Abstract
Culled plantain pulp starch, peel starch, and peel flour were characterized for their value-addition. Scanning electron microscopy articulated the smooth irregular with elongated and spheroid shaped granules of both culled plantain pulp and peel starch. Culled plantain pulp starch has the highest purity and contained ash (0.1 g/100 g), fat (0.02 g/100 g), protein (0.9 g/100 g), and crude fibre (0.1 g/100 g) with 98 g/100 g whiteness (L* value). The culled plantain contains 40 g/100 g amylose and 77 g /100 g resistant starch. Similar type of viscosity values were found in culled plantain pulp and peel starch with same pasting temperature (84.9°C) measured by Rapid Visco Analyzer. But, very low viscosity values were obtained in peel flour. Highest amount of swelling was 17.4 g/g in culled plantain pulp starch whereas highest amount of solubility (14.6 g/100 g) was recorded in peel flour at 90°C among three samples. Maximum water holding capacity (14.6 g/g) was found in culled plantain pulp starch, followed by 13.5 g/g in peel starch and 8.8 g/g in peel flour at 90°C. The high value of exudates from starch samples indicated unsuitability to use in frozen foods.
INTRODUCTION
Plantain (Musa paradisiaca Normalis) is one of the most important and abundantly produced fruits in developing tropical and subtropical countries. It is usually cultivated for its carbohydrate content and can be consumed as unripe fruit or when ripe.[Citation1] Undersized and partially injured plantains called “cull,” comprising more than 25 g/100 g during postharvest are removed from the bunch before marketing. On the other hand, plantain peels represent 40 g/100 g of the total weight of fresh bananas.[Citation2] These rejected plantains and peels are used as animal feed and some products such as chips, flakes, and powders in a limited extent due to the low value of such products. Huge amount of culled plantains and peels are disposed of improperly every day as waste which makes qualitative and quantitative loses and causes environmental pollution. Plantain starch has good potential acceptability because of its bland flavor and exhibits a limited digestibility due to their high resistant starch content (about 75 g/100 g) which controls the sugar and cholesterol level in blood.[Citation3] On the other hand, plantain peel powder contains 39.3 g/100 g starch[Citation4] including resistant starch and dietary fiber.
Plantain known as Kluai Namwa is the mostly grown variety in Thailand which is also grown in other Asian countries but in different names. Approximately 82 g/100 g of the total banana production in Thailand is Kluai Namwa (Musa ABB group)[Citation5] and is consumed domestically. It is sold in very low price during the peak season and hence, Kluai Namwa fruits become spoiled without proper utilization. There have been reports on the properties of plantain pulp starches[Citation6−Citation10] and on partial characterization of plantain peel flour.[Citation4,Citation11] But there is no report on the production and/or characterization of Kluai Namwa plantain peel starch or culled plantain pulp starch. Some chemical properties of Kluai Namwa plantain pulp starch have been studied but not much on functional properties. The purpose of this study is to produce and to characterize the culled plantain pulp starch, peel starch, and peel flour of Kluai Namwa variety for its appropriate and value-addition applications.
MATERIALS AND METHODS
Extraction of Starch from Culled Plantain Pulp and Peel
Kluai Namwa plantain aged 100–110 days (first stage of maturity) after anthesis was collected from a local market (Talad Thai, Pathumthani, Thailand). The last two bunches (under sized, called cull) having a green peel and sharp edges were collected for further extraction of starch. Bananas used for starch isolation are generally at the first stage of the ripening process.[Citation12] A modified method of Lii et al.[Citation13] was used to extract the starch from culled plantain pulp and peels. Injured portion (if any) of culled plantain was removed and peeled. The peeled pulp and peel were cut into slices (1.5 ± 0.3 cm thickness) and rinsed in a 1 g/100 g (w/v) citric acid solution. Slices were disintegrated with 0.05 g/100 g NaOH solution (1:2 ratios by volume). The ground pulp and peel were steeped for one and two hours, respectively. Water was added to the slurry (5:1 ratio by volume) and passed through 60 and 200 mesh screens to remove any fibrous materials. The slurry was washed with distilled water and left to settle the starch by gravity. Starch was dried at 40°C overnight in a hot air oven, was then ground and passed through 100 mesh sieves. Culled plantain pulp and peel starch were packed into high density polyethylene bags by vacuum sealing and stored in desiccators until further used.
Production of Flour from Plantain Peel
Plantain peels were cut into slices (1.5 ± 0.3 cm thickness) and dipped into 1 g/100 g (w/v) citric acid solution. Slices were dried overnight at 60°C by hot air oven. The flour was ground and passed through 40 mesh screen and packed into high density polyethylene bags by vacuum sealing and stored in desiccators for further use.
Chemical Analysis
Moisture content, ash, protein, fat, crude fiber, and amylose of culled plantain pulp starch, peel starch, and peel flour were determined by standard procedures of International Institute of Tropical Agriculture[Citation14] Carbohydrate content was calculated by subtracting protein, lipids, ash, and fiber content of the sample from 100. Amylopectin was obtained by subtracting amylose content from 100. Resistant starch and non-resistant starch of the samples were calculated according to McCleary and Monaghan,[Citation15] with some modifications. Samples were incubated in a shaking water bath with 1 M sodium maleate buffer (pH 6.0) having pancreatic α-amylase (10 mg/ml) and amyloglucosidase (3 U/ml) for 16 h at 37°C to solubilise and hydrolyse the non-resistant starch part to glucose. The enzymatic reaction was stopped by adding ethanol and the resistant starch was obtained as pellet by centrifugation (at 3000 rpm for 10 min at room temperature). The pellets were further washed with ethanol (50 g/100 g v/v) and centrifuged at 3000 rpm for 10 min at room temperature. Resistant starch pellets were suspended in 2 M KOH, followed by addition of 1.2 M sodium acetate buffer pH 3.8 (8 ml) and 0.1 ml of amyloglucosidase (3300 U/ml). The mixture samples were further incubated at 50°C with continuous shaking for 30 min. The liberated glucose was determined by the glucose oxidase assay.
Color Spectra Analysis
Color of culled plantain pulp starch, peel starch, and peel flour was measured by a Hunter Lab Spectrocolorimeter (Model TC-P III A, Tokyo Denshoku Co., Ltd., Japan). Samples (10 g) were kept in the glass container of the instrument and put over the slit of the equipment for taking photographs. CIE Lab system was used where L* (L* = null means black and L* = 100 means white), a* (−a* = greenness and + a* = redness) and b* (−b* = blueness and + b* = yellowness) values were measured.
Microstructure Analysis
Micro structural images of culled plantain pulp starch, peel starch, and peel flour were acquired by JSM-6301F Scanning Electron Microscope (SEM) according to Nwokocha and Williams.[Citation8] The sample particles were sprayed over the adhesive carbon tape fixed with a circular brass stub. Then it was kept in a vacuum chamber and when reaching the vacuum of 8 Pa and plasma current of 15 mA, the gold splattering on the particles was done for 90 s. The stub with gold splattered samples was then kept in the SEM chamber for imaging. While the size of the granules was determined by using optical microscope connected with digital camera and calibrated micrometer scale. For the measurement of size, 100 particles were counted for each sample.
Fourier Transform Infrared (FT-IR)
Infrared spectra of the culled plantain pulp starch, peel starch, and peel flour were measured according to Nasrin and Anal[Citation16] on an attenuated total reflectance (ATR) FT-IR (Nicolet Avatar 360) with spectra-Tech HATR accessory. Powdered samples were used directly for measurement of spectrum from 500 to 4000 cm−1 wave numbers. Each spectrum was the average of eight scans. All measurements were conducted under ambient conditions.
Pasting Properties
Pasting properties of the culled plantain pulp starch, peel starch and peel flour were tested according to Li et al.[Citation17] by Rapid Visco Analyzer (RVA) (Model 4, Newport Scientific Pvt., Ltd. Australia). Samples (2.5 g) were kept into the canister and mixed thoroughly with 25 ml of distilled water. The sample suspension was treated at 50°C for one min and then temperature was increased until reached to 95°C and kept for 3.2 min and finally, decreased to 50°C. Samples were mixed and homogenized with 960 rpm for 10 s during starting of test, and then speed was reduced to 160 rpm and continued it throughout the test. The total analysis time was 13 min.
Swelling Power and Solubility
The swelling power and solubility were calculated according to the method of Konik et al.[Citation18] and Koksel et al.[Citation19] with slight modifications. The sample (1 g) was dispersed in 50 ml distilled water placed in the centrifuge tubes. The tubes were then heated in a water bath at different temperatures (60–95°C) for 30 min with continuous stirring. After cooling to room temperature the tubes were centrifuged (model EBA 8S, Hettich, Germany) at 3000 rpm for 15 min. The supernatant was dried at 105°C about 5 h for determining solubility. Alternatively, the weight of the wet sample (sediments) into the test tube was taken to determine the swelling power. The swelling power and solubility were calculated by using the following formula:
Water Holding Capacity
Water holding capacity was determined according to the method of Rodriguez-Ambriz et al.[Citation20] with some modifications. Sample (1 g) was dispersed in 50 ml distilled water in the centrifuge tubes. The tubes were heated in a water bath at different temperatures (40–90°C) for 30 min with continuous stirring. After cooling to room temperature, the tubes were centrifuged at 3000 rpm for 20 min. The supernatant was discarded and the paste was weighed, dried, and the water holding capacity was determined according to the following formula:
Freeze–Thaw Stability
The freeze–thaw stability was calculated following the method described by Singhal and Kulkarni[Citation21] with some modifications. Starch or flour (5 g/100 g in distilled water) solution was heated at 95°C for 30 min with constant stirring. Paste (10 ml) was placed into centrifuge tubes, cooled, and treated with alternate freezing and thawing cycles (18 h and 3 h, respectively) for eight consecutive days. After each thawing, tubes were centrifuged at 5000 rpm for 10 min at room temperature. The percentage of water exudates was calculated by the difference of the weight of paste between two consecutive cycles.
Statistical Analysis
The experimental data was analyzed with MSTAT-C. Comparisons among the samples were done by Duncan’s Multiple Range Test (DMRT) at 95% confidence level.
RESULTS AND DISCUSSION
Chemical Composition
Chemical composition of culled plantain pulp starch, peel starch, and peel flour are shown in . The yield of plantain peel flour was 12.9 g/100 g (w/w), followed by 6.6 g/100 g (w/w) of culled plantain pulp starch and 0.7 g/100 g (w/w) of peel starch. Nwokocha and Williams[Citation8] found that the yield of white and yellow plantain pulp starch was 4.5 and 6.8 g/100 g, respectively. These results are similar to the yield of 6.57 g/100 g for culled plantain pulp starch, but Perez-Sira[Citation22] found the higher values of plantain pulp starch yield between 10 and 12 g/100 g for the samples. The moisture content was 10 g/100 g (w/w) for all the samples.
TABLE 1 Yield and compositions of culled plantain pulp starch, peel starch, and peel flour
Ash contents of culled plantain pulp starch, peel starch, and peel flour were 0.1 g/100 g, 0.2 g/100 g, and 9.7 g/100 g respectively. The ash content of culled plantain pulp starch was lower than that reported by Bello-Pe´rez et al.[Citation9] representing 1.3 g/100 g in macho and 0.4 g/100 g in criollo unripe banana pulp starch. On the other hand, Nimsung et al.[Citation6] reported 0.06 g/100 g ash in different variety of Thai plantain pulp starches. The ash content of plantain and banana peels from 6.4 g/100 g to 12.8 g/100 g was reported by Emaga et al.[Citation4] Small amounts of fat were recorded from culled plantain pulp and peel starch while 6 g/100 g from peel flour. Nwokocha and Williams[Citation8] reported 0.3 g/100 g and 0.4 g/100 g fat in white and yellow plantain pulp starch, respectively. Protein content in culled plantain pulp starch was 0.9 g/100 g that is lower than that reported by Bello-Pe´rez et al.[Citation9] Peel flour had 4.2 g/100 g protein whereas Ramli et al.[Citation11] reported 7.1 g/100 g and 7.3 g/100 g protein in that of Cavendish and Dream green banana, respectively. The amylose content was 39.8 g/100 g in culled plantain pulp starch, 32.5 g/100 g in peel starch, and 17.6 g/100 g in peel flour. Vatanasuchart et al.[Citation3] also reported 43.8 g/100 g amylose in the starch from Kluai Namwa plantain. C-1: Avoid % for concentration, use more clear? g/100 g starch etc.
Maximum (76.8 g/100 g) resistant starch was recorded in culled plantain pulp starch followed by 63.8 g/100 g in peel starch and 8.6 g/100 g in peel flour. There was 75.6 g/100 g resistant starch and 88.9 g/100 g total starch in Kluai Namwa plantain pulp starch.[Citation3] The total starch content was almost same for plantain pulp and peel starch that was approximately eight times more than that of peel flour representing 11.7 g/100 g only. The resistant starch and total starch content of green banana peel flour were 8.2 g/100 g and 11.5 g/100 g, respectively.[Citation11] The chemical compositions observed in this research are not similar in all cases as reported in previous reports. These differences may be due to the variety of plantain, stage of maturity, and production procedures. All the previous studies were conducted with either unripe or ripe banana pulp, whereas this study focused on culled unripe banana pulps and peels which are the wastes from banana processing industries.
Microstructure Analysis
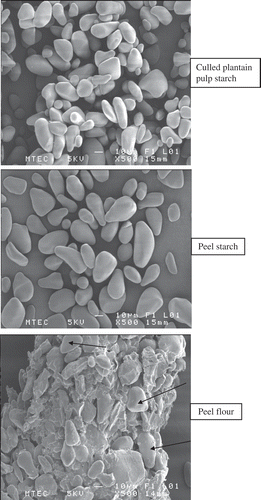
The shape of starch particles from culled plantain pulp and peel starch is similar, smooth with elongated and spheroid shaped as observed from . Particle size diameter of peel starch was ranged from 10 to 44 μm and from 19 to 61 μm for pulp starch. Yellow plantain pulp starch had elliptical smooth granules with 11–41 μm sizes.[Citation8] Some particles found in peel flour were bigger (152.99 μm) and irregular shape with rough surfaces.[Citation10]
FT-IR Analysis
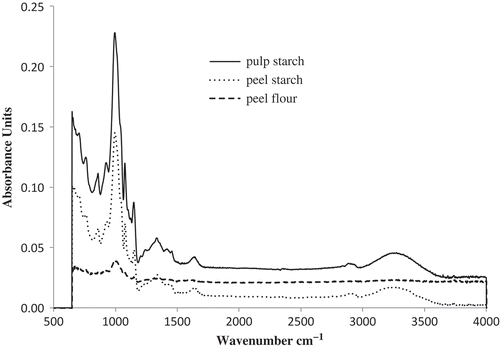
FT-IR spectra of culled plantain pulp starch, peel starch, and peel flour are illustrated in . The entire spectra of starch can be divided into three regions, the OH- stretching region (3560–3000 cm−1), the C-H stretching region (3000–2800 cm−1), and the fingerprint region (1600–650 cm−1).[Citation23] In all spectra, the region below 800 cm−1 demonstrated composite variational mode as a result of the pyranose rings in the glucose unit. The peak representing the properties of anhydrous glucose ring O-C stretching region at around 987 cm−1 to 1006 cm−1 wave number was found in culled plantain pulp starch, 981 cm−1 to 1016 cm−1 in peel starch, and between 1025 cm−1 and 973 cm−1 in peel flour. The highest peak was shown in culled plantain pulp starch followed by peel starch and peel flour. Fanga et al.[Citation24] found the peak for O-C bond at 990 to1030 cm−1 wave number in potato starch. Peak of OH- bond was detected at 3216 cm−1 to 3297 cm−1 in culled plantain pulp starch and at 3212 cm−1 to 3266 cm−1 in peel starch. In the case of peel flour, broader peak ranges from 3187 cm−1 to 3355 cm−1 wave numbers was found. In pre gelatinized native starch, Irudayaraj et al.[Citation25] observed the absorbance peak at 3389 cm−1 and 2930 cm−1 would be allocated to OH- and C-H bond, respectively in FT-IR analysis.
Color Spectra Analysis
Any color substance or presence of other components in starch reduces its quality and acceptability.[Citation26] A little value of chroma and a high value of whiteness are preferred for the starch to gain the preference of the consumer. The highest value of whiteness (L* = 97.8) was found in culled plantain pulp starch followed by L* = 93.3 in peel starch and L* = 60.8 in peel flour which were also statistically differences at 5% level of significance as indicated in . Alternatively, highest value of chroma (a* = 2.6 and b* = 18. 6) was contained by peel flour than peel starch (a* = 0.8 and b* = 5.4) and culled plantain pulp starch (a* = 0.08 and b* = 2.2), accordingly. The color values of L* = 97.9, a* = –0.2, and b* = 0.8 were found in cassava starch.[Citation27] In case of banana peel flour, the range of color values were (L* = 34.8–48.7, a* = 3.8–6.4, and b* = 21–27).[Citation28]
TABLE 2 Color values of culled plantain pulp starch, peel starch, and peel flour
Pasting Properties
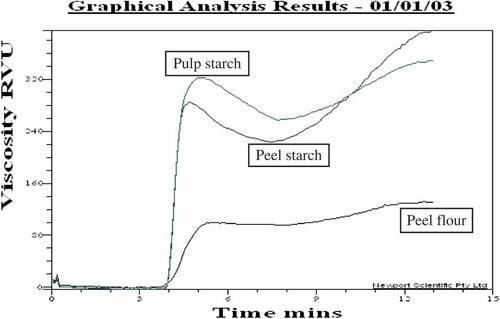
Pasting properties of culled plantain pulp starch, peel starch, and peel flour analysed by RVA are illustrated in and . Pasting properties of starch solution during heating represents the changes of viscosity, swelling, transparency, solubility, loss of birefringence, etc. For culled plantain pulp starch, pasting was initiated at 84.9°C, reached a peak viscosity at 315.7 RVU in 5 min, retained at 95°C the viscosity fell to 252.1 RVU and finally attained to 377.9 RVU at 50°C. A similar type of pasting profile was found for peel starch with statistically lower values (of peak viscosity, trough, break down viscosity, and peak time) than that of culled plantain pulp starch. The peak viscosity, breakdown, setback, and final viscosity were found in the range of 356.8–432.5, 118.2–426.6, 74.58–396.9, and 361.1–542.9 RVU, respectively for different varieties of Thai plantain pulp starch.[Citation6] In the case of peel flour, at 83.8°C pasting started and after 5.4 min, it gained the peak viscosity (103.6 RVU), that is less than half of the culled plantain pulp and peel starch. This is due to pulp and peel starch being mostly composed of starch particles which swell significantly during heating with water and finally increases viscosity, granule rupture, granule alignments, etc.[Citation29] Peel flour is made up of different particles which generally do not puff up much.
TABLE 3 Pasting properties of plantain pulp starch, peel starch, and peel flour by RVA
Swelling Power and Solubility
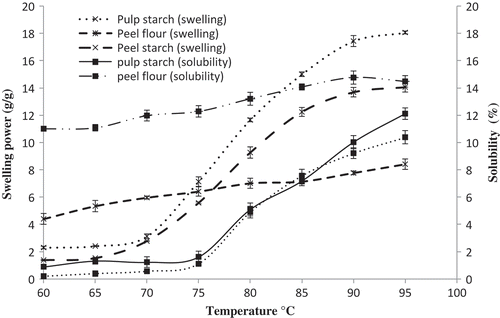
The swelling and solubility trends of culled plantain pulp starch, peel starch and peel flour are shown in . Swelling and solubility indicate the extent of interaction within the amorphous and crystalline areas of starch granules that greatly depends on amylose-amylopectin ratio, degree of branching, length of branches, configuration of the molecules, etc.[Citation30] Lipid-amylose complex and protein-amylose complex also confine swelling and solubility of granules.[Citation31] Culled plantain pulp and peel starch had two stages (from 60–70°C and from 75–95°C) of swelling curve while peel flour had a single stage as shown in . At 60°C, small amount of swelling (only 2.3 g/g and 1.7 g/g) had occurred while 17.4 g/g and 13.9 g/g were observed at 90°C for culled plantain pulp and peel starch, respectively. These values specify that pulp starch contains a weaker granule structure than peel starch. At first stage of swelling, starch granules obtain thermal energy during the initial period of heating which loosen the intra-granular bonds and the granules soak up water and swell; the lower molecular weight amylose solubilises and leaches out of the granule into the surrounding medium. This augments the thermal shakeup creating the fragile internal bonds, and finally in the second stage, enormous swelling happens. In peel flour, a gradual swelling was observed from 5 g/g at 60°C and 8.2 g/g at 95°C. The complex formation between proteins (4.6 g/100 g), lipid (6 g/100g) with the amylose presence in peel flour confined its swelling.
The solubility of culled plantain pulp starch, peel starch, and peel flour followed the same trends like the swelling power as shown in the . The same amount of solubility was observed at 75 to 85°C in culled plantain pulp and peel starch while at 95°C, the values were 11.9 and 10.1 g/100 g, respectively. Maximum (14.6 g/100 g) solubility was recorded in peel flour among the three samples at 90°C. The swelling power and solubility of plantain pulp starches were around 17.5 g/g and 7 g/100 g, respectively at 90°C.[Citation8]
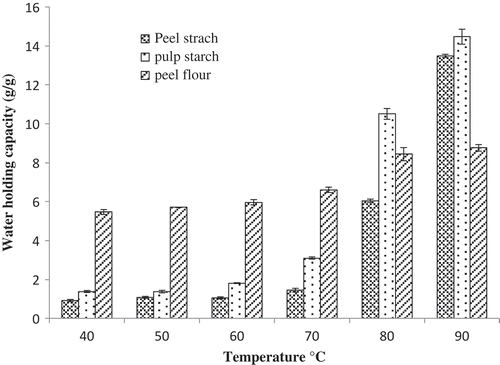
Water Holding Capacity
Water holding capacity is greatly affected by the physical conditions of starch molecules besides dietary fibre, protein, and percentage of amylose in the sample.[Citation20] The water holding capacity of culled plantain pulp starch, peel starch, and peel flour was presented at different temperatures from 40 to 90°C (). Two stages of the water holding capacity graph was observed with culled plantain pulp and peel starch and one stage for peel flour, same as for swelling power and solubility. Maximum water holding capacity (14.6 g/g) was found in culled plantain pulp starch, followed by 13.5 g/g in peel starch, and 8.8 g/g in peel flour at 90°C (maximum temperature). According to Kannadhason and Muthukumarappan,[Citation32] breakdown of starch molecules is enhanced with increasing moisture contents and temperatures by the development of more expansible matrix which consequences in elevated water holding capacity. Water holding capacity of ripe and green banana peel flour was amplified with temperature and the values were from 4.1 to 8.2 g/g, respectively.[Citation28]
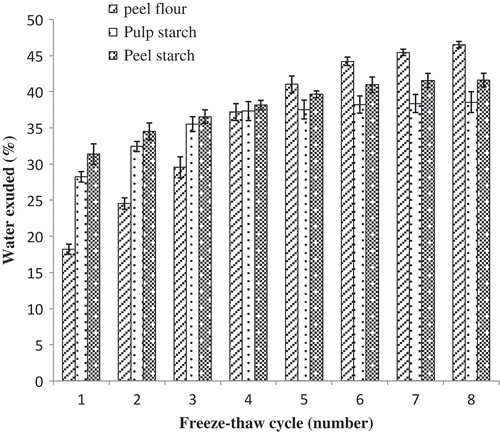
Freeze–Thaw Stability
Freeze-thaw stability is an important property of starch, and provides information for its suitability to produce frozen products. Reformation of starch molecules during freezing may result in syneresis affecting the functional properties such as viscosity or in gel behavior.[Citation17] No syneresis (freeze-thaw stable) or very a small amount of syneresis of a starch paste indicate that it can be applied in frozen foods. illustrates the freeze-thaw stability of culled plantain pulp starch, peel starch, and peel flour at different alternate freeze-thaw cycles. The exudates increased with increasing number of freeze-thaw cycles. At the first cycle, peel starch showed the maximum amount of syneresis (31.4 g/100 g), followed by 28.2 g/100 g for culled plantain pulp starch, and 18.2 g/100 g for peel flour. The high value of exudates from starch samples indicated unsuitability in frozen foods. The exudates were 14.4 g/100 g and 26.5 g/100 g from yellow and white plantain pulp starch, respectively at the first freeze-thaw cycle.[Citation8]
CONCLUSIONS
Culled plantain pulp starch and peel starch may satisfy the consumer due to the presence of low amounts of protein, fat, ash, crude fiber, and especially the absence of colorant. Comparatively, larger starch granules (19 to 61 μm) were found in culled plantain pulp starch than that of peel starch (10 to 43 μm) with similar type of smooth oval shape in most cases. High amounts of amylose (39.8 g/100 g), resistant starch (76.83 g/100 g), and larger granule size may make the culled plantain pulp starch as well as peel starch an important ingredient in low glycemic index food products that may be used for both human beings and animals. Peel flour can be used in animal feed, as it contains 6 g/100 g fat and 4.2 g/100 g protein. High pasting temperature (84.9°C), peak viscosity (315.7 RVU), swelling power (18.1 g/g), water holding capacity (14.1 g/g), and solubility (12.1 g/100 g) of pulp starch are required in the products which need high temperature during processing such as canned food, soup, sauce, and baby food. Peel starch also can be used to produce these products as it has the above mention colloidal properties with similar, or slightly higher or lower values. Being less freeze-thaw stable, pulp and peel starch are not suitable in any frozen food production. Resistant starch (Type III) produced from plantain waste can be used in encapsulation of functional food preparation to enhance the stability and targeted delivery of bioactive molecules. Research is being conducted for similar applications of starch obtained from plantain waste. As no literature has been found on plantain peel starch, similar and more research can be done on this topic to verify these results and the proper utilization of it.
REFERENCES
- Ahenkora, K.; Kyei, M.A.; Marfo, E.K.; Banful, B. Nutritional composition of False Horn Apantupa plantain during ripening and processing. African Crop Science Journal 1996, 4, 243–247.
- Tchobanoglous, G.; Theisen, H.M., Vigil, S.A. Integrated Solid Waste Management: Engineering Principals and Management Issues, McGraw-Hill: New York, 1993, 3–22.
- Vatanasuchart, N.; Niyomwit, B.; Wongkrajang, K. Resistant starch content in vitro starch digestibility and physico-chemical properties of flour and starch from Thai Bananas. Maejo International Journal of Science and Technology 2012, 6 (2), 259–271.
- Emaga, T.H.; Andrianaivo, R.H.; Wathelet, B.; Tchango, J.T.; Paquot, M. Effects of the stage of maturation and varieties on the chemical composition of banana and plantain peels. Food Chemistry 2007, 103, 590–600.
- Boonyanuphap, J.; Wattanachaiyingcharoen, D.; Sakurai, K. GIS-based land suitability assessment for Musa (ABB group) plantation. Journal of Applied Horticulture 2004, 6 (1), 3–10.
- Nimsung, P.; Thongngam, M.; Naivikul, O. Compositions, morphological, and thermal properties of green banana flour and starch. Kasetsart Journal: Natural Science 2007, 41, 324–339.
- Nunez-Santiago, M.C.; Bello-Perez, L.A.; Tecante, A. Swelling-solubility characteristics, granule size distribution, and rheological behavior of banana (Musa paradisiaca) starch. Carbohydrate Polymer 2004, 56, 65–75.
- Nwokocha, L.M.; Williams, P.A. Some properties of white and yellow plantain (Musa paradisiaca, Normalis) starches. Carbohydrate Polymer 2009, 76 (1), 133–138.
- Bello-Pe´rez, L.A.; Agama-Acevedo, E.; Sanchez-Hernandez, L.; Paredez-Lopez, O. Isolation and partial characterization of banana starches. Journal of Agricultural and Food Chemistry 1999, 47 (3), 854–857.
- Eggleston, G.; Swennen, R.; Akoni, S. Physicochemical studies on starches isolated from plantain cultivars, plantain hybrids, and cooking bananas. Starch/Starke 1992, 44, 121–128.
- Ramli, S.; Ismail, N.; Fadhl, A.; Alkarkhi, M.; Easa, A.M. The use of principal component and cluster analysis to differentiate banana peel flours based on their starch and dietary fibre components. Tropical Life Science Research 2010, 21 (1), 91–100.
- Von Loesecke, H.W. Banana: Chemistry, Physiology, Technology, 2nd Ed; Interscience: New York, 1950, pp. 173.
- Lii, C.Y.; Chang, S.M.; Young, Y.L. Investigation of the physical and chemical properties of banana starches. Journal of Food Science 1982, 47, 1493–1497.
- International Institute of Tropical Agriculture. Selected Laboratory Methods for Maize Quality Evaluation. International Institute of Tropical Agriculture, (IITA) Press: Ibadan, Nigeria, 1995, 1–60.
- McCleary, B.V.; Monaghan, D.A. Measurement of resistant starch. Journal of AOAC International 2002, 85 (3), 665–675.
- Nasrin, T.A.A.; Anal, A.K. Resistant starch III from culled banana and its functional properties in fish oil emulsion. Food Hydrocolloids 2013, doi: 10.1016/j.foodhyd.2013.06.019.
- Li, G.; Zeng, J.; Gao, H; Li, X. Characterization of phosphate monoester resistant starch. International Journal of Food Properties 2011, 14 (5), 978–987.
- Konik, C.M.; Kiskelly, D.M.; Gras, P.W. Starch: Swelling power, grain hardness, and protein relation to sensory properties of Japanese noodles. Starch/Starke 1993, 45, 139–144.
- Koksel, H.; Basman, A.; Kahraman, K.; Ozturk, S. Effect of acid modification and heat treatments on resistant starch formation and functional properties of corn starch. International Journal of Food Properties 2007, 10, 691–702.
- Rodrıguez-Ambriz, S.L.; Islas-Hernandez, J.J.; Agama-Acevedo, E.; Tovar, J.; Bello-Perez, L.A. Characterization of a fibre-rich powder prepared by liquefaction of unripe banana flour. Food Chemistry 2008, 107, 1515–1521.
- Singhal, R.S.; Kulkarni, P.R. Some properties of Amaranthus Paniculatas (Rajgeera) starch pastes. Starch/Starke 1990, 42 (1), 5–7.
- Perez-Sira, E. Characterization of starch isolated from plantain (Musa Paradisiaca normalis). Starch/Starke 1997, 49, 45–49.
- Santha, N.; Sudha, K.G; Vijayakumari, K.P.; Nayar, V.U.; Moorthy, S.N. Raman and infrared spectra of starch samples of sweet potato and cassava. Journal of Chemical Sciences 1990, 102 (5), 705–712.
- Fanga, J.M.; Fowler, P.A.; Tomkinson, J.; Hill, C.A.S. The preparation and characterization of a series of chemically modified potato starch. Carbohydrate Polymer 2002, 47 (3), 245–252.
- Irudayaraj, J.; Yang, H. Depth profiling of a heterogeneous food-packing model using step-scan Fourier Transform Infrared photoacoustic spectroscopy. Journal of Food Engineering 2002, 55, 25–33.
- Galvez, F.C.F.; Resurreccion, A.V.A. Reliability of the focus group technique in determining the quality characteristics of Mung bean noodles. Journal Sensory Study 1992, 7, 315–326.
- Oduro-Yeboah, C.; Johnson, P.N.T.; Sakyi-Dawson, E.; Budu, A. Effect of processing procedures on the colorimetry and viscoelastic properties of cassava starch, flour, and cassava-plantain fufu flour. International Food Research Journal 2010, 17, 699–709.
- Alkarkhi, A.F.M.; Ramli, S.B.; Yong, Y.S.; Easa, A.M. Physicochemical properties of banana peel flour as influenced by variety and stage of ripeness: Multivariate statistical analysis. Asian Journal of Food and Agro-Industry 2003, 3 (3), 349–362.
- Adewole, E.; Oso, O.A.; Talabi, J.Y.; Ogunmodede, O.T.; Ajiboye, B.O.; Ojo, O.A.1.; Onikanni, S.A.1.; Adewumi, F.D. Pasting characteristics of plantain (Balbisiana hybrids) and banana (Musa acuminata) starches. Der Chemica Sinica 2012, 3 (2), 313–317.
- Ratnayake, W.S.; Hoover, R.; Warkentin, T. Pea starch: Composition, structure, and properties-A review. Starch/Starke 2002, 54, 217–234.
- Han, X.Z.; Hamaker, B.R. Partial leaching of granule-associated proteins from rice starch during alkaline extraction and subsequent gelatinization. Starch/Starke 2002, 54, 454–460.
- Kannadhason, S.; Muthukumarappan, K. Effect of starch sources on properties of extrudates containing DDGS. International Journal of Food Properties 2010, 13, 1012–1034.