Abstract
To compare the immunogenicity and digestibility in-vitro simulated gastric fluid, the protein extracts of white-leg shrimp, Chinese shrimp (female and male shrimp were separated), Southern rough shrimp, Acetes chinensis, Mantis shrimp and crawfish were analyzed by SDS-PAGE, indirect ELISA and Western blotting. The results showed that except for Acetes chinensis, the larger proportion of molecular mass were near to 25 kDa (sarcoplasmic calcium-binding protein) and 38 kDa (tropomyosin). White-leg shrimp protein and Southern rough shrimp protein had a maximum similarity of 68.2%. White-leg shrimp protein had higher immunogenicity than others. There were much more IgE-binding bands in male Chinese shrimp protein, while Crawfish just had only one IgE-binding band (38 kDa, tropomyosin). In simulated gastric fluid system, the protein such as 25 kDa was rapidly degraded within 0.5 mins, while tropomyosin (38 kDa) was relatively resistant to pepsin digestion. The Western blotting results indicated that the allergenicity of those crustacean protein extracts still remained even they were digested with simulated gastric fluid for 60 min.
INTRODUCTION
A large variety of crustaceans are widely used for human consumption. White-leg shrimp (Penaeus vannamei), Chinese shrimp (Penaeus chinensis), Southern rough shrimp (Trachypenaeus curvirostris), Acetes chinensis, Mantis shrimp (Squilla oratoria), and crawfish (Procambarus clarkii) are extensively consumed in many areas of Asia, especially in China, due to their delicious taste and nutritional value. In particular, the consumption in Qingdao area is very high. With increasing production and consumption of crustaceans and their products, reports about allergy induced by ingestion of such kind of foods are frequent.[Citation1,Citation2]
Crustaceans’ food, e.g., shrimp, prawn, crab, lobster, and crawfish, are listed as one of “the big eight” allergies mediated by IgE antibodies[Citation3] and considered as the most common cause of food allergies worldwide.[Citation4] In crustacean sensitized individuals, it is characterized by the development of urticaria, vomiting, diarrhea, angioedema, asthma, and even life-threatening anaphylactic reactions.[Citation5] More and more individuals are at risk of having hypersensitive responses upon ingestion of shellfish.[Citation6] Since seafood trading increased, immediate hypersensitivity reactions to these products have become an important health issue and the detail between different varieties are very significant in the seafood allergy research.
Tropomyosin, a myofibrillar protein with molecular mass of 35-38kDa has been identified as the major allergen of crustaceans in Indian white shrimp (Penaeus indicus),[Citation7] brown shrimp (Penaeus aztecus),[Citation8] sand shrimp (Metapenaeus ensis),[Citation9] American lobster (Homarus americanus), and spiny lobster (Panulinus stimpsoni),[Citation10] etc. In addition, some proteins including triosephosphate isomerase,[Citation11] sarcoplasmic calcium-binding protein,[Citation12,Citation13] myosin light chain,[Citation14] Troponin C,[Citation14] and arginine kinase[Citation15CitationCitationCitationCitation−Citation20] have been reported in some crustacean species causing an allergic reaction in hypersensitive individuals. Therefore, it is worthy to study the allergenic activity of different crustacean species in order to develop hypoallergenic crustacean foods.
Stability during digestion is considered as an important factor in determining the allergenicity of food proteins.[Citation21] Mud crab (Scylla serrata),[Citation22] grass prawn (Penaeus monodon), and Pacific white shrimp (Litopenaeus vannamei)[Citation23] in simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) digestion assay system was investigated and comparatively studied. Although many crustaceans have been studied and reported to be one of the most common causes of IgE-mediated allergic reactions, there are less reports about the characterization and comparison of protein extracts from White-leg shrimp (Penaeus vannamei), Chinese shrimp (Penaeus chinensis), Southern rough shrimp (Trachypenaeus curvirostris), Acetes chinensis, Mantis shrimp (Squilla oratoria), and crawfish (Procambarus clarkii). In this study, it was reported that the protein composition, immunological analyses, and digestive stability in SGF of above crustacean species.
MATERIALS AND METHODS
Crustaceans and Chemicals
White-leg shrimp (Penaeus vannamei), Chinese shrimp (Penaeus chinensis) (the female and male shrimp were separated), Southern rough shrimp (Trachypenaeus curvirostris), Acetes chinensis, Mantis shrimp (Squilla oratoria), and crawfish (Procambarus clarkii) were purchased alive from the local market and their shells and heads were removed. The raw crustacean extracts were stored at –20°C until use.
Unless otherwise stated, all reagents were of analytical grade. Buffers and reagents used for Western blotting were as follows: Phosphate buffer saline (PBS): 0.01 mol/L phosphate buffer, pH 7.4, containing 0.15 mol/L NaCl; PBS-Tween (PBST): 0.01 mol/L phosphate buffer, pH 7.4, containing 0.15 mol/L NaCl and 0.05% Tween 20. Buffers and reagents used for indirect ELISA were as follows: Blocking buffer: 0.01 mol/L phosphate buffer, pH 7.4, containing 1% BSA (bovine serum albumin), 0.15 mol/L NaCl; washing buffer: The same with PBST. Goat anti-human IgE conjugated with peroxidase and goat anti-rabbit IgG conjugated with peroxidase (Sigma, Missouri, USA) were used in Western blotting and indirect ELISA. Rabbit antiserum against tropomyosin was prepared by Beijing Genomics Institute. Solid-phase enzyme immunoassays were performed in 96-well microtiter plates (Nunc, Denmark) using Multiskan MK3 ELISA reader (Thermo Labsystems).
Pepsin was purchased from Sigma (St. Louis, MO). The proteolytic activity of pepsin was estimated by measuring trichloroacetic acid and bovine hemoglobin. The specific activity of pepsin was 806.3 U/mg. Protein standards for SDS-polyacrylamide gel electrophoresis (SDS-PAGE) were from Fermentas (Lithuania) or Bio-Rad (Richmond, CA, USA). Sera from 10 patients were obtained from the affiliated hospital of Medical College of Qingdao University. The patients were selected on the basis of their past clinical history of having crustacean allergy, such as urticaria and diarrhea, after ingestion of crustaceans. All sera were stored at –80°C until used.
Extraction of Crustacean Proteins
Peeled crustacean was weighed and homogenized in 4-fold (W/V) of 0.9% sodium chloride (NaCl). The homogenate was then subjected to cold acetone three times to make acetone powder with the method of Greaser and Gergely[Citation24] with minor modifications. Then, the acetone powder was extracted overnight with 10-fold 1 mol/L potassium chloride (KCl) and 5 mmol/L dithiothreitol (DTT). The mixture was then centrifuged at 10,000 rpm for 30 min and the resulting supernatant was dialyzed against double distilled water for 48 hr. The solution was lyophilized and stored at –20°C. The protein concentration of crustacean proteins were determined using bicinchoninic acid by measuring the absorbance at 590 nm and using BSA as standard.[Citation25]
Western Blotting with Patient’s Sera
Denaturing protein electrophoresis (SDS-PAGE) was performed according to the method of Laemmli.[Citation26] Samples were mixed 4:1 (V:V) with 1 × Laemmli buffer (2% SDS, 25% glycerol, 14.4 mmol/L β-mercaptoethanol, and 0.1% bromphenol blue in 1 mol/L Tris-HCl, pH 6.8), heated at 100°C for 7 min and applied on a 15% analytical SDS-polyacrylamide gel employing a vertical electrophoresis system (Bio-Rad) according to the manufacturer’s recommendations. Gels were either stained with Coomassie Brilliant Blue R-250[Citation27] or transferred to polyvinylidene fluoride (PVDF) membrane (450 nm, Pall-Gelman, USA) through Western blotting. The software of quantity-one was used to determine the molecular mass. For immune blotting, samples separated by SDS-PAGE were transferred to PVDF membrane by electro blotting at constant current of 0.8 mA/cm2 membrane for 3.5 hr, according to Towbin and Gordon[Citation28] with some changes. After the membrane was stained with Ponceau S and verified the protein transfer, blots were blocked with 5% skimmed milk in PBST (pH 7.4) for 2 hr at 37°C, and then incubated with patient’s sera (1:20 dilution with blocking buffer) overnight at 4°C. A polyclonal goat anti-human IgE antibody conjugated with peroxidase (1:1000 in blocking buffer) was added after washing. Immunoreactive bands were developed using enhanced chemiluminescent (ECL) after final washing with PBST. Non-specific binding of the anti-IgE antibody conjugate was measured in a similar blotting procedure, omitting the incubation step with patient sera. Coomassie blue stained gels and developed PVDF membranes were scanned using Tanon-4200 automatic translation of the digital gel image analysis system. The low-range pre-stained SDS-PAGE protein mixture (Fermantas, Lithuania) was used as standard.
Indirect ELISA to Determine Tropomyosin in Protein Extract
Protein extracts (1 μg/well) were immobilized on microtiter plates using 100 μl coating buffer (carbonate buffer pH 9.6) and incubated overnight at 4°C. The plates were washed and free binding sites were blocked with blocking buffer for 2 hr at 37°C. After washing three times with washing buffer/PBST, polyclonal antibodies/rabbit anti-tropomyosin antibodies (1:20,000 in PBS) was added and incubated for 1.5 hr at 4°C. The plates were washed again and 100 μL of goat anti-rabbit IgG conjugated with peroxidase (1:10,000 in PBS) was added and incubated for 1 hr at 37°C. Plates were washed with PBST followed by the addition of 3,3′,5,5′-tetramethylbenzidine (TMB) as substrate. The plates were incubated in the dark for 15 min, and read by ELISA reader at the following wave lengths: 450 nm. All ELISA experiments were performed in triplicate and the mean was determined.
SGF Digestion Stability Assay
The digestibility of crustacean allergen proteins in SGF was examined according to the method[Citation29] with some modifications. SGF was prepared as described in United States Pharmacopoeia[Citation30] and consists of porcine pepsin A (806.3U/mg) prepared in 35 mM NaCl and 0.084 mM HCl, pH 1.2. A centrifuge tube of 2 mL for each sample containing SGF was preheated at 37°C prior to the addition of test protein. A ratio of 10 U of pepsin activity/μg protein was selected and 1.6 mL final volume was set for reaction solution. Digestion was performed at 37°C with continuous rocking. Aliquots of 200 μL were withdrawn at different time intervals (0.5, 1, 2, 5, 10, 15, 30, and 60 min) and digestion was immediately terminated by adding 70 μL of 200 mM Na2CO3 and 67.5 μL of 1 × Laemmli buffer. Samples were heated at 100°C for 7 min and analyzed by SDS-PAGE, or Western blot. For the 0 min sample preparation, protein samples were mixed with SGF that had been inactivated by neutralization with Na2CO3. In control experiments, each protein sample was dissolved in the reaction buffer without pepsin and carried out as described above. All the experiments were repeated three or more times and no obvious differences were identified.
RESULTS
Protein Composition of Crustacean Protein Extracts
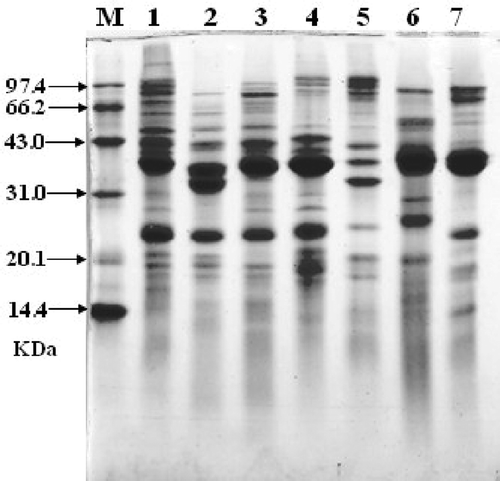
The composition of crustacean protein extracts was examined by SDS-PAGE/Coomassie blue-staining. The bands of all seven crustacean proteins are different with each other (). All the crustacean species have more than 10 protein bands. Protein bands of Acetes chinensis were well distributed, but the other species have thick protein bands and the apparent molecular masses were ˜ 38 and ˜ 25 kDa, representing most probably tropomyosin and sarcoplasmic calcium-binding protein, respectively.
FIGURE 1 SDS-PAGE/Coomassie blue-staining of seven crustaceans protein extracts with same protein concentration. M, molecular weight proteins; (1) White-leg shrimp (Penaeus vannamei); (2) Female Chinese shrimp (Penaeus chinensis); (3) Male Chinese shrimp (Penaeus chinensis); (4) Southern rough shrimp (Trachypenaeus curvirostris); (5) Acetes chinensis; (6) Mantis shrimp (Squilla oratoria); (7) Crawfish (Procambarus clarkii).
Western Blot Analysis of Reactivity of Crustacean Protein Extracts
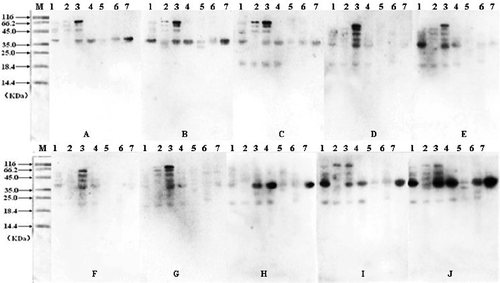
The allergenicity of protein extracts was confirmed by immuno blotting analyses. Electrophoretically separated extracts from those protein samples were tested for their allergenicity with 10 human sera of crustacean allergy patients. shows the immuno blotting results that different crustacean species have different immunoreaction. The male Chinese shrimp (Penaeus chinensis) had high reactivity activity and had immune response to all of the 10 human sera, while Acetes chinensis just had immune response to three patients’ sera. In , the molecular mass of most of the bands was near to 38 kDa (probably tropomyosin).
TABLE 1 The molecular mass (kDa) of western blotting bands of seven crustacean protein extracts to 10 crustacean allergy patients sera
FIGURE 2 Western blotting detection of the crustacean protein extracts from seven different varieties. Samples were separated by SDS-PAGE followed by immunological detection using serum of 10 crustacean allergic patients (A,B,C, … J). M, molecular weight proteins; (1) White-leg shrimp (Penaeus vannamei); (2) Female Chinese shrimp (Penaeus chinensis); (3) Male Chinese shrimp (Penaeus chinensis); (4) Southern rough shrimp (Trachypenaeus curvirostris); (5) Acetes chinensis; (6) Mantis shrimp (Squilla oratoria); (7) Crawfish (Procambarus clarkii).
Allergenicity of Tropomyosin in Crustacean Protein Extracts
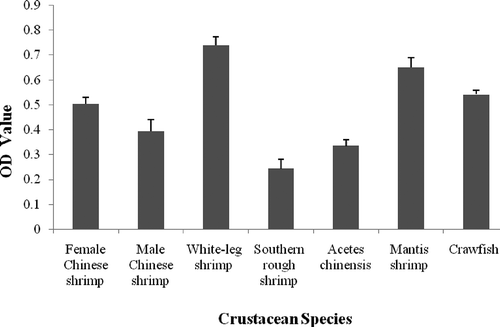
The allergenicity of different crustacean protein extracts was measured by indirect ELISA using specific IgE from rabbit antiserum against white-leg shrimp tropomyosin. For comparison, the allergenicity of tropomyosin in protein extracted from crustaceans was also investigated. As shown in , the IgE-binding ability of white-leg shrimp tropomyosin was higher than other varieties, while southern rough shrimp tropomyosin has the lowest IgE-binding ability.
Digestibility of Crustacean Protein Extracts by SGF
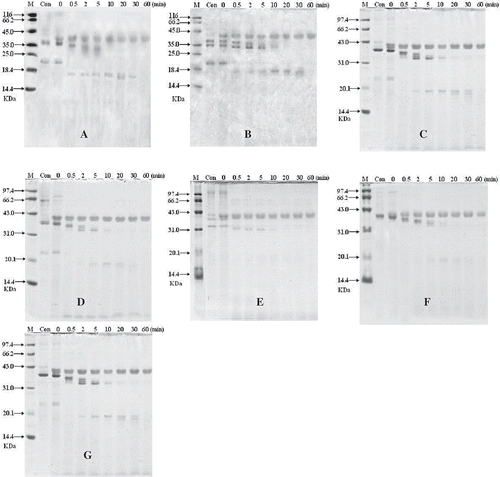
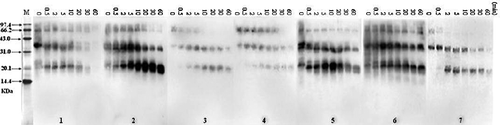
The digestibility of crustacean protein extracts was examined by SDS-PAGE. The results of seven crustacean protein extracts are shown in and the digestion of those varieties had the same state. The result showed that the representative band of tropomyosin (38 kDa) was not present in all of the samples after 10 min, while bands of other proteins disappeared quickly in SGF. The bands of tropomyosin were slowly got thinner than before and many minor degraded fragments became visible, except Acetes chinensis which had unclear fragments ().
FIGURE 4 SDS-PAGE analysis of SGF digestion on crustacean protein extracts. In the control experiments (Con) pepsin was excluded. The digest was withdrawn at different time intervals (0.5, 1, 2, 5, 10, 15, 30, and 60 min). M, molecular weight proteins; Con, the eatracts of no pepsin; (A) White-leg shrimp (Penaeus vannamei); (B) female Chinese shrimp (Penaeus chinensis); (C) male Chinese shrimp(Penaeus chinensis); (D) Southern rough shrimp (Trachypenaeus curvirostris); (E) Acetes chinensis; (F) Mantis shrimp (Squilla oratoria); (G) Crawfish (Procambarus clarkii).
IgE-Binding of Crustacean Protein Extracts by SGF Digested
The IgE-binding of SGF digested crustacean protein extracts was analyzed by means of immuno blotting (). Blots treated with rabbit polyclonal IgE antibody showed that it strongly reacted with the protein bands of 69 kDa, 38 kDa and a new band (20 kDa) that appeared after pepsin digestion. However, no IgE reactivity was observed against smaller fragment (23 kDa) which was original band of crustacean protein extracts. Possibly, IgE-binding epitopes in these fragments were destroyed by trypsin digestion. With the time of pepsin digestion, the immune band of 38 kDa (tropomyosin) became weak gradually, and disappeared in 60 min. The reactivity of a new band (20 kDa) that appeared after pepsin digestion with the antibody was more powerful than the other bands (-, -5, 5-6, 5-7). Although the thickness of the bands was reduced in 60 min, the IgE-binding band still existed.
FIGURE 5 IgE-Western blot (B) using rabbit polyclonal antibodies to detect the IgE-binding of crustacean protein extracts after digestion by SGF. The digest was withdrawn at different time intervals (0.5, 1, 2, 5, 10, 15, 30, and 60 min). M, molecular weight proteins; (1) White shrimp (Penaeus vannamei); (2) female Chinese shrimp (Penaeus chinensis); (3) male Chinese shrimp (Penaeus chinensis); (4) Southern rough shrimp (Trachypenaeus curvirostris); (5) Acetes chinensis; (6) Mantis shrimp (Squilla oratoria); (7) Crawfish (Procambarus clarkii).
DISCUSSION
The characteristics of the major allergen tropomyosin of crustaceans have been widely studied.[Citation31CitationCitation−Citation34] However, detailed information concerning all the proteins of crustacean is still not available. In this study, the proteins extracted from seven crustacean species were investigated. Allergenic proteins, which act as denominators of allergic reactions in majority (more than 50%) of patients, are termed “major allergens;” while those which elicit allergic reactions only in a minor fraction (less than 50%) of patients are called “minor allergens.”[Citation35] The tropomyosin was the major allergen in all species, except female Chinese shrimp (). The male Chinese shrimp not only had the immune bands at 37 kDa (tropomyosin), 23 kDa (sarcoplasmic calcium-binding protein), and 42 kDa (arginine kinase), but also had two IgE-reactive bands with the molecular masses 69 and 81 kDa which were not reported in previous studies. It was possible that the bands were the dimer of tropomyosin and arginine kinase respectively, but it was not investigated in this study. It would be helpful if Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF-MS) is used to analyze the characteristic of these two proteins after purification.
SGF digestion assay was also performed and different time intervals were chosen to synchronize the conditions of food digestion in stomach. Phe or Tyr residues are the peptide bonds which can be cleaved by pepsin easily. Results from in vitro simulated digestion studies with purified proteins are routinely used to assess the sensitivity of potential allergens. These results showed that all of the protein bands from seven crustaceans had the same digestive pattern in SGF and comport with the previous studies, except the resistibility to digestion of tropomyosin (). The tropomyosin was more stable than the other proteins extracted from the selected species in SGF. These results suggest that the ability of tropomyosin to survive stomach digestion increases its potential ability to produce allergy. Similar results have been reported in other studies for mud crab (Scylla serrata),[Citation22] grass prawn (Penaeus monodon), and pacific white shrimp (Litopenaeus vannamei).[Citation23]
FUNDING
This work was supported by Nature Science Foundation of China (Grants 30800859), Earmarked Fund for Modern Agro-industry Technology Research System (nycytx-50-G09), Supporting Program in Public Area, Qingdao (09-1-1-6-nsh).
ACKNOWLEDGMENTS
The authors thank Dr. Chen Guan-zhi from Qingdao Medical College for initiating studies on the prevalence of seafood allergies in China. Also the assistance of Dr. M. Aslam Buzdar with the linguistics in this article is gratefully acknowledged.
REFERENCES
- FAO/WHO. Evaluation of Allergenicity of Genetically Modified Foods, in Report of a Joint FAO/WHO Expert Consultation of Allergenicity of Foods Derived from Biotechnology. World Health Organization, Geneva, 2001; 22–25.
- Lopata, A.L.; Lehrer, S.B. New insights into seafood allergy. Current Opinion in Allergy and Clinical Immunology 2009, 9 (3), 270–277.
- Leung, N.Y.; Wai, C.Y.; Shu, S.; Wang, J.; Kenny, T.P.; Chu, K.H.; Leung, P.S. Current Immunological and Molecular Biological Perspectives on Seafood Allergy: A Comprehensive Review, Clinical Reviews in Allergy and Immunology, 2012; 1–18.
- Burks, A.W.; Tang, M.; Sicherer, S.; Muraro, A.; Eigenmann, P.A.; Ebisawa, M.; Fiocchi, A.; Chiang, W.; Beyer, K.; Wood, R.; Hourihane, J.; Jones, S.M.; Lack, G.; Sampson, H.A. ICON: Food allergy. The Journal of Allergy and Clinical Immunology 2012, 129 (4), 906–920.
- Tsabouri, S.; Triga, M.; Makris, M.; Kalogeromitros, D.; Church, M.K.; Priftis, K.N. Fish and shellfish allergy in children: Review of a persistent food allergy. Pediatric Allergy and Immunology 2012, 23 (7), 608–615.
- Wild, L.G.; Lehrer, S.B. Fish and shellfish allergy. Current Allergy and Asthma Reports 2005, 5, 74–79.
- Shanti, K.N.; Martin, B.M.; Nagpal, S.; Metcalfe, D.D.; Rao, P.V. Identification of Tropomyosin as the major shrimp allergen and characterization of its IgE-binding epitopes. The Journal of Immunology 1993, 151 (10), 5354–5363.
- Daul, C.B.; Slattery, M.; Reese, G.; Lehrer, S.B. Identification of the major brown shrimp (Penaeus aztecus) allergen as the muscle protein tropomyosin. International Archives of Allergy And Immunology 1994, 105 (1), 49–55.
- Leung, P.S.; Chu, K.H.; Chow, W.K.; Ansari, A.; Bandea, C.I.; Kwan, H.S.; Nagy, S.M.; Gershwin, M.E. Cloning, expression, and primary structure of Metapenaeus Ensis tropomyosin, the major heat-stable shrimp allergen. Journal of Allergy and Clinical Immunology 1994, 94 (5), 882–890.
- Leung, P.S.C.; Chen, Y.S.; Mykles, D.L.; Chow, W.K.; Li, C.P.; Chu, K.H. Molecular identification of the lobster muscle protein tropomyosin as a seafood allergen. Molecular Marine Biology and Biotechnology 1998, 7, 12–20.
- Bauermeister, K.; Wangorsch, A.; Perono Garoffo, L.; Reuter, A.; Conti, A.; Falk, S.; Taylor, S.L.; Vieths, S.; Holzhauser, T.; Ballmer-Weber, B.K.; Reese, G. Novel crustacean allergens identified in North sea shrimp Crangon and other crustacean species-sarcoplasmic calcium-binding protein, troponin c, troponin i, triosephosphate isomerase, and myosin light chain. 2008. http://www.ncbi.nlm.nih.gov/protein/238477329 (accessed May 28, 2010).
- Shiomi, K.; Sato, Y.; Hamamoto, S.; Mita, H.; Shimakura, K. Sarcoplasmic calcium binding protein: Identification as a new allergen of the Black Tiger shrimp Penaeus monodon. International Archives of Allergy and Immunology. 2008, 146 (2), 91–98.
- Ayuso, R.; Grishina, G.; Ibáňez, M.D.; Blanco, C.; Carrillo, T.; Bencharitiwong, R.; Sánchez, S.; Nowak-Wegrzyn, A.; Sampson, H.A. Sarcoplasmic calcium-binding protein is an EF-hand-type protein identified as a new shrimp allergen. Journal of Allergy and Clinical Immunology 2009, 124 (1), 114–120.
- Ayuso, R.; Grishina, G.; Bardina, L.; Carrillo, T.; Blanco, C.; Ibaňez, M.D.; Sampson, H.A.; Beyer, K. Myosin light chain is a novel shrimp allergen, lit v 3. Journal of Allergy and Clinical Immunology 2008, 122 (4), 795–802.
- Yu, C.J.; Lin, Y.F.; Chiang, B.L.; Chow, L.P. Proteomics and immunological analysis of a novel shrimp allergen, Pen m 2. The Journal of Immunology 2003, 170 (1), 445–453.
- Garćıa-Orozco, K.D.; Aispuro-Hern´andez, E.; Yepiz-Plascencia, G.; Caldeŕon-de-la-Barca, A.M.; Sotelo-Mundo, R.R. Molecular characterization of arginine kinase, an allergen from the shrimp Litopenaeus Vannamei. International Archives of Allergy and Immunology. 2007, 144 (1), 23–28.
- Huang, Y.Y.; Liu, G.M.; Cai, Q.F.; Weng, W.Y.; Maleki, S.J.; Su, W.J.; Cao, M.J. Stability of major allergen tropomyosin and other food proteins of mud crab (Scylla serrata) by in vitro gastrointestinal digestion. Food and Chemical Toxicology 2010, 48 (5), 1196–1201.
- France, R.M.; Sellers, D.S.; Grossman, S.H. Purification, characterization, and hydrodynamic properties of Arginine Kinase from Gulf shrimp (Penaeus aztecus). Archives of Biochemistry and Biophysics 1997, 345 (1), 73–78.
- Yao, C.L.; Wu, C.G.; Xiang, J.H.; Dong, B. Molecular cloning and response to laminarin stimulation of Arginine Kinase in Haemolymph in Chinese shrimp, Fenneropenaeus Chinensis. Fish and Shellfish Immunology 2005, 19 (4), 317–329.
- Ortea, I.; Caňas, B.; Gallardo, J.M. Mass spectrometry characterization of species-specific peptides from Arginine Kinase for the identification of commercially relevant shrimp species. Journal of Proteome Research 2009, 8 (11), 5356–5362.
- Taylor, S.L.; Lehrer, S.B. Principles and characteristics of food allergens. Critical Reviews in Food Science and Nutrition. 1996, 36 (S1), 91–118.
- Huang, Y.Y.; Liu, G.M.; Cai, Q.F.; Weng, W.Y.; Maleki, S.J.; Su, W.J.; Cao, M.J. Stability of major allergen tropomyosin and other food proteins of mud crab (Scylla serrata) by in vitro gastrointestinal digestion. Food and Chemical Toxicology 2010, 48 (5), 1196–1201.
- Liu, G.M.; Huang, Y.Y.; Cai, Q.F.; Weng, W.Y.; Su, W.J.; Cao, M.J. Comparative study of in vitro digestibility of major allergen, tropomyosin and other proteins between grass prawn (Penaeus monodon) and Pacific white shrimp (Litopenaeus vannamei). Journal of the Science of Food and Agriculture 2011, 91 (1), 163–170.
- Greaser, M.L.; Gergely, J. Reconstitution of troponin activity from three protein components. Journal of Biological Chemistry 1971, 246 (13), 4226–4233.
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Analytical Biochemistry 1987, 150 (1), 76–85.
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227 (5259), 680–685.
- Smith, I.; Cromie, R.; Stainsby, K. Seeing gel wells well. Analytical Biochemistry 1988, 169 (2), 370–371.
- Towbin, H.; Gordon, J. Immuno blotting and dot immuno binding: Current status and outlook. Journal of Immunological Methods 1984, 72 (2), 312–340.
- Thomas, K.; Aalbers, M.; Bannon, G.A.; Bartels, M.; Dearman, R.J.; Esdaile, D.J.; Fu, T.J.; Glatt, C.M.; Hadfield, N.; Hatzos, C.; Hefle, S.L.; Heylings, J.R.; Goodman, R.E.; Henry, B.; Herouet, C.; Holsapple, M.; Ladics, G.S.; Landry, T.D.; MacIntosh, S.C.; Rice, E.A.; Privalle, L.S.; Steiner, H.Y.; Teshima, R.; VanRee, R.; Woolhiser, M.; Zawodny, J. A Multi-laboratory evaluation of a common in vitro pepsin digestion assay protocol used in assessing the safety of novel proteins. Regulatory Toxicology and Pharmacology 2004, 39 (2), 87–98.
- Leung, P.S.C.; Chow, W.K.; Duffey, S.; Kwan, H.S.; Gershwin, M.E.; Chu, K.H. IgE reactivity against a cross-reactive allergen in crustacea and mollusca: Evidence for tropomyosin as the common allergen. Journal of Allergy and Clinical Immunology 1996, 98 (5), 954–961.
- US Pharmacopoeia, the National Formulary, simulated gastric fluid and simulated intestinal fluid, TS. In: The United States Pharmacopeia 23, the National Formulary 18; The United States Pharmacopeial Convention Inc.: Rockville, MD, 1995; 2053.
- Leung, P.S.C.; Chen, Y.S.; Gershwin, M.E.; Wong, S.H.; Kwan, H.S.; Chu, K.H. Identification and molecular characterization of Charybdis Feriatus tropomyosin, the major crab allergen. Journal of Allergy and Clinical Immunology 1998, 102 (5), 847–852.
- Shanti, K.N.; Martin, B.M.; Nagpal, S.; Metcalfe, D.D.; Rao, P.V. Identification of tropomyosin as the major shrimp allergen and characterization of its IgE binding epitopes. The Journal of Immunology 1993, 151 (10), 5354–5363.
- Motoyama, K.; Suma, Y.; Ishizaki, S.; Nagashima, Y.; Shiomi, K. Molecular cloning of tropomyosins identified as allergens in six species of crustaceans. Journal of Agricultural and Food Chemistry 2007, 55 (3), 985–991.
- Bredehorst, R.; David, K. What establishes a protein as an allergen? Journal of Chromatography B: Biomedical Sciences and Applications 2001, 756 (1), 33–40.