Abstract
Recently, glycidyl fatty acid esters (glycidyl esters) were found widely spread in processed oils and foodstuffs. In this article, several edible oil samples marketed in China, i.e., extra virgin olive oil, organic flax seed oil, cold-pressed peanut oil, soybean oil, butter, and camellia oil were evaluated using a gas chromatography/mass spectrometry (GC/MS) method and their glycidyl ester levels were compared. Results showed that the glycidyl ester levels of the olive oil, flax seed oil, peanut oil, and butter were below the detection of limit, while the glycidyl ester levels of two camellia oil samples were 32.7 and 20.1 mg/kg, respectively. To further find out the source for the relatively high glycidyl ester level in the camellia oil, camellia oils with different refining degrees were evaluated. Results indicated that the glycidyl ester levels were about 12˜13 mg/kg in crude camellia oil, degummed camellia oil, deacidified camellia oil, and bleached camellia oil, while the glycidyl ester level in deodored camellia oil was about 27.0 mg/kg. Based on the results, it was suggested that the source of glycidyl ester in camellia oil was from the crude oil and the deodorization step doubled the glycidyl ester level, therefore, it is of significance to eliminate the glycidyl ester in crude camellia oil in order to control the glycidyl ester level in the final camellia oil product.
INTRODUCTION
Glycidol is a monoglyceride with an epoxide bridge in position 1 and 3 of the glycerine molecule. Although there is no epidemiological data indicating the relationship between human cancer and glycidol exposure, glycidol is viewed as a mutagen and multisite carcinogen based on sufficient evidence of carcinogenicity from experimental animal studies.[Citation1] Recently, the ester bound forms of glycidol, glycidyl fatty acid esters (glycidyl esters) were found widely spread in processed oils and foodstuffs.[Citation2,Citation3] Little is known about their effects on animals and human,[Citation4] but in theory they could be hydrolyzed to form the harmful glycidol by lipases in the gastrointestinal tract.[Citation5] Therefore, glycidyl esters immediately drew the attention of scientists since they were found in foodstuffs.[Citation6]
In the previous studies, glycidyl esters were detected ubiquitously in refined oils while they have never been detected in cold-processed virgin or crude oils.[Citation2,Citation7,Citation8] Several studies indicated that glycidyl esters were generated by heat treatment during the oil refining procedure, especially by the deodorization step.[Citation9−Citation11] It was shown that glycidyl esters were formed from diacylglycerols (DAG) at high temperature (up to 280℃) by elimination of a free fatty acid residue.[Citation12,Citation13] To the best of the authors knowledge, there is no report about the determination of the glycidyl ester levels in the edible oil samples marketed in China. In China, peanut oil, soybean oil, olive oil, butter, flax seed oil, and camellia oil are most commonly used edible oil categories. The manufacturing process of edible oils in China was different from other countries in raw material, processing method and processing conditions. Therefore, it is of significance to evaluate the glycidyl ester levels of edible oils marketed in China, not only for the safety concern, but also for the better improvement in its refining technique.
As for the quantitative determination, glycidyl esters are considered as forming substances of another food process contaminant, 3-monochloro-1, 2-propanediol ester (3-MCPD ester). Several reports on the quantitative analysis of glycidyl esters were published. In the first report,[Citation2] glycidyl esters were indirectly detected using a gas chromatography/mass spectrometry (GC/MS) method. In this method, samples were determined twice (before and after an acidic treatment), and the difference between the two determinations was assumed to be the amount of glycidol. The method was adopted by the German Society of Fat Science (DGF) as an official method.[Citation14] However, the method was proved as inadequate due to incomplete removal or generation of glycidol esters during the determination.[Citation15] Kaze et al. further indicated that in the course of DGF official method there was bidirectional conversion between 3-MCPD and glycidol, which would account for the unreliable results of glycidol esters.[Citation16] Kuhlmann proposed another method based on an improved alkaline catalysed release of MCPD and glycidol, followed by glycidol transformation into monobromopropanediol (MBPD) and then detected by GC/MS.[Citation7] A collaborative study indicated that both the DGF method and the method by Kuhlmann could be used to estimate the 3-MCPD levels in fats and oils assuming that there were no other 3-MCPD forming substances except glycidol.[Citation17] In the meantime, methods based on liquid chromatography/mass spectrometry (LC/MS)[Citation5,Citation18] or liquid chromatography/time-of-flight mass spectrometry (LC/TOFMS)[Citation19] that were able to directly detect the glycidyl ester contents were published. However, the accuracy and precision of the LC based method were not sufficient. Moreover, liquid chromatography (LC) determination was relatively expensive because a lot of different glycidyl ester standards were required in order to perform quantitative analysis. In this study, a modified GC/MS method was applied to determine the glycidyl fatty acid ester levels in camellia oil and other edible oil samples marketed in China. The source of the glycidyl esters was also discussed.
MATERIALS AND METHODS
Standards and Reagents
An internal standard glycidyl stearate-d5 was purchased from Kewei Chemical Technology Co. Ltd (Shanghai, China). Glycidyl stearate was obtained from J & K Scientific Ltd (Guangdong, China). Acetone, methyl tert-butyl ether (MTBE), methanol, hexane and phenylboronic acid (PBA) were of chromatographical pure grade. Sodium chloride, 96% sulfuric acid, glacial acetic acid, and sodium methoxide were of analytical pure grade. All the reagents were purchased from Jingke Co. Ltd (Guangzhou, China). Stock solutions (1 mg/mL) of glycidyl stearate-d5 as well as glycidyl stearate were prepared in MTBE. Working solutions (2, 10, 50, 100, and 200 μg/mL) of glycidyl stearate and working solution of glycidyl stearate-d5 (10 μg/mL) were prepared before use by further diluting the stock solutions with MTBE.
Samples
Edible oil samples
Edible oil samples including extra virgin olive oil (Monte Brand, Italy), organic flax seed oil (Changbai Gongfang Brand, China), cold-pressed peanut oil (Luhua Brand, China), soybean oil (Jin Long Yu Brand, China), butter (Anchor Brand, New Zealand), and two camellia oil samples (Jin Long Yu Brand, China; Taiyuan, China) were purchased from the supermarket in Guangzhou. Samples were stored at 4℃ and were allowed to warm up at room temperature before use.
Preparation of camellia oil samples with different refining degrees
Preparation of camellia oil samples with different refining degrees representative of the typical manufacturing process of camellia oil was as follows: Crude camellia oil (CCO): CCO was obtained by pressing the seeds of camellia (Camellia oleifera, Theaceae) in an automatic horizontal hydraulic oil press (Model 6YY-360, Zhengzhou, China). Degummed camellia oil (DGCO): The hydratable phospholipids were removed by adding 3% (w/w) deionized water to the CCO under 80℃ with stirring at 300 rpm for 30 min. DGCO was collected after centrifugation at 3000 rpm for 15 min at 55℃.[Citation20] Deacidified camellia oil (DACO): 3000 g of DGCO (acid value was 2.67 mg KOH/g) was heated to 85℃ and 45 mL of 25% NaOH solution was added with rapid stirring (300 rpm) to form soaps with free fatty acids. Subsequently, the soap was allowed to precipitate at the same temperature and at a lower stirring speed (80 rpm), then the DACO (acid value was 0.173 mg KOH/g) was collected. Bleached camellia oil (BCO): 60 g of activated bleaching earth was added to the above DACO and reacted for 30 min (80℃, 0.08 MPa). Then, activated bleaching earth was removed by filtering and BCO was collected.[Citation21] Deodored camellia oil (DOCO): the BCO was heated to 250℃ at 190 Pa for 2 h and yielded the DOCO.[Citation21]
Sample Preparation for GC/MS Detection
Sample preparation was performed by converting the glycidol ester into 3-methoxypropane-1, 2-diol (3-MPD) prior to GC/MS analysis.[Citation22] In brief, 200 μL of oil sample was added to a screw-capped glass tube and then 200 μL of 10 μg/mL glycidyl stearate-d5 and 1.8 mL of MTBE were added, followed by vigorous shaking. Afterwards, 500 μL of concentrated H2SO4/CH3OH solution (v: v = 1:100) was added while shaking, and heated for 30 min at 50℃. In this step, the glycidol ester in the oil sample was converted into 3-MPD. Then, 200 μL of sodium methoxide (20% w/v in methanol) was added and mixed for 2 min to allow the breaking reaction of ester bonds. Two hundred microliter of glacial acetic acid and 2 mL of 20% NaCl solution were added to stop the reaction with vigorous shaking. After phase separation, the upper organic layer was discarded. Subsequently, 200 μL of PBA solution (25% w/v in acetone) was added to the lower aqueous layer with vigorous shaking. The resulting cyclic phenylboronate derivative was then extracted with 2 mL of hexane. The organic layer was separated and subjected to GC/MS analysis.
Instrumentation and Operation Conditions
GC/MS analysis was performed on an Agilent 7890 gas chromatograph equipped with a 5975C mass selective detector and operated in the selective ion monitoring (SIM) mode. The analytes were separated in a 5 MS capillary column (length 30 m, inner diameter 0.25 mm, film thickness 0.25 μm). The injector was run at 250℃ in the splitless mode; injection volume was 1 μL. Helium gas was at a flow rate of 1 mL/min. The oven temperature was programmed from 60 (3 min isotime) to 270℃ with a heating rate of 30℃/min. The temperature for the transfer line and ion source was 270 and 230℃, respectively. The software MSD Chemstation (Agilent, version E.01.00.237) was used for analysis. Quantitative analysis was carried out by monitoring characteristic ions at m/z 147 (3-MPD) and m/z 150 (3-MPD-d5). Contents of glycidyl esters were calculated as the ratio of the peak area for 3-MPD and 3-MPD-d5 for the blank, calibration solutions and oil samples as well. Ions at m/z 192 (3-MPD) and m/z 197 (3-MPD-d5) were used as qualifiers.
RESULTS AND DISCUSSION
Method Validation
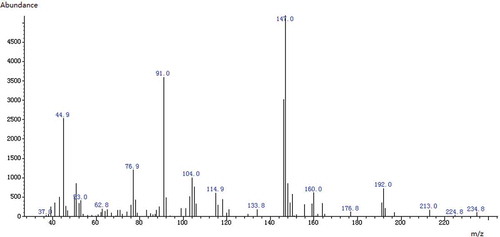
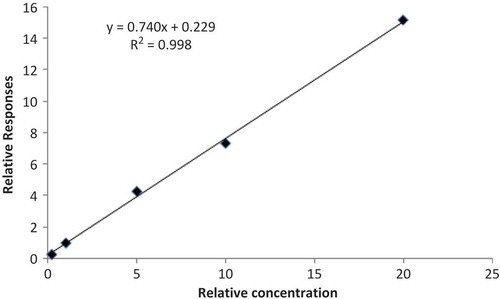
Identification of 3-MPD-d5 and 3-MPD were verified by their retention times. The characteristic peak area ratios of the m/z 147, 192 for 3-MPD and m/z 150, 197 for 3-MPD-d5 were used for quantification. A full scan electron ionization (EI) mass spectrum of PBA derivative of 3-MPD was shown in . Method validations were performed with regard to the linearity, limit of detection (LOD), limit of quantitation (LOQ), precision, repeatability and recoveries. The peak area ratios of the analytes (3-MPD) against corresponding internal standard (3-MPD-d5) were determined. The linearity of the calibration standard curve was determined with correlation coefficient >0.998 for glycidyl stearate (). The LOD value and LOQ value were calculated by standard solutions related to a ratio of signal to noise ≥3 and ≥10, respectively. Precision and repeatability was determined by analysis (n = 10) of soybean oil. A summary of the validation data was displayed in . The recoveries percentage ranged from 95 to 102% (average 98%) for soybean oil spiked with different levels of glycidyl stearate (). Since the method validations showed that the method was adequate for the determination, seven edible oil samples were analyzed for their glycidyl ester levels with this method.
TABLE 1 Summary of validation data
TABLE 2 Recoveries of glycidyl stearate spiked into soybean oil
Results of Edible Oil Samples
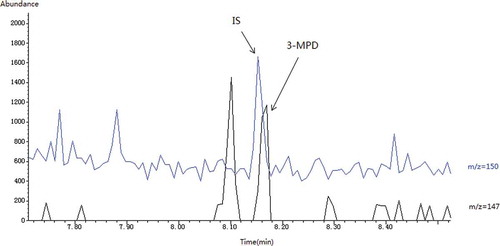
Results for the seven edible oil samples purchased from the supermarket of China showed that the glycidyl ester contents of the extra virgin olive oil, organic flax seed oil, cold-pressed peanut oil, and butter were below the LOD, while the glycidyl ester content of the soybean oil was about 9.6 mg/kg (). A GC/MS chromatogram of an oil sample was shown in . The olive oil, flax seed oil, and peanut oil for the analysis were manufactured using cold-pressed technique and did not undergo deodorization step. Also, during the production of butter, high temperature was not applied. The results were quite consistent with the previous studies that glycidyl esters have never been detected in cold-processed virgin or crude oils.[Citation2,Citation7,Citation8] It is not surprising that glycidyl ester was detected in the soybean oil sample. In the manufacturing process of soybean oil, different processes including degumming, alkali refining (also called deacidifying), bleaching and deodorization were employed. The result might confirm the previous studies that glycidyl esters existed ubiquitously in refined oil; especially when deodorization step was included.[Citation9−Citation11] For the two camellia oil samples, the glycidyl ester contents were 32.7 and 20.1 mg/kg, respectively (). Camellia oil is important as cooking oil in southern China when compared to olive oil.[Citation23] The unexpected high amount of glycidyl ester in camellia oil urged us to go into its forming sources.
TABLE 3 Levels of glycidyl ester in several edible oils marketed in China
Camellia Oil Samples with Different Refining Degrees
Five samples of camellia oil with different refining degrees were prepared. And the results of their glycidyl ester levels were displayed in . For the five samples, the glycidyl ester levels were almost constant for CCO, DGCO, DACO, and BCO (about 12˜13 mg/kg), while the value for DOCO was twice (about 27 mg/kg) as those for the other four samples. It is not surprising that the glycidyl ester level of oil sample increased after the deodorization, as it is known from previous results, deodorization step is the most critical point for the formation of glycidyl esters in edible oils.[Citation10] However, it was unusual to detect high level of glycidyl ester in the cold-pressed CCO, as glycidyl ester is not supposed to exist in crude oil.[Citation2,Citation7,Citation8] It could also be seen from the results that the glycidyl ester levels were relatively constant after the crude oil underwent degumming, deacidification, and/or bleaching. As a consequence, the results suggested the source of glycidyl ester in camellia oil was from the crude oil, and the deodorization step doubled the glycidyl ester level. Although the forming mechanism of glycidyl ester in CCO is not clear, it is of significance to eliminate the glycidyl ester in CCO in order to control the glycidyl ester level in the final camellia oil product. The research on the forming mechanism and elimination method of the glycidyl ester in CCO is ongoing.
TABLE 4 Levels of glycidyl ester in camellia oil of different refining degrees
CONCLUSIONS
In this report, a GC/MS method was applied to detect the glycidyl ester levels in camellia oil samples and other edible oil samples marketed in China. Results showed that the glycidyl ester levels in most of the commonly used edible oil categories were low (below the LOD), including extra virgin olive oil, organic flax seed oil, cold-pressed peanut oil, and butter. Relatively high levels of glycidyl ester were found in soybean oil and camellia oil. The results from camellia oils with different refining degrees showed that there was high amount of glycidyl ester in CCO and this was responsible for the high glycidyl ester level in the final camellia oil product. Further studies are needed to provide more detailed information on the forming mechanism of glycidyl ester in the CCO so as to improve its refining technique.
FUNDING
This work was supported by the Fundamental Research Funds for the Central Universities, SCUT (No. 2011ZM0097 and No. 2011ZZ0018).
Additional information
Funding
REFERENCES
- National Toxicology Program: Toxicology and carcinogenesis studies of glycidol (CAS No. 556-52-5) in F344/N Rats and B6C3F1 Mice (Gavage Studies). National Toxicology Program Technical Report Series 1990, 374, 1–229.
- Weißhaar, R.; Perz. R. Fatty acid esters of glycidol in refined fats and oils. European Journal of Lipid Science and Technology 2010, 112, 158–165.
- Zelinková, Z.; Svejkovská, B.; Velišek, J.; Doležal, M. Fatty acid esters of 3-chloropropane-1, 2-diol in edible oils. Food Additives & Contaminants 2010, 23, 1290–1298.
- Schilter, B.; Scholz, G.; Seefelder, W. Fatty acid esters of chloropropanols and related compounds in food: Toxicological aspects. European Journal of Lipid Science and Technology 2011, 113, 309–313.
- Becalski, A.; Feng, S.Y.; Lau, B.P.-Y.; Zhao, T. Glycidyl fatty acid esters in food by LC-MS/MS: Method development. Analytical and Bioanalytical Chemistry 2012. DOI:10.1007/s00216-012-5932-8.
- Matthäus, B. 3-MCPD and glycidyl fatty acid esters: What is the knowledge today? European Journal of Lipid Science and Technology 2011, 113, 277–278.
- Kuhlmann, J. Determination of bound 2, 3-epoxy-1-propanol (glycidol) and bound monochloropropanediol (MCPD) in refined oils. European Journal of Lipid Science and Technology 2011, 113, 335–344.
- Shimizu, M.; Moriwaki, J.; Shiiba, D.; Nohara, H.; Kudo, N.; Katsuragi, Y. Elimination of glycidyl palmitate in diolein by treatment with activated bleaching earth. Journal of Oleo Science 2012, 61, 23–28.
- Franke, K.; Strijowski, U.; Fleck, G.; Pudel, F. Influence of chemical refining process and oil type on bound 3-chloro-1, 2-propanediol contents in palm oil and rapeseed oil. LWT-Food Science and Technology 2009, 42, 1751–1754.
- Pudel, F.; Benecke, P.; Fehling, P.; Freudenstein, A.; Matthäus, B.; Schwaf, A. On the necessity of edible oil refining and possible sources of 3-MCPD and glycidyl esters. European Journal of Lipid Science and Technology 2011, 113, 368–373.
- Strijowski, U.; Heinz, V.; Franke, K. Removal of 3-MCPD esters and related substances after refining by adsorbent material. European Journal of Lipid Science and Technology 2011, 113, 387–392.
- Craft, B.D.; Nagy, K.; Seefelder, W.; Dubois, M.; Destaillats, F. Glycidyl esters in refined palm (Elaeis guineensis) oil and related fractions. Part II: Practical recommendations for effective mitigation. Food Chemistry 2012, 132, 73–79.
- Destaillats, F.; Craft, B.D.; Dubois, M.; Nagy, K. Glycidyl esters in refined palm (Elaeis guineensis) oil and related fractions. Part I: Formation mechanism. Food Chemistry 2012, 131, 1391–1398.
- DGF. Ester-bound 3-chloropropane-1, 2-diol (3-MCPD esters) and glycidol (glycidol esters). Deutsche Gesellschaft fűr Fettwissenschaft: DGF Standard Methods (14. Supplement) C-III 18, 2009.
- Shimizu, M.; Kudo, N.; Shiro, H.; Yasunaga, K.; Masukawa, Y.; Katsuragi, Y.; Yasumasu, T. Comparison of indirect and direct quantification of glycidol fatty acid ester in edible oils. Journal of Oleo Science 2010, 59, 535–539.
- Kaze, N.; Sato, H.; Yamamoto, H.; Watanabe, Y. Bidirectional conversion between 3-monochloro-1, 2-propanediol and glycidol in course of the procedure of DGF standard methods. Journal of the American Oil Chemists’ Society 2011, 88, 1143–1151.
- Fleblg, H.J. Determination of ester-bound 3-chloro-1, 2-propanediol and glycidol in fats and oils—A collaborative study. European Journal of Lipid Science and Technology 2011, 113, 393–399.
- Masukawa, Y.; Shiro, H.; Nakamura, S.; Kondo, N.; Jin, N.; Suzuki, N.; Ooi, N.; Kudo, N. A new analytical method for the quantification of glycidol fatty acid esters in edible oils. Journal of Oleo Science 2010, 59, 81–88.
- Haines, T.D.; Adlaf, K.J.; Pierceall, R.M.; Lee, I.; Venkitasubramanian, P.; Collison, M.W. Direct determination of MCPD fatty acid esters and glycidyl fatty acid esters in vegetable oils by LC-TOFMS. Journal of the American Oil Chemists’ Society 2010. DOI:10.1007/s11746-010-1732-5.
- Indira, T.; Hemavathy, J.; Khatoon, S.; Krishna, A.G.; Bhattacharya, S. Water degumming of rice bran oil: a response surface approach. Journal of Food Engineering 2000, 43, 83–90.
- Wang, W.; Li, T.; Ning, Z.; Wang, Y.; Yang, B.; Ma, Y.; Yang, X. A process for the synthesis of PUFA-enriched triglycerides from high-acid crude fish oil. Journal of Food Engineering 2012, 109, 366–371.
- Küsters, M.; Bimber, U.; Reeser, S.; Gallitzendörfer, R.; Gerhartz, M. Simultaneous determination and differentiation of glycidyl esters and 3-monochloropropane-1, 2-diol (MCPD) esters in different foodstuffs by GC-MS. Journal of Agricultural and Food Chemistry 2011, 59, 6263–6270.
- Zhang, W.G.; Zhang, D.C.; Chen, X.Y. A novel process for extraction of tea oil from Camellia oleifera seed kernels by combination of microwave puffing and aqueous enzymatic oil extraction. European Journal of Lipid Science and Technology 2012, 114, 352–356.