Abstract
Sesame oil is an edible vegetable oil derived from the sesame seed that has been used as a flavor enhancer in Southeast Asian cuisine. This highly valuable oil can be subjected to adulterations with lower price oils in order to gain economical profit. Among 10 vegetable oils evaluated using fatty acid profiles with principal component analysis, corn oil has the closest similarity in fatty acids combined together with sesame oil; therefore, corn oil is a potential adulterant in sesame oil. FTIR spectra at 1072−935 cm−1 was chosen for quantitative analysis with acceptable values of coefficient determination (R2), root mean square errors of calibration and prediction. These combined methods using first derivative FTIR spectra in partial least square showed well quantified corn oil in sesame oil with R2 (0.992), root mean square errors of calibration (0.53% v/v) and root mean square errors of prediction (1.31% v/v) values. Moreover, the Coomans plot based on Mahalanobis distance were able to discriminate between sesame oil with adulterated oils such as corn oil, grape seed oil, and rice bran oil.
INTRODUCTION
Sesame oil (SeO) is derived from a plant species called Sesamum Indicum L.[Citation1] SeO is one of the highly valuable edible oils due to its special characteristic flavors that acts as a source of food oils in many Asian countries. It is commonly used as a cooking and flavor enhancer. Studies have been conducted in order to investigate the health-benefit effects of sesame and its effectiveness against various diseases including atherosclerosis and hypertension, and also possesses an anti-aging effect.[Citation2,Citation3] The retail price for pure SeO is higher (approximately 10–15 times) than other common vegetable oils such as corn and palm oils (PO); consequently, unscrupulous producers tried to mix SeO with the lower priced oil and pass it off as the real thing.
Several analytical authentication techniques of SeO have been developed such as gas chromatography with flame ionization detection for analysis of fatty acid, (FA) changes during adulteration action and carbon isotope ratio,[Citation4] high-performance liquid chromatography for analysis of triglycerides,[Citation5,Citation6] and electronic nose for aroma profile analysis in several fields.[Citation7,Citation8] Some of these methods are laborious and involve hazardous solvents and reagents. Therefore, the analytical techniques offering an environmentally friendly solvent are continuously being developed by fat and oil researchers. One of the methods which can overcome some of the analytical problems is FTIR spectroscopy.
Recent studies showed that FTIR spectroscopy has been widely used in the food industry due to its simple, rapid, cost effective, and environmentally friendly analysis method. Nowadays, the progress in computer technology and multivariate chemometric analysis has made it frequently used for quantitative determination with the inclusion of fats and oils.[Citation9,Citation10] Multivariate calibrations are one of the most successfully statistical methods that have been used in the food industry, especially in the field of food analysis.[Citation11]
The research group has continually investigated the development of rapid and technologically advanced methods using FTIR spectroscopy for detecting the presence of lard in mixture of animal fats[Citation12] determining a range of adulteration problems in food products such as lard content in cakes and chocolates[Citation13] and authenticating an extra virgin olive oil (EVOO) from PO.[Citation14] Recently, SeO in EVOO having longer in Mahalanobis distance using FTIR and gas chromatography (GC) has been reported;[Citation15] however, there is no report available regarding the combination of FTIR characterization with chemometric for quantitative analysis of CO in SeO, which are shorter in mahalanobis distance and similarity in FA compositions. Therefore, the present study is a report of the first example of FTIR spectroscopy combined with multivariate calibration of partial least square (PLS) and discriminant analysis (DA) as a method for determination of oil adulteration with the closest similarities to SeO.
MATERIALS AND METHODS
Sesame (SeO), corn (CO), rice bran (RBO), and grape seed (GSO), palm (PO), pumpkin (PKO), extra virgin olive (EVOO), soybean (SO), sunflower (SFO), canola (CaO), walnut (WO) and virgin coconut (VCO) oils were bought from local markets in Serdang, Selangor Malaysia. FA compositions of these vegetable oils were quantitatively determined using gas chromatography coupled with flame ionization detection as described in Rohman and Che Man.[Citation16] The standard of 37 FA methyl esters (FAME) was purchased from Sigma, and then was used to compare the retention samples of FAs in the evaluated oils. All reagents and solvents used throughout this study were of analytical grade.
Calibration and Prediction Samples for Quantification of Co in SeO
For calibration, 20 samples consisting of CO in binary mixtures with SeO in the concentration range of 0.5–50% (volume/volume) were analytically prepared. To evaluate the capability of calibration samples to predict the unknown samples, the independent samples were also made. In order to ensure the homogeneity, these sample mixtures were vigorously shaken using vortex for one min. All samples were further analyzed using FTIR spectrometer.
DA
In the first experiment of DA, SeO and CO were blended in chloroform for obtaining a series of trained sets or standard of 10 unadulterated (pure SeO) and 18 adulterated samples containing 5–50% of CO in SeO. The SeO samples containing CO were marked as adulterated samples; meanwhile, a series of pure SeO in chloroform was assigned as unadulterated ones. Both of classes were subjected to DA using their FTIR spectra. In the second experiment, SeO and three vegetable oils whose FAs are relatively similar with SeO (CO, RBO, and GSO) were prepared. Pure SeO and SeO mixed with three vegetable oils were classified using DA.
FTIR Spectra Acquisition
FTIR spectrometer of Nicolet 6700 (Thermo Nicolet, Madison, WI) was used during spectra acquisition. This instrument was equipped with a detector of deuterated triglycine sulphate and beam splitter of potassium bromide. The software of the OMNIC operating system (Version 7.0 Thermo Nicolet) included in the FTIR spectrometer was used to obtain the spectra and to manipulate them. Using a Pasteur pipette, an approximately 1.0 mL of oil samples was properly placed on attenuated total reflectance (ATR) crystal. During measurement, FTIR spectra was obtained at frequency regions of 4,000−650 cm−1 and co-added at 32 scans and at 4 cm−1 resolution. FTIR spectra were displayed as absorbance values, at least in three measurements.
Statistical Analysis
FA profiles of SeO, RBO, CO, and GSO were subjected to principal component analysis (PCA) using Minitab software Release 15 (Minitab, State College, PA, USA). Multivariate calibration and DA was carried using the software of TQ AnalystTM version 6 from Thermo electron Corporation, Madison, WI, USA) included in FTIR spectrometer.
RESULTS AND DISCUSSION
PCA Classification of SeO and CO
FA compositions of SeO and others can be seen in the previous reports.[Citation17] These FAs were subsequently subjected to chemometrics of PCA. PCA was used in order to classify SeO and other vegetable oils. PCA is unsupervised pattern recognition technique which projects the original data in reduced dimensions defined by the principal components (PC). This technique is useful when there is a correlation present among data.[Citation18,Citation19]
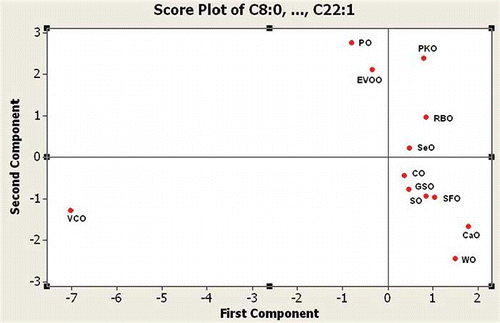
exhibits PCA score plot of the first principal component (PC1) and second principal components (PC2) using FA compositions (C8:0; C10:0; C12:0; C14:0; C16:0; C18:0; C18:1; C18:2; C18:3, and C20:0; C20:1; C22:0; and C22:1). Among vegetable oils shown in the score plot, the PC1 and PC2 of SeO have the closest distance with CO. It means that CO is similar to SeO in chemical compositions and FA profiles. Therefore, CO can act as a potential adulterant in SeO.
Figure 1 PCA score plot for classification of SeO and other oils. SeO = sesame oil; CO = corn oil; RBO = rice bran oil; GSO = grapeseed oil; PO = palm oil; PKO = pumpkin oil; EVOO = extra virgin olive oil; SO = soybean oil; SFO = sunflower oil; CaO = canola oil; WO = walnut oil; VCO = virgin coconut oil.
Quantification of CO in SeO
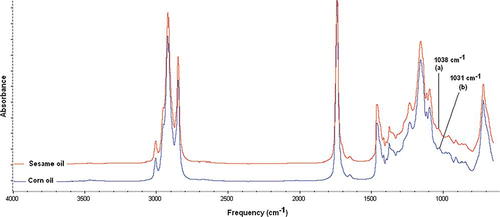
Triglyceride is a principal component in fats and oils which dominate their FTIR spectra. shows FTIR spectra of SeO and CO from 4000–500 cm−1. The entire region of spectra displayed very similar for both oils. However, there was a minor difference between two spectra which can be observed at wavenumber of 1038 cm−1 caused by =C–O–C symmetric stretching for SeO and at 1031 cm−1 assigned to C–O stretching for CO. The differences between SeO and CO were exploited for their quantitative analysis. Finally, the optimization process at wavenumber of 1072–935 cm− Citation1 is selected to be used for quantification of CO in SeO due to its ability to provide the high value of correlation (R 2) and the low value of error.
Calibration and Validation Using PLS Model
PLS is one of the multivariate calibration models in which the concentration of analytes is correlated with the PCs or factor, rather than original variables (FTIR spectra). This technique can reduce the amount of data (FTIR spectra) which insignificantly contributed for PLS model.[Citation20] lists the values obtained from PLS calibration models for analysis of CO in SeO in terms of R 2, root mean square error of calibration (RMSEC) and root mean square error of prediction (RMSEP) either for normal spectra or its derivatives. It is known that FTIR normal spectra and its first derivative offered the acceptable R 2 (0.992), RMSEC (0.53% v/v) and RMSEP (1.31% v/v) values. In addition, second derivative spectra exhibited the highest values of R Citation2 and the lowest values of RMSEC. However, the RMSEP value obtained here was too high; therefore, PLS of the second derivative is not chosen for such quantification. This is an indication that overfitting occurred when using the second derivative spectra. Overfitting is the main problem when using PLS model, in which PLS gives the over-optimistic in calibration model and inversely in prediction model.[Citation20]
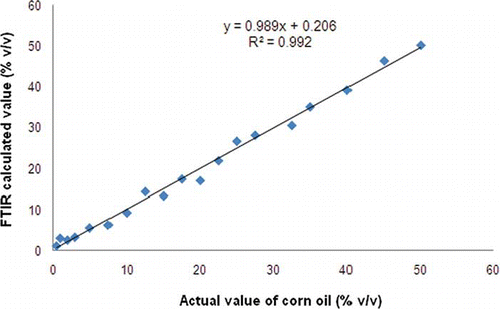
exhibits a scatter plot for the relationship between actual value (x-axis) and FTIR predicted value (y-axis) of CO in SeO in PLS using the first derivative spectra. The developed model was further used to predict the independent samples which are different from calibration samples. This result suggests that FTIR spectroscopy combined with PLS can be a promising method for quantification of CO in SeO. These results indicated that FTIR spectroscopy in conjunction with multivariate calibration of PLS is well suited for determination of CO in SeO.
Table 1 The statistical parameters for quantification of CO in SeO using PLS model
DA
DA can be used to determine the class of oil which is similar to oil adulterants by calculating the distance from each class center in Mahalanobis distance unit. Once classification model has been obtained, the membership of unknown objects to one of the defined classes can be predicted.[Citation21] The classification of pure SeO and those adulterated with CO was performed using DA. Firstly, pure SeO, and that mixed with adulterant CO, were classified into two groups known as pure SeO and adulterated oil. Both classes (SeO and that adulterated with CO) were classified using DA and Coomans plot. Coomans plot is useful for visualizing the classification results. It is formed by calculating two independent PC models and plotting the residual distances of samples of each two model,[Citation22] meanwhile, the Mahalanobis distance obtained from the Coomans plot is useful to assign whether a set of unknown value samples is similar to a group of known measured samples.
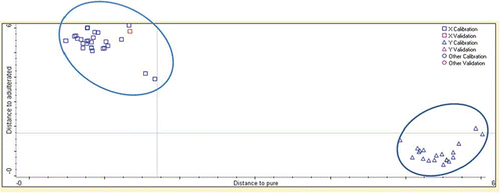
displays the Coomans plot for classification of SeO and SeO adulterated with CO. The x-axis shows Mahalanobis distance to SeO, while the y-axis shows the distance to SeO blended with CO. In this study, the DA model classified 100% of all samples accurately according to its group. These results indicate that no samples are misclassified into the wrong group, which sometimes could happen because of the similarities in the chemical compositions between the classes.[Citation23]
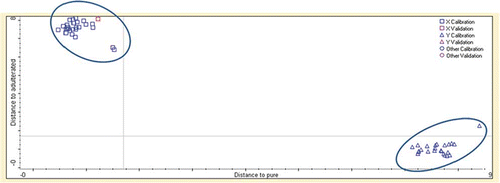
Further study was conducted to investigate the ability of DA to discriminate pure SeO samples, and that added with the mixture of CO, RBO, and GSO. shows the Cooman plot of SeO and adulterated with other vegetable oils. The models successfully classified SeO samples and other adulterated oils. However, the Mahalanobis distances were longer to each other than those in . Within the adulterated class, it is noted that the samples containing a higher percentage of adulterants were more inclined toward the right side of the plot, indicating longer distance from the SeO axis. This can be explained that the addition of other oils (RBO and GSO) in CO as adulterants in SeO can make easier discrimination.
CONCLUSION
In conclusion, FTIR spectroscopy combined with chemometric analysis indicates a useful tool for quantitative determination of adulterated SeO with CO. PLS regression at selected fingerprint region of 1072–935 cm−1 can be successfully used for determining CO in SeO with RMSEC and RMSEP values of 0.53% and 1.31% (v/v), respectively. DA can be perfectly used to classify SeO and potential adulterants of vegetable oils such as CO, RBO, and GSO. These techniques are fast, reliable and inexpensive.
ACKNOWLEDGEMENTS
The authors would like to thank the Ministry of Science, Technology, and Innovation (MOSTI), Malaysia for funding this study through Research Grant (Science Fund 05-01-04-SF0285) awarded to Professor Dr. Y. B. Che Man. The authors also thank Dr. Hendrik Oktendy Lintang (Ibnu Sina Institute for Fundamental Science Studies, Universiti Teknologi Malaysia) for advice and discussions during the preparation of this manuscript.
Notes
Color versions of one or more of the figures in the article can be found online at www.tandfonline.com/ljfp.
REFERENCES
- Nuchanart , R. , Nanthanit , P. , Chulabhorn , M. , Wasana , W. , Kanya , S. , Sumontha , N. and Jutamaad , S. 2010 . Variation of sesamin, sesamolin, and tocopherols in sesame (Sesamum indicum L.) . seeds and oil products in Thailand. Food Chemistry , 122 : 724 – 730 .
- Kang , M.H. , Kawai , Y. , Naito , M. and Osawa , T. 1999 . Dietary defatted sesame flour decreases susceptibility to oxidative stress in hypercholesterolemic Rabbits . Journal of Nutrition , 129 : 1885 – 1890 .
- Fukuda , Y. , Osawa , T. , Namiki , M. and Ozaki , T. 1985 . Studies on antioxidative substances in sesame seed . Agriculture and Biological Chemistry , 49 : 301 – 306 .
- Seo , H.Y. , Ha , J. , Sin , D.B. , Shim , S.L. , No , K.M. , Kim , K.S. , Lee , K.B. and Han , S.B. 2010 . Detection of corn oil in adulterated sesame oil by chromatography and carbon isotope analysis . Journal of the American Oil Chemists’ Society , 87 : 621 – 626 .
- Yamajaki , M. , Nagao , A. and Yamamoto , H. 1993 . Estimation of the mixing ratio of foreign oil in adulterated sesame oil II . Rice bran oil or rapeseed oil. Journal of Japan Oil Chemists’ Society , 43 : 10 – 17 .
- Lee , D.S. , Lee , E.S. , Kim , H.J. , Kim , S.O. and Kim , K. 2001 . Reversed phase liquid chromatographic determination of triacylglycerol composition in sesame oils and the chemometric detection of adulteration . Analytica Chemica Acta , 429 : 321 – 330 .
- Hai , Z. and Wang , J. 2006 . Electronic nose and data analysis for detection of maize oil adulteration in sesame oil . Sensor Actuator B , 119 : 449 – 455 .
- Hai , Z. and Wang , J. 2006 . Detection of adulteration in camellia seed oil and sesame oil using an electronic nose . European Journal of Lipid Science and Technology , 108 : 116 – 124 .
- Haaland , D.M. and Thomas , E.V. 1988 . Partial least squares method for spectral analysis . I. Relation to other quantitative calibration methods and the extraction of qualitative information. Analytical Chemistry , 60 : 1193 – 1202 .
- Guillen , M.D. and Cabo , N. 1997 . Infrared spectroscopy in the study of edible oils and fats . Journal of Science of Food and Agriculture , 75 : 1 – 11 .
- Cadet , F. , Bertrand , D. , Robert , P. , Maillot , J. , Dieudonne , J. and Rouch , C. 1990 . Quantitative determination of sugar cane sucrose by multidimensional statistical analysis of their mid infrared attenuated total reflectance spectra . Applied Spectroscopy , 45 : 166 – 170 .
- Che Man , Y.B. and Mirghani , M.E.S. 2001 . Detection of lard mixed with body fats of chicken, lamb, and cow by Fourier transform infrared spectroscopy . Journal of the American Oil Chemists’ Society , 78 : 753 – 761 .
- Che Man , Y.B. , Mirghani , M.E.S. , Syahariza , Z.A. , Selamat , J. and Bakar , J. 2005 . Detection of lard adulteration in cake formulation by Fourier transformed infrared spectroscopy . Food Chemistry , 92 : 365 – 371 .
- Che Man , Y.B. , Rohman , A. and Mansor , T.S.T. 2010 . Differentiation of lard from other edible oils by means of Fourier transform infrared spectroscopy and chemometrics . Journal of the American Oil Chemists’ Society , 88 : 187 – 192 .
- Rohman , A. and Che Man , Y.B. 2011 . “ International Journal of Food Properties ” . In Authentication of extra virgin olive oil from sesame oil using FTIR spectroscopy and gas chromatography DOI:10.1080/10942912.2010.521607
- Rohman , A. and Che Man , Y.B. 2009 . Monitoring of virgin coconut oil (VCO) adulteration with palm oil using fourier transform infrared (FTIR) spectroscopy . Journal of Food Lipids , 16 : 618 – 628 .
- Cserháti , T. 2009 . Review: Data evaluation in chromatography by principal component analysis . Biomedical Chromatography , 24 : 20 – 28 .
- Shao , Y. , He , Y. , Yidan , B. and Mao , J. 2010 . Near-infrared spectroscopy for classification of oranges and prediction of the sugar content . International Journal of Food Properties , 12 : 644 – 658 .
- He , Y. , Li , X. and Shao , Y. 2007 . Fast discrimination of apple varieties using Vis/NIR spectroscopy . International Journal of Food Properties , 10 : 9 – 18 .
- Miller , J.N. and Miller , J.C. Statistics and Chemometrics for Analytical Chemistry , 5th , Harlow : Pearson Education Limited, 2005 . EdEdinburgh Gate
- Rohman , A. and Che Man , Y.B. 2009 . Analysis of cod liver oil adulteration using Fourier transform infrared (FTIR) spectroscopy . Journal of the American Oil Chemists’ Society , 86 : 1149 – 1153 .
- Ballabio , D. and Todeschini , R. 2008 . Multivariate classification for qualitative analysis in infrared spectroscopy for food quality: Analysis and control. Elsevier 83 – 104 . New York , USA
- Manaf , M.A. , Che Man , Y.B. , Hamid , N.S.A. , Ismail , A. and Syahariza , Z.A. 2007 . Analysis of adulteration of virgin coconut oil by palm kernel olein using fourier transformed infrared spectroscopy . Journal of Food Lipids , 14 : 111 – 121 .