- Parkerizing , Nippon . 2010 . Co. Ltd. Additive for use in cold rolling oil, and cold rolling oil. Japan Kokai Tokkyo Koho P2010 – 189461A .
- Steel , Nippon . 2010 . Corp., Nippon Parkerizing Co. Ltd. Cold rolling oil for steel plate and cold rolling method. Japan Kokai Tokkyo Koho P2010 – 43146A .
- Shimadzu Home Page(accessed October 17, 2011). http://www.shimadzu.com/ (http://www.shimadzu.com/)
- Formulaction Smart Scientific Analysis Home Page(accessed October 17, 2011). http://www.formulaction.com/index.html (http://www.formulaction.com/index.html)
- Chemicals , Mitsubishi . 2006 . Corp. Emulsion stabilizer containing diglycerin fatty acid ester, organic acid mono glyceride and alkali metal salt. Japan Tokkyo Koho P3840903
- Chemicals , Mitsubishi . 2007 . Corp. Coffee extract solution obtained from roasted coffee bean with L value of not more than 24 and milk component-containing homogeneous coffee. Japan Tokkyo Koho P3989927
- Chemicals , Mitsubishi . 2006 . Corp. Method for producing milk coffee containing coffee extract obtained from roasted coffee bean with L value of 24 or below, and milk component. Japan Kokai Tokkyo Koho P2006 – 94867A .
- Capobiangoa , M. , Bizzottoa , C.S. , Biasuttia , E.A.R. and Silvestrea , M.P.C. 2006 . Action of pepsin on emulsifying properties of globin . International Journal of Food Properties , 9 ( 2 ) : 357 – 364 .
- Chobert , J.M. , Bertrand-Harb , C. and Nicolas , M.G. 1988 . Solubility and emulsifying properties of caseins and whey proteins modified enzymically by trypsin . Journal of Agricultural and Food Chemistry , 36 ( 5 ) : 883 – 892 .
- Frentcela , M. , Shwartza , R. and Garti , N. 1982 . Turbidity measurements as a technique for evaluation of water-in-oil emulsion stability . Journal of Dispersion Science and Technology , 3 ( 2 ) : 195 – 207 .
- Horie , K. , Tanaka , S. and Akabori , T. 1978 . Determination of emulsion stability by spectral absorption. I: Relationship between surfactant type, concentration, and stability index. Cosmetics and Toiletries Vol. 93 , 53 – 63 .
- Horie , K. , Tanaka , S. and Akabori , T. 1978 . Determination of emulsion stability by spectral absorption; (Part 2) Relation between surfactant type, oil, phase concentration and stability index . Journal of Society Cosmetic Chemists Japan , 12 ( 1 ) : 53 – 59 .
- Kaufman , V.R. and Garti , N. 1981 . Spectral absorption measurements for determination of ease of formation and stability of oil in water emulsions . Journal of Dispersion Science and Technology , 2 ( 4 ) : 475 – 490 .
- Pearce , K.N. and Kinsella , J.E. 1978 . Emulsifying properties of proteins: Evaluation of a turbidimetric technique. Journal of Agricultural and Food Chemistry , 26 ( 3 ) : 716 – 723 .
- Song , M.-G. , Jho , S.-H. , Kim , J.-Y. and Kim , J.-D. 2000 . Rapid evaluation of water-in-oil (w/o) emulsion stability by turbidity ratio measurements . Journal of Colloid and Interface Science , 230 ( 1 ) : 213 – 215 .
- Song , M.-G. , Cho , S.-Ho. , Kim , J.-Y. and Kim , J.-D. 2002 . Novel evaluation method for the water- in-oil (W/O) emulsion stability by turbidity ratio measurements. Korean Journal of Chemical Engineering , 19 ( 3 ) : 425 – 430 .
- Khuwijitjarua , P. , Anantanasuwonga , S. and Adachi , S. 2010 . Emulsifying and foaming properties of defatted soy meal extracts obtained by subcritical water treatment . International Journal of Food Properties , 14 ( 1 ) : 9 – 16 .
- Jiang , S.-J. and Zhao , X.-H. 2012 . “ Cross-linking and glucosamine conjugation of casein by transglutaminase and the emulsifying property and digestibility in vitro of the modified product ” . In International Journal of Food Properties , 15(6), 1286–1299 .
- Atkins , P. and Paula , J.D. 2010 . Atkins’ Physical Chemistry; , 790 – 792 . Oxford : Oxford University Press .
- Nash , J.C. 1987 . Nonlinear Parameter Estimation: An Integrated System in Basic; , New York : Marcel Dekker .
- Okumura , H. 1991 . The Recent Computer Algorithms in C (in Japanese); Gijyutsu–Hyouronsha: Tokyo 260
- Tankei , K. , Okumura , H. , Sato , T. , Kobayashi , M. , Press , W.H. , Flannery , B.P. , Teukolsky , S.A. and Vetterling , W.T. 1993 . Numerical Recipes in C , Japanese. Transl : Gijyutsu–Hyouronsha: Tokyo .
- Miyawaki , N. and Sakai , K. 2004 . Basic Data Analysis by Using MS-Excel (in Japanese); Baifukan Co., Ltd: Tokyo 205