Abstract
The Citrus L. genus is a major source of limonene, which includes vitamin C and can be used in food and traditional medicinal plants. The polymerization method has been employed to study the preparation of Citrus nanocapsules by employing the polyesteric triblock copolymer polyethylene glycol–poly butylene adipate–polyethylene glycol in the shell, and olive oil is used as the core. In our current study, a specific amount of polymer, limonene, and olive oil were initially mixed first with acetone and ethanol and then added to water. The solvent was then removed from under vacuum and put in a freeze dryer, resulting in the residual nanocapsules being produced. Our study indicated that the size of the nanocapsules was a function of a variety of factors, such as the concentration of oil, polymer, and plant extract. Finally, we used scanning electron microscopy, particle size analysis report, Fourier-transform infrared spectroscopy, and nuclear magnetic resonance to visualize the nanocapsules.
INTRODUCTION
Monoterpenes are important chemical constituents found in the essential oils of many plants. They have widespread applications in medicine and flavoring.[Citation1,Citation2] Nanocapsules coated with medicinal plant extracts have multiple applications in the drug manufacturing process. In biological systems, pectins serve the role of cement in the cell wall of higher plants and are present in variable amounts and qualities in fruits. Globally, the major pectin producers are located in the citrus fruit producing regions of Europe and Latin America. Pectin is isolated from the citrus peel, orange peel, and from the residues following the extraction of citrus juice, citrus oil, and apple juice. Hot aqueous mineral acid is used to extract the raw material with the objective of obtaining a high concentration of pectins with a high molar mass. A wide range of pectins are available in the market.[Citation3,Citation4] Finally, increasing the content of the drug load increases the rate of drug release.[Citation5,Citation6] In the fourth type of copolymers, i.e., star block copolymers, unit A has a multi-arm functionality and copolymerizes with the blocks of B, creating a star-like shape. The number of arms of the star block copolymer depend on the number of functional groups on block A.[Citation7] The synthetic structure leading to the formation of the copolymers should possess several properties for the block copolymers to have well-defined properties and structure. First, the molecular weight of the backbone chain/arm of the star block copolymers, along with the single unit block, should be adjustable. Second, the copolymers, polymer block, and arms of the star block should have a very narrow molecular weight distribution. Third, the types, numbers, and arrangements of the polymer blocks and the arms of the star block should be known and the chemical structure should be as uniform as possible. Finally, the polymer should not contain any nonremovable impurities.[Citation8]
Over the past 30 years, scientists have conducted extensive research to synthesize desirable biodegradable block copolymers to be used for drug delivery. For this purpose, hydro gels, nanoparticles, micro particles, implants, and micelles have all been investigated. Block copolymers are advantageous because they can be polymerized by a large number of well-known polymerization techniques, including anionic, radical, cationic, ring opening, photo, group transfer, and Ziegler/Natta polymerization. Living polymerization is the most widely used technique because it allows for the molecular weight of the individual blocks (variation of initiator monomer/monomer ratio), as well as the block arrangement (AB, ABA, BAB), to be adjusted as required.[Citation9,Citation10] Various properties of block copolymer micelles render them to be suitable drug carriers. First, hydrophobic drugs can be physically encapsulated by the core of the block and carried at rates that exceed their intrinsic water solubility rate. Second, hydrophilic blocks can form hydrogen bonds within the aqueous surrounding, which creates a tight shell around the micelle core.[Citation11] Furthermore, nanoemulsions have uniform and extremely small droplet sizes, typically in the range of 20–200 nm. In addition, high kinetic stability, low viscosity, and optical transparency make them an attractive system for industrial applications.[Citation12] Surfactants stabilize nanoemulsion droplets, and the application of low-energy emulsification methods for the preparation of nanoemulsions stabilized by nonionic surfactants has been widely studied.[Citation13] An oil-in-water emulsion was fabricated using a conventional emulsification technique, and the subsequent addition of water to the system causes the solvent to diffuse from emulsion droplets, which results in the formation of nanocapsules.[Citation14−Citation17]
MATERIALS AND METHODS
Materials
Adipoyl chloride, butylene glycol, polyethylene glycol 2000, triethylamine, diethyl ether (purchased from Labscan Ltd., Dublin, Ireland), Citrus extract, acetone, and pure water were used in this study.
Plant Material
In this study, citrus (300 g) (Voucher No. 1860) fruits were gathered from Amool (15 km) (Mazandaran Province), Iran, in June 2012. Voucher specimens were deposited at the Herbarium of the Research Institute of Forests and Rangelands (TARI), Tehran, Iran. The fruits were peeled to separate the external part, resulting in a yield of 45% (w/w) of the peel with respect to the whole fruit; this material was employed in the extraction process.
Oil Isolation
Hydro-distillation with a Clevenger-type apparatus was employed to extract the fresh citrus. The essential oils were collected, dried under anhydrous sodium sulfate, and stored at 4°C until used.
Analysis
The 1H–13C-NMR spectra were obtained in CDCl3 on a Bruker DRX-500 NMR spectrometer operating at 500 and 125 MHz. The chemical shift values have been reported as ppm (δ) using tetramethylsilane (TMS) as the internal standard, whereas coupling constants are expressed in Hz.
Synthesis of the Triblock Copolymer Polyethylene Glycol–Poly Butylene Adipate–Polyethylene Glycol (PEG–PBA–PEG)
First, 1.7 mL of adipoylchloride and 9.8 mL of butylene glycol were poured into a balloon with a magnetic stirrer at 85°C and three drops of triethylamine were added to the mixture. The reaction was conducted for approximately 24 h, until the release of HCl stopped. An additional amount of PEG (2000) was then added to the prepared polyester at this stage and the reaction was maintained at 85°C. The reaction was identified to be complete when no further HCl was released. In the subsequent stage, 40 mL of diethylether was added to the balloon at 15°C, followed by three washes with distilled water to wash the deposits. Finally, the resulting white copolymer was centrifuged and then dried at a reduced temperature for 10 h.
Plant Materials and Extraction and Isolation
Limonene was isolated and purified from the aerial parts of Citrus.[Citation18] IR spectra were recorded in KBr using a Pye infrared spectrometer, and a Perkin-Elmer Polarimeter Model 241 MC was employed to obtain specific rotations at an ambient temperature.
Preparation of Core-Shell Nanoparticles by Emulsion Diffusion
Synthesis of PEG–PBA–PEG Nanocapsules Containing the Extract in the Absence of the Surfactant
First, a specific amount of the PEG–PBA–PEG copolymer and olive oil were dissolved in 10 mL of acetone, whereas limonene was separately dissolved in 10 mL ethanol. The two mixtures were then combined to create an organic solution. This organic solution was added drop-wise to 100 mL of water being stirred at room temperature. A suspension solution was then prepared and exposed to ultrasound, following which the solvents were released under vacuum and the suspension solution containing the nanocapsules was passed through a syringe-shaped filter containing cellulose acetate. This helped separate the stacked mass without contributing to the extraction in the nanoparticles.
Synthesis of Nanocapsules with Different Concentration of Oil
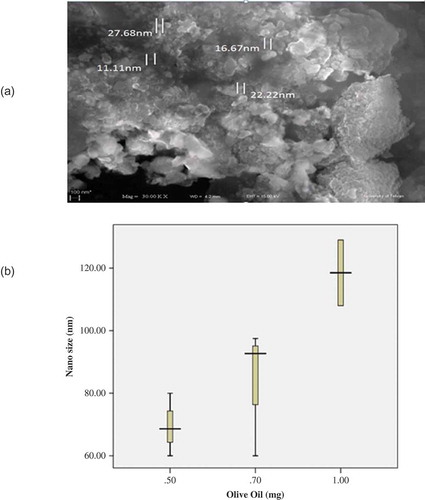
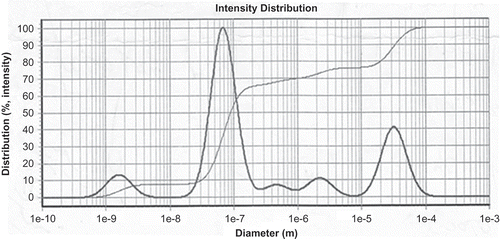
To study the effects of oil concentration on the behavior of the nanocapsules, three samples containing different concentrations of oil based on the weight ratio of oil/polymer were prepared, and 3.0 mg of polymer and 0.1 mg of extract were used in the samples. The polymer and oil were then dissolved in 10 mL of acetone and the extract was dissolved in 10 mL of ethanol. They were then added drop-wise to 100 mL of water while stirring.[Citation18] The results are presented in and .
TABLE 1 Result of nanoparticle size for different concentrations of oil [polymer/oil (1/3)]
Synthesis of Nanocapsules with Different Concentrations of Polymer
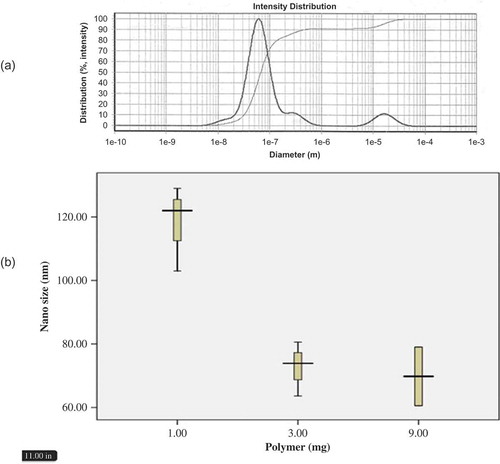
To study the effects of polymer concentration on the behavior of the nanocapsules, four samples containing different concentrations of polymer were prepared by maintaining a constant concentration of the oil and extract at 0.5 and 0.1 mg, respectively. The polymer concentration was then set at 1.0, 5.0, 9.0, and 10.0 mg. The polymer and oil were dissolved in 10 mL of acetone and the extract was dissolved in 10 mL of ethanol. They were then added drop-wise to 100 mL of the aqueous phase. The results are presented in and .
TABLE 2 Result of nanoparticle size for different concentrations of polymer [extract/oil (1/5)]
Synthesis of Nanocapsules with Different Limonene Concentrations in Citrus
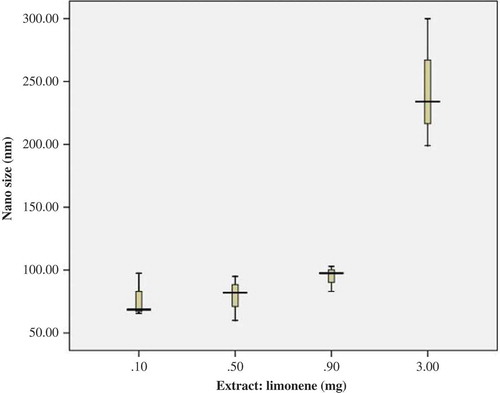
To study the effect of the drug on the behavior of nanocapsules, four samples with different amounts of the Citrus extract were prepared. In these nanocapsules, the amount of the polymer and oil was fixed, whereas the concentration of the extract was altered. The amount of polymer and oil was set at 3.0 and 0.5 mg, respectively, whereas the concentration of the extract in the samples was set at 0.1, 0.5, 0.9, and 3.0 mg. The polymer and oil were dissolved in acetone and the extract was dissolved in ethanol and then added drop-wise to 100 mL of the aqueous phase. The results are presented in and and .
TABLE 3 Result of nanoparticle size for different concentrations of limonene [polymer/oil (3/5)]
Synthesis of Nanocapsules with a Different Type of Oil
To study the effect of the type of drug on the behavior of the nanocapsules, one of the experiments was repeated, this time with home oil rather than industrial oil. The amount of polymer, oil, and extract were set at 3.0, 0.5, and 0.1 mg, respectively. The results are presented in .
Synthesis of Nanocapsules Using Binary Solvents (Acetone:Ethanol)
To prepare the nanocapsules, 9 mg of polymer, 0.5 mg of olive oil, and 0.1 mg of limonene were dissolved in 20 mL of a mixture composed of acetone and ethanol at different ratios (100:0, 90:10, 70:30, 60:40, 50:50, 40:60, 30:70, and 10:90). While stirring, each organic phase was added drop-wise to 100 mL of distilled water. Each primary emulsion was then exposed to ultrasound for 3 min, and poured into 300 mL of deionized water. The solvent was eliminated under reduced pressure and at room temperature. The results are presented in .
RESULTS AND DISCUSSION
In our present research, Citrus samples were collected from Amool, from the province of Mazandaran, Iran, and were separately subjected to distillation for 4 h. After decanting and drying over anhydrous sodium sulfate, yellowish colored oils were recovered. We separated limonene from the Citrus L. samples. This study was designed to employ emulsion diffusion to fabricate nanocapsules with a core-shell structure, employing the PEG–PBA–PEG copolymer, Citrus extract, olive oil, and acetone to form an organic solution and then adding the solution drop-wise to water spinning at a moderate speed at room temperature. The final product obtained was a comminuted powder consisting of core-shell nanocapsules with a polymeric shell surrounded by the hydrophilic part of the copolymer and enclosing the extracted plant material. The formation of nanocapsules occurs at the diffusion step, when the oil droplets lose the solvent while retaining the immiscible polymer and oil.[Citation14]
Synthesis of Core-Shell Nanoparticles by Emulsion Diffusion
The reaction was initiated by mixing adipoylchloride (1.7 mL) and butylene glycol (0.98 mL, 11 mmol) at 85°C in a balloon, with a magnetic stirrer, followed by the addition of three drops of triethylamine. The aggregate polymerization reaction for preparing polyester continued for approximately 24 h; subsequently, HCl was no longer released. At this stage, the proportion of alcohol acid chloride polyester to HCl was determined to be 1:1.5. For the next stage, the preparation of the triblock, additional PEG (2000) was added to the polyester, and the reaction was maintained at the same temperature. The end of the reaction was estimated on the basis of the absence of HCl release. The polymer was precipitated in diethyl ether (40 mL) at 15°C; theresulting deposits were washed thrice with distilled water and separated by centrifugation.
Nanocapsules with a core-shell structure are advantageous in comparison with nanoemulsions because the core-shell structure prevents direct contact between the encapsulated drug and the environment. Therefore, the drug is better protected from degradation because fast interactions between the drug and the physiological surrounding are minimized, thereby reducing irritation to the drug user. The polymeric shell is also responsible for the long-term stability of the particles, which eases storage.[Citation15] Soon after, the organic phase enters the aqueous phase, which leads to polymer precipitation around the oil particles. This process is designed to achieve consistent nanocapsules with a core-shell structure. Citrus extract and olive oil are mixed in the acetone solvent and this organic solution is added drop-wise to water while stirring at room temperature. In our study, olive oil was used because of its biocompatibility and similarity with nutraceutical oil as well as its ability to dissolve hydrophobic compounds.[Citation16]
Synthesis of Nanoparticles with Different Concentrations of Oil
Following an increase in the amount of oil, an increase in particle size was observed. This increase in the size of the nanoparticles is caused by the increased viscosity of the organic phase resulting from the increased oil. Results are shown in and . Loading efficiency is a function of the extract dispersion in a polymeric matrix in the solid state, and depends on the extract–polymer interaction.[Citation19–Citation21] However, for nanocapsules prepared by the emulsion process, loading efficiency seems to be a function of the partition coefficient of the plant extract between the organic and aqueous phases, employed in the preparation of the nanocapsules.[Citation20] The size of the particles of Onopordon leptolepis and M. recutita in this study were smaller compared with previous studies.[Citation14]
Synthesis of Nanoparticles with a Higher Polymer Concentration
The nanoparticle size demonstrated a continuous decrease when we increased the amount of polymer from 0.1 to 0.9 mg, yet further addition of polymer increased the size of the nanoparticles. Thus, the smallest nanoparticles were obtained when we used 0.9 mg of polymer, whereas higher polymer concentrations led to a decrease in the polymer–polymer interactions, resulting in the separation of the polymer chains during the emulsification process, consequently increasing the particle size. We had previously investigated how different concentrations of a polymer affect O. leptolepis and M. recutita.[Citation16,Citation17] Increasing the polymer concentration in the organic phase promotes the viscosity of the phase, effectively decreasing the diffusion of the solvent in the aqueous phase. This leads to a larger drop size in the nanoemulsion. In other words, increasing the concentration of the polymer in the organic phase will diminish the contribution of the aqueous solvent in the emulsification, thereby increasing the particle size. The results are presented in and .
Synthesis of Nanoparticles with Different Concentrations of Limonene Extracted from Citrus
Limonene isolated from the Citrus L. samples was separately subjected to distillation for 4 h, and yellowish colored oils were recovered after decanting and drying over anhydrous sodium sulfate. 1H and 13C-NMR spectral data yielded the following: 1H-NMR: 1.23 (s, 3 H), 1.70 (s, 3 H), 1.4–2.0 (m, 5 H), 2.1–2.3 (m, 1 H), 3.61 (t, J = 2.9 Hz, 1 Heq), 4.70 (s, 2 H); 13C-NMR: 21.1 (q), 26.2 (t), 26.5 (q), 33.7 (t), 34.0 (t), 37.5 (d), 71.4 (s), 73.9 (d), 109.0 (t), 149.3 (s). IR spectrum data: 3012–3084 cm−1, n(CH, sp2); 2836–2966 cm−1, n(CH, sp3); 2836–2966 cm−1, n(C=C, alkene); 1645 cm−1, n(C=C, alkene); 1377, 1453 cm−1, d(CH2, CH3, bend); 888 cm−1, oop, mono subst. alkene; 797 cm−1, oop, tri subst. alkene. Because larger carriers often do not meet the therapeutic requirements, there is a need for nano-sized carriers. In particular, for parenteral applications, nanoparticles are superior to microparticles as they can be administered without any risk of embolism. Furthermore, only nano-sized carriers can circumvent the high food dependency or insufficient bioavailability after the processing period. Our results have shown that by increasing the drug concentration, the size of the particles increases. This increase depends on the concentration of the extract; when the amount of extract in the nanocapsules increases, the particles’ size increases and stability is decreased. The results are presented in and .
Previous studies conducted with a novel PEG-block–poly aspartic acid-stat–phenylalanine copolymer for a micelle drug carrier demonstrated that the presence of a significant amount of drug in the carrier causes an increase in the micelle size. Recent experimental work by other groups suggests that increasing the amounts of both, the plant extract and the drug, will increase the particle size.[Citation16,Citation17] In accordance, polymer-coated particles are widely used for the preparation of paints, inks, pharmaceuticals, and cosmetics. In the present study, we have coated limonene with PEG–PBA–PEG to improve the rate of absorption on skin and increase the medicinal effectiveness of limonene.
Synthesis of Nanocapsules Using Binary Solvents (Acetone:Ethanol)
We used two solvents in order to prepare nanocapsules with a controlled size. Therefore, we investigated the influence of ethanol and observed that the smallest particles were obtained when using 70% ethanol and 30% acetone (86 nm), whereas 70% acetone and 30% ethanol (159 nm) produced the largest particles. The use of ethanol at volumes higher than 70% led to the precipitation of the polymer because it is insoluble in ethanol; this led to an increase in the size of the nanocapsules. Previous reports had stated that increasing the ethanol concentration decreased the particle diameter and polydispersity. These results concurred with the results of our current study and it was found that increasing the ethanol concentration decreases the size of the nanoparticles. The results are shown in . The size of nanoemulsions and polymeric nanoparticles was thought to be influenced by the interfacial instability between the organic and the aqueous phases caused by the use of ethanol. There was an exponential correlation between the particle diameter and the surface tension gradient coefficient multiplied by the ethanol molar concentration in the organic phases.[Citation22,Citation23]
TABLE 4 Results of nanoparticle size with different amounts of ethanol
CONCLUSION
Nanocapsules containing medicinal plants have several applications in food flavoring and in drug manufacturing. In the present study, the effects of parameters, such as the polymer, the oil, and the extract concentrations, have been investigated on the size of the nanocapsules. Using the binary solvent system led to better results and smaller nanoparticles. It has been observed that the volume of ethanol also has a considerable effect on particle size. Furthermore, scanning electron microscopy identified the coating of the nanocapsules by limonene; this coating renders the nanocapsules suitable for a variety of applications, ranging from medicinal to cosmetic products.
REFERENCES
- Esmaeili, A.; Mohebi, N.; Suzangarzade, S. Experimental and theoretical determination of the antioxidant properties of aromatic monoterpenes of thymol and 2,5,6-trifluorothymol. International Journal of Food Properties 2014, 17, 1162–1168.
- Sowndhararajan, K.; Joseph, J.M.; Manian, S. Antioxidant and free radical scavenging activities of Indian Acacias: Acacia leucophloea (Roxb.) Willd., Acacia ferruginea DC., Acacia dealbata Link. and Acacia pennata (L.) Willd. International Journal of Food Properties 2013, 16 (8), 1717–1729.
- Manojlovic, V.; Nedovic, V.; Kailasapathy, K.; Zuidam, N.J. Encapsulation of probiotics for use in food products. In Encapsulation Technologies for Active Food Ingredients and Food Processing, Vol. 3; Zuidam, N.J., Nedovic, V.A., Eds.; Springer: London, UK, 2010; 52.
- Bigger, J.; Morizet, G.; Aubert, H.; Chacun, M.J.; Terrier-Lacombe, J.; Bigger, J.; Morizet, G.; Aubert, H.; Chacun, M.J.; Terrier-Lacombe, J. Poly(ethylene glycol)-coated hexadecylcyanoacrylate nanospheres display a combined effect for brain tumor targeting. Journal of Pharmacology and Experimental Therapeutics 2002, 303, 928–936.
- Hollins, A.J.; Benboutera, M.; Omidi, Y.; Zinselmeyhegbu, I.F.; Akhtar, S. Toxicogenomics of drug delivery systems: Exploiting delivery system-induced changes in target gene expression to enhance siRNA activity. Pharmaceutical Research 2004, 21, 458–462.
- Luo, Y.; Yao, X.; Yuan, J.; Ding, T.; Gao, Q. Preparation and drug controlled-release of polyion complex micelles as drug delivery systems. Colloids and Surfaces B: Biointerfaces 2009, 68, 218–224.
- Bromberg, L.E.; Ron, E.S. Temperature-responsive gels and thermogelling polymer matrices for protein and peptide delivery. Advanced Drug Delivery Reviews 1998, 31, 197–200.
- Esmaeili, A.; Saremnia, B. Preparation and characterization of germacranolide from Onopordum leptolepis DC. extract loaded nanocapsules. Industrial Crops and Products 2012, 37, 259–263.
- Néeraj, K.; Majeti, N.V.; Ravikumarb, A.J. Biodegradable block copolymers. Advanced Drug Delivery Reviews 2001, 53, 23–44.
- Penco, M.; Marhcioni, S.; Ferruti, P.; D’Antone, S.; Deghenghi, R. Degradation behaviour of block copolymers containing poly(lactic-glycolic acid) and poly(ethylene glycol) segments. Biomaterials 1996, 17, 1583–1590.
- Kennedy, J.P.; Price, G.L. Mechanisms and catalysis in cyclophosphazene polymerization. In: A Key to Advances in Applied Polymer Science; Vandenberg, E.J.; Salamone, J.C.; Eds.; ACS Symposium Series No. 496; American Chemical Society: Washington, DC, 1992; 236–247.
- Rosler, W.; Guido, M.; Vandermeulen, H.A.K. Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Advanced Drug Delivery Reviews 2001, 53, 95–108.
- Wang, L.; Li, X.; Zhang, G.; Dong, J.; Eastoe, J. Oil in water nanoemulsion for pesticides formulations. Journal of Colloid and Interface Science 2007, 314, 230–235.
- Maestro, A.; Solè, I.; Gonzalez, C.; Solans, C.; Gutiérrez, J.M. Influence of the phase behavior on the properties of ionic nanoemulsions prepared by the phase inversion composition method. Journal of Colloid and Interface Science 2008, 327, 433–439.
- Moinard-Chécot, D.; Chevalier, Y.; Briançon, S.; Beney, L.; Fessi, H. Mechanism of nanocapsules formation by the emulsion-diffusion process. Journal of Colloid and Interface Science 2008, 317, 458–468.
- Couvreur, P.; Couarraze, G.; Devissaguet, J.; Puisieux, F. Nano-particles: Preparation and characterization. In: Microencapsulation: Methods and Industrial Applications; Benita, S.; Ed.; Marcel Dekker: New York, 1996; 183–211.
- Wulff-Perez, M.; Torcello-Gomez, A.; Galvez-Ruiz, M.J.; Martin-Rodriguez, A. Stability of emulsions for parenteral feeding: preparation and characterization of o/w nanoemulsions with natural oils and Pluronic f68 as surfactant. Food Hydrocolloids 2009, 23, 1096–1102.
- Esmaeili, A.; Saremnia, B.; Koohian, A.; Rezazadeh, S. Mechanism of nanocapsules of Matricaria recutita L. extract formation by the emulsion–diffusion process. Superlattices and Microstructures 2011, 50, 340–349.
- Esmaeili, A.; Moazami, N.; Rustaiyan, A. Biotransformation of germacranolide from Onopordon leptolepies by Aspergillus niger. Pakistan Journal of Pharmaceutical Sciences 2011, 25 (1), 155–159.
- Matsumoto, Y.; Matsukawa, T.; Suzuki, H.; Yoshino, J. Drug release characteristics of multi-reservoir type microspheres with poly (dl-lactide-co-glycolide) and poly(dl-lactide). Journal of Controlled Release 2005, 106, 172–180.
- Freiberg, S.; Zhu, X.X. Polymer microspheres for controlled drug release. International Journal of Pharmaceutics 2004, 282, 1–18.
- Fujiyama, Y.; Nakase, K.; Osaki, C.H.; Sakakura, H.; Ymagishi, A. Cisplatin incorporated in microspheres: Development and fundamental studies for its clinical application, Hagiwara. Journal of Controlled Release 2003, 89, 397–408.
- Poletto, F.S.; Fiel, L.A.; Donida, B.; Re, M.I.; Guterres, S.S.; Pohlmann, A.R. Polymeric nanocapsules: Concepts and applications. Colloids and Surfaces A: Physicochemical and Engineering 2008, 324, 105–112.