Abstract
A supercritical fluid chromatography method for the determination of seven pyrethroid insecticides (allethrin, resmethrin, phenothrin, permethrin, tetramethrin, cypermethrin, deltamethrin) and one of their common metabolites, phenoxybenzyl alcohol, in whole and peeled potatoes and mixed vegetables was developed. The efficiencies of the two extraction techniques, supercritical fluid extraction and microwave-assisted extraction, for the extraction of pyrethroids from vegetable samples were also compared. The retention times of various pyrethroids ranged from 8.4 to 22.9 min, while all of the peaks were well-resolved and distinctly identified. The limits of detection of pyrethroid insecticides ranged between 0.31 and 0.54 ppm, whereas the limits of detection of phenoxybenzyl alcohol was 0.62 ppm. The recoveries of pyrethroid insecticides from whole potatoes, peeled potatoes, and mixed vegetables ranged as 93.83–99.8%, 92.3–105.8%, and 93.67–102.7%, respectively, with the use of supercritical fluid extraction. The corresponding recovery ranges while using microwave-assisted extraction were 94.2–102%, 96.6–101.2%, and 96–103.2%. These findings suggested that supercritical fluid chromatography was a sensitive and rapid technique for the analysis of pyrethroids in complex matrices, such as vegetables, fruits, and other agricultural products.
INTRODUCTION
Pyrethroids are synthetic compounds that are mainly used for the protection of crops and food storage against insects and acarids. Detection of environmental chemicals in vegetable samples[Citation1−Citation4] has raised concerns about the human exposure of these contaminants through food consumption. Several chromatographic methods have been reported for the separation, detection, and determination of pyrethroids in edible products. Portoles et al.[Citation5] have studied the potential of atmospheric pressure chemical ionization combined with gas chromatography tandem mass spectrometry (GC-MS/MS) for estimation of eight pyrethroids (bifenthrin, cyfluthrin, cypermethrin, permethrin, λ-cyhalothrin, fluvalinate, fenvalerate, and deltamethrin) in fruits and vegetables. Ananda Gowda and Somashekar[Citation6] screened 50 vegetable samples (beans, brinjal, cabbage, and carrot) from Karnataka, India and observed the domination of organochlorines (97%) followed by organophosphates (83%) and pyrethroids (60%), while 58% of the samples were found to contain the residues of these insecticides above their respective maximum permissible limits. Boulaid et al.[Citation7] determined the residue levels of pyrifenox, pyridaben, and tralomethrin in unprocessed and processed tomatoes, grown in an experimental greenhouse, to evaluate the effect of different household processes, including washing and peeling.
Shen et al.[Citation8] developed a sensitive gas chromatography/mass spectrometry (GC-MS) method in negative chemical ionization for the determination of 17 pyrethroid pesticide residues in troublesome matrices, including garlic, onion, spring onion, and chili. The limits of detection ranged from 0.02 to 6 μg/kg and recovery yields were from 54.0 to 129.8% at three spiked levels (20, 40, and 60 μg/kg for chili, and 10, 20, and 30 μg/kg for others in four different matrices depending on the compounds determined).[Citation8] Beltran et al.[Citation9] developed a solid-phase microextraction (SPME) method for the determination of seven pyrethroid insecticides (bifenthrin, lambda-cyhalothrin, permethrin, cyfluthrin, cypermethrin, fenvalerate, and tau-fluvalinate) in water, vegetable (tomato), and fruit (strawberry) samples, based on direct immersion mode and subsequent desorption into the injection port of a GC/MS. Detection limits for tomato samples were between 0.003 and 0.025 mg/kg with relative standard deviations around 25%.[Citation9] Another gas chromatographic method was used for the simultaneous determination of 12 pyrethroids (tefluthrin, bifenthrin, fenpropathrin, cyhalothrin, permethrin, cyfluthrin, cypermethrin, alpha-cypermethrin, flucythrinate, fenvalerate, fluvalinate, and deltamethrin) in tomato puree, peach nectar, orange juice, and canned peas with the mean recoveries ranging from 70.2 to 96.0% and the quantitation limits <0.010 mg/kg.[Citation10] Colume et al.[Citation11] screened the agricultural samples to determine 17 synthetic pyrethroids using GC-MS with LODs varying between 0.1 and 0.8 ng/ml (except for piperonyl butoxide, 25 ng/ml) and the recoveries of pyrethroids from the samples fortified at levels of 20–100 ng/g ranging from 66 to 102%. Metabolism of pyrethroids in the human body results in the urinary excretion of their metabolites, such as 3-phenoxybenzyl alcohol (3-PBA), which is considered as a suitable biomarker for monitoring of pyrethroids exposure. Recently, it has been found that people with frequent consumption of both raw and cooked vegetables have significantly higher levels of 3-PBA in their urine than subjects consuming lesser amount of vegetables.[Citation12]
In recent years, supercritical fluid chromatography (SFC) has emerged as a powerful green technology in the area of separation science. SFC has several advantages over other conventional chromatographic techniques; in particular, it provides rapid separations without the use of organic solvents. The superiority of SFC over conventional high performance liquid chromatography (HPLC) has been shown in terms of high resolution, lower analysis time, and better separation potential for drugs and their metabolites.[Citation13,Citation14] Toribio et al.[Citation15] have performed chiral separation of six triazole pesticides using SFC equipped with a Chiralpak AD column. Dost et al.[Citation16] have developed a sensitive SFC-mass spectrometry method for separation and quantification of three classes of pesticides, including triazines, carbamates, and sulfonylureas, in soil samples. A multi-residue method based on SFC and solid-phase extraction has been reported for the analysis of 35 common contaminants, including pesticides in water with the detection limits ranging from 0.4 to 2.6 μg/L.[Citation17] SFC coupled with supercritical fluid extraction (SFE) has been used for the analysis of sulfonylureas, their precursors, and metabolites in complex matrices, such as soil, vegetation, and cell culture medium.[Citation18] In view of several advantages of SFC over conventional chromatography, we developed a SFC method coupled with ultraviolet detection (UVD) for separation and quantitation of pyrethroids in crude and processed vegetable samples. We also optimized conditions for the extraction of pyrethroids using SFE and microwave-assisted extraction (MAE).
MATERIALS AND METHODS
Pesticides Standards
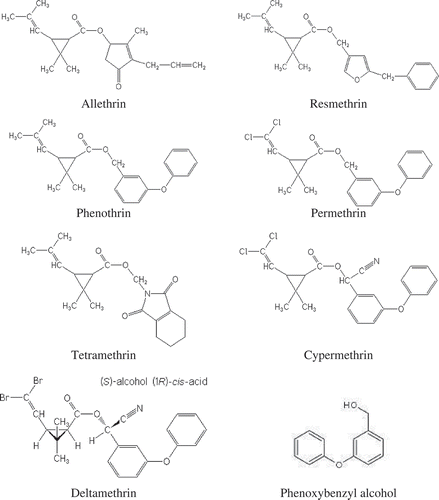
The standards of seven pyrethroid insecticides (allethrin, resmethrin, phenothrin, permethrin, tetramethrin, cypermethrin, and deltamethrin) and one metabolite (phenoxybenzyl alcohol) were obtained from ChemService Inc., USA. The chemical structures of these compounds are given in . The standard solutions were prepared in chromatography grade methanol to give a concentration range of 0.001–10 ppm. All of the standards as well as samples were filtered with 0.2-μm filter discs prior to analysis.
Vegetable Samples
The samples, including the raw whole potatoes, peeled potatoes, and frozen mixed vegetables (carrots, potatoes, green beans, peas, lima beans, okra, corn, onion, and celery), were purchased from a local market. All of the food products were cleaned, washed, chopped, homogenized, and dehydrated at 50°C overnight in an electric oven under vacuum, prior to the extraction procedure. The powdered food samples were stored in glass containers and kept at --5°C until analyzed.
Spiking of Vegetable Samples with Pyrethroids
Dried vegetable samples were spiked with known amounts (0.16–1.60 μg/g) of pyrethroids. The spiked vegetable samples were extracted by using SFE or MAE for pesticide residue analysis using SFC-UVD.
Supercritical Fluid Extraction (SFE)
The supercritical fluid extraction apparatus (model 7680T, Hewlett Packard, USA) was comprised of an automated restrictor and a solid phase sorbent trap prepacked with 30 μm Hypersil ODS into which the CO2 extraction solvent was decompressed during collection. The extraction method of Khan[Citation19] was used after modifications for optimal conditions for pyrethroids extraction. A known amount of food sample (5 g) was transferred into the extraction thimble. The extraction process was carried out in three steps that lasted for 55 min. The first extraction step was conducted for 5 min to extract only the hydrocarbons and other nonpolar compounds without extracting the spiked pesticides. The second extraction step was continued for 40 min to mainly extract the pesticides from the vegetable samples. The final step was conducted for 10 min to ensure complete extraction of pesticides. The optimized conditions for each step were as follows: modifier (0, 0, 30%), CO2 density (0.25, 0.67, 0.67 g/ml), chamber temperature (40, 80, 80°C), flow rate (1.0, 2.5, 2.5 ml/min), and pressure (1117, 3469, 3469 psi). The extracted sample was eluted from the trap with 1.5 ml of methanol at a flow rate of 0.4 ml/min and a trap temperature of 40°C and was collected in an autosampler vial.
Microwave-Assisted Extraction (MAE)
A microwave solvent extraction system (model MES-1000, CEM Corporation, Matthews, NC, USA) consisting of lined extraction vessels was used. Double-walled extraction vessels made of inner Teflon PFA liner and Ultem polyetherimide outer bode, suitable for use with organic solvents were used. Pre-weighed food samples (5 g) were extracted with 60 ml of solvent (acetone-hexane, 3:2) for 30 min. The extraction conditions were as follows: microwave power, 75%; temperature, 125°C; and pressure, 85 psi. After extraction, the samples were filtered and concentrated using a rotary evaporator. The residue on the filter paper was re-extracted thrice with 10 ml of methanol to ensure a complete extraction of pesticides. The filtrates were concentrated in a rotary evaporator.
Determination of Pyrethroids by SFC-UVD
A Hewlett Packard SFC (model G 1205A) attached to an HP 1050 diode array detector, modifier pump, and a silica column (Alltec Hypersil APS, 25 micron, length 205 mm, ID 4.6 mm) was used. We used a previously reported method[Citation20] with some modifications. Chromatographic conditions were optimized for the analysis of pyrethroids as follows: oven temperature, 60°C; pressure, 130–200 bar; flow rate, 1–3 ml/min; and 2% methanol as modifier. Pyrethroids were detected at a wavelength of 220 nm.
TABLE 1 Retention times (RT) and lower limits of detection (LOD) for different pyrethroid insecticides
RESULTS
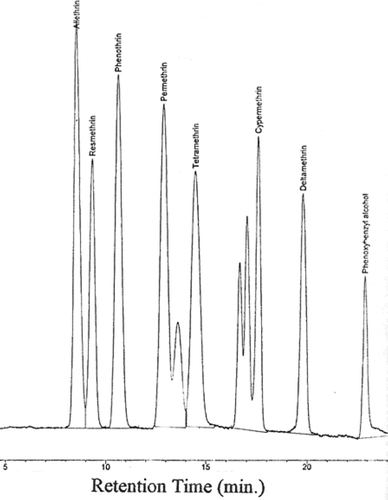
All the pyrethroids were clearly separated in the form of well-resolved peaks (). The retention times (RTs) and lower limits of detection (LOD) for various pyrethroids are given in . The RTs ranged from 8.4 to 22.9 min in the following order of increasing RTs: allethrin (8.4 min), resmethrin (9.2 min), phenothrin (10.5 min), permethrin (12.8 and 13.5 min; two isomers), tetramethrin (14.5 min), cypermethrin (16.6, 17.1, and 17.8 min; three isomers), deltamethrin (19.9 min), and phenoxybenzyl alcohol (22.9 min). The LODs of various pyrethroid insecticides ranged between 0.31 and 0.54 ppm, whereas the LOD of the metabolite, phenoxybenzyl alcohol, was 0.62 ppm (). The recoveries of pyrethroid insecticides from whole potatoes, peeled potatoes, and mixed vegetables ranged from 93.83–99.8%, 92.3–105.8%, and 93.67–102.7%, respectively, when SFE was used for their extraction (). The corresponding recovery ranges in these samples were found to be 94.2–102%, 96.6–101.2%, and 96–103.2%, when MAE was applied for extraction (). The recoveries of the common metabolite of pyrethroids, phenoxybenzyl alcohol, were comparatively higher than pyrethroids, using both of the extraction methods ().
TABLE 2 Recoveries (%) of pyrethroids from spiked vegetable samples using two different extraction methods (SFE and MAE) (values are the mean of three replicates ± standard deviation)
DISCUSSION
The results of this study clearly showed the potential of SFC for the separation and determination of pyrethroid insecticides in vegetable samples (). We compared the two extraction methods (SFE and MAE) for the extraction of pyrethroids from vegetable samples prior to their analysis by SFC. Both SFE and MAE resulted in high recoveries of pyrethroids from spiked vegetable samples (). Although analytical extraction approaches have been improved, most of them rely on time-consuming procedures, such as Soxhlet extraction, which requires large volumes of expensive and toxic solvents.[Citation21,Citation22] SFE is rather a new technique for the extraction of analytes from sample matrices. The main advantages of SFE are related to the properties of supercritical fluids that are inexpensive, contaminant free, and less costly to safely dispose of than organic solvents. The main advantages of MAE are reductions in extraction time and solvent quantity; hence, this technique is also environmentally friendly. MAE has been reported as superior to ultrasonic as well as Soxhlet methods for the extraction of chlorinated pesticides from animal feed.[Citation23] A comparative study of five extraction techniques revealed the ability of MAE to retrieve high concentration of pesticides from dietary composites.[Citation24] Otake et al.[Citation25] have optimized the conditions for MAE and observed the mean recoveries of 16 organophosphates and 10 pyrethroid pesticides between 72–108% for a 1.0 pg spiking level and 70–119% for a 0.2 μg level.
Ye et al.[Citation26] have reported a simple and rapid pressurized isocratic capillary electrochromatography method for separation and estimation of six pyrethroid pesticides in cabbage. The limits of quantification using this method ranged from 0.5 to 0.8 μg/ml (corresponding to 0.05 and 0.08 mg/kg in the vegetable sample).[Citation26] Dong et al.[Citation27] have developed a novel method for separating and enriching of pyrethroid pesticides from vegetables by solvent sublation and their determination by HPLC. The LODs ranged between 1.4 μg/kg (bifenthrin) and 4.2 μg/kg (fenpropathin) and the recoveries from spiked vegetable samples were from 85.7 to 110.4%.[Citation27] Vazquez et al.[Citation28] have validated a sensitive and efficient SPME method for the determination of seven pyrethroid insecticides, including fenpropathrin, lambda-cyhalothrin, deltamethrin, fenvalerate, permethrin, tau-fluvalinate, and bifenthrin in cucumber and watermelon samples using HPLC combined with post-column photochemically-induced fluorimetry derivatization and fluorescence detection. Limits of quantifications were between 1.5 and 5 μg/kg for cucumber and between 1.3 and 5 μg/kg for watermelon samples.[Citation28] A quantitative method consisting of solid-phase extraction (SPE) followed by liquid chromatography/electrospray ionization ion trap mass spectrometry (LC/ESI-ITMS) analysis has been developed for the identification and quantitation of 10 pyrethroid pesticides (tetramethrin, allethrin, fenpropathrin, lambda-cyhalothrin, cypermethrin, deltamethrin, fenvalerate, bioresmethrin, permethrin, and bifenthrin) commonly used in vegetables. This method was successfully applied for the determination of pyrethroids in six vegetables with the limits of quantification ranging from 0.03 to 0.1 mg/kg.[Citation29] Park et al.[Citation30] applied a competitive enzyme-linked immunosorbent assay (ELISA) method for determination of cypermethrin and permethrin in agricultural products. The matrix interferences were minimized by direct dilution of the extracts without any further cleanup. The mean percentage recoveries of permethrin spiked in apple, banana, cucumber, lettuce, onion, and peach were 99.2, 105, 70.2, 97.5, 94.4, and 89.4%, respectively.[Citation30] For liquid chromatographic separation of enantiomers of pyrethroid insecticides, the use of cyano-bonded columns with chiral stationary phases (CSPs) has been recommended.[Citation31] Girelli et al.[Citation32] have indicated that CSPs based on cellulose derivatives are more appropriate for the stereoisomer separation of cis-biphenthrin, resmethrin, and (1R)-phenothrin, whereas multiple-interaction CSPs, like (S)-1-(alpha-naphthyl)-ethylamine/(S)-tert-leucine, are more selective for cyfluthrin. Nishikawa[Citation33] studied the temperature dependencies of the cis and trans isomers of permethrin and phenothrin on chromatographic retention times. For both the pyrethroids, the curves crossed between 120 and 150°C with a reversal in the elution order of geometrical isomers occurring at this point. Above this temperature, the volatility contribution dominated for the retention and cis-isomers eluted earlier, whereas below this temperature trans-isomers eluted earlier owing to the solvation contribution.[Citation33]
In conclusion, our findings showed higher recoveries of pyrethroids using both SFE and MAE suggesting the suitability of these two methods for rapid and efficient extraction of pyrethroids from vegetable samples. The chromatographic separation and determination of pyrethroids using SFC offered the advantages of high sensitivity, greater resolution, and minimal use of organic solvents.
FUNDING
This study was supported by the Deanship of Scientific Research, Research Center, College of Food and Agricultural Sciences, King Saud University, Riyadh, Saudi Arabia.
REFERENCES
- Banerjee, T.; Banerjee, D.; Roy, S.; Banerjee, H.; Pal, S. A comparative study on the persistence of imidacloprid and beta-cyfluthrin in vegetables. Bulletin of Environmental Contamination and Toxicology 2012, 89, 193–196.
- Fujita, M.; Yajima, T.; Iijima, K.; Sato, K. Comparison of the variability in the levels of pesticide residue observed in Japanese cabbage and grape units. Journal of Agricultural and Food Chemistry 2012, 60, 1516–1521.
- Randhawa, M.A.; Anjum, F.M.; Asi, M.R.; Ahmed, A.; Nawaz, H. Field incurred endosulfan residues in fresh and processed vegetables and dietary intake assessment. International Journal of Food Properties 2014, 17, 1109–1115.
- Khan, H.A.; Arif, I.A.; Al Homaidan, A.A. Distribution pattern of eight heavy metals in the outer and inner tissues of ten commonly used vegetables. International Journal of Food Properties 2012, 15, 1212–1219.
- Portoles, T.; Mol, J.G.; Sancho, J.V.; Hernández, F. Advantages of atmospheric pressure chemical ionization in gas chromatography tandem mass spectrometry: Pyrethroid insecticides as a case study. Analytical Chemistry 2012, 84, 9802–9810.
- Ananda Gowda, S.R.; Somashekar, R.K. Evaluation of pesticide residues in farmgate samples of vegetables in Karnataka, India. Bulletin of Environmental Contamination and Toxicology 2012, 89, 626–632.
- Boulaid, M.; Aguilera, A.; Camacho, F.; Soussi, M.; Valverde, A. Effect of household processing and unit-to-unit variability of pyrifenox, pyridaben, and tralomethrin residues in tomatoes. Journal of Agricultural and Food Chemistry 2005, 53, 4054–4058.
- Shen, C.Y.; Cao, X.W.; Shen, W.J.; Jiang, Y.; Zhao, Z.Y.; Wu, B.; Yu, K.Y.; Liu, H.; Lian, H.Z. Determination of 17 pyrethroid residues in troublesome matrices by gas chromatography/ mass spectrometry with negative chemical ionization. Talanta 2011, 84, 141–147.
- Beltran, J.; Peruga, A.; Pitarch, E.; López, F.J.; Hernández, F. Application of solid-phase microextraction for the determination of pyrethroid residues in vegetable samples by GC-MS. Analytical and Bioanalytical Chemistry 2003, 376, 502–511.
- Sannino, A.; Bandini, M.; Bolzoni, L. Determination of pyrethroid pesticide residues in processed fruits and vegetables by gas chromatography with electron capture and mass spectrometric detection. Journal of AOAC International 2003, 86, 101–108.
- Colume, A.; Cardenas, S.; Gallego, M.; Valcarcel, M. Selective enrichment of 17 pyrethroids from lyophilised agricultural samples. Journal of Chromatography A 2001, 912, 83–90.
- Fortes, C.; Mastroeni, S.; Pilla, M.A.; Antonelli, G.; Lunghini, L.; Aprea, C. The relation between dietary habits and urinary levels of 3-phenoxybenzoic acid, a pyrethroid metabolite. Food and Chemical Toxicology 2013, 52, 91–96.
- Toribio, L.; del Nozal, M.J.; Bernal, J.L.; Alonso, C.; Jimenez, J.J. Comparative study of the enantioselective separation of several antiulcer drugs by high-performance liquid chromatography and supercritical fluid chromatography. Journal of Chromatography A 2005, 1091, 118–123.
- Wang, Z.; Li, S.; Jonca, M.; Lambros, T.; Ferguson, S.; Goodnow, R.; Ho, C.T. Comparison of supercritical fluid chromatography and liquid chromatography for the separation of urinary metabolites of nobiletin with chiral and non-chiral stationary phases. Biomedical Chromatography 2006, 20, 1206–1215.
- Toribio, L.; del Nozal, M.J.; Bernal, J.L.; Jimenez, J.J.; Alonso, C. Chiral separation of some triazole pesticides by supercritical fluid chromatography. Journal of Chromatography A 2004, 1046, 249–253.
- Dost, K.; Jones, D.C.; Auerbach, R.; Davidson, G. Determination of pesticides in soil samples by supercritical fluid chromatography-atmospheric pressure chemical ionisation mass spectrometric detection. Analyst 2000, 125, 1751–1755.
- Toribio, L.; del Nozal, M.J.; Bernal, J.L.; Jimenez, J.J.; Serna, M.L. Packed-column supercritical fluid chromatography coupled with solid-phase extraction for the determination of organic microcontaminants in water. Journal of Chromatography A 1998, 823, 163–170.
- McNally, M.E.; Wheeler, J.R. Supercritical fluid extraction coupled with supercritical fluid chromatography for the separation of sulfonylurea herbicides and their metabolites from complex matrices. Journal of Chromatography 1988, 435, 63–71.
- Khan, S.U. Supercritical fluid extraction of bound pesticide residues from soil and food commodities. Journal of Agricultural and Food Chemistry 1995, 43, 1718–1723.
- Nishikawa, Y. Enantiomer separation of synthetic pyrethroids by subcritical and supercritical fluid chromatography with chiral stationary phases. Analytical Sciences 1993, 9, 33–37.
- Antunes, P.; Gil, O.; Gil, M.G. Supercritical fluid extraction of organochlorines from fish muscle with different sample preparation. Journal of Supercitical Fluids 2003, 25, 135–142.
- Luque de Castro, M.D.; Garcia Ayuso, L.E. Soxhlet extraction of solid materials: An outdated technique with a promising future. Analytica Chimica Acta 1998, 369, 1–10.
- Gfrerer, M.; Chen, S.; Lankmayr, E.P.; Quan, X.; Yang, F. Comparison of different extraction techniques for the determination of chlorinated pesticides in animal feed. Analytical and Bioanalytical Chemistry 2004, 378, 1861–1867.
- Rosenblum, L.; Garris, S.T.; Morgan, J.N. Comparison of five extraction methods for determination of incurred and added pesticides in dietary composites. Journal of AOAC International 2002, 85, 1167–1176.
- Otake, T.; Kuroda, Y.; Aoyagi, Y.; Yarita, T. Evaluation of microwave-assisted extraction for the analysis of organophosphorus and pyrethroid pesticides in green onions. Journal of AOAC International 2012, 95, 232–237.
- Ye, F.; Xie, Z.; Wu, X.; Lin, X. Determination of pyrethroid pesticide residues in vegetables by pressurized capillary electrochromatography. Talanta 2006, 69, 97–102.
- Dong, H.; Bi, P.; Xi, Y. Determination of pyrethroid pesticide residues in vegetables by solvent sublation followed by high-performance liquid chromatography. Journal of Chromatographic Science 2008, 46, 622–626.
- Vazquez, P.P.; Mughari, A.R.; Galera, M.M. Solid-phase microextraction (SPME) for the determination of pyrethroids in cucumber and watermelon using liquid chromatography combined with post-column photochemically induced fluorimetry derivatization and fluorescence detection. Analytica Chimica Acta 2008, 607, 74–82.
- Chen, T.; Chen, G. Identification and quantitation of pyrethroid pesticide residues in vegetables by solid-phase extraction and liquid chromatography/electrospray ionization ion trap mass spectrometry. Rapid Communications in Mass Spectrometry 2007, 21, 1848–1854.
- Park, E.K.; Kim, J.H.; Gee, S.J.; Watanabe, T.; Ahn, K.C.; Hammock, B.D. Determination of pyrethroid residues in agricultural products by an enzyme-linked immunosorbent assay. Journal of Agricultural and Food Chemistry 2004, 52, 5572–5576.
- Cayley, G.R.; Simpson, B.W. Separation of pyrethroid enantiomers by chiral high-performance liquid chromatography. Journal of Chromatography 1986, 356, 123–134.
- Girelli, A.M.; Messina, A.; Sinibaldi, M. A study on the separation of synthetic pyrethroid stereoisomers by HPLC. Annali de Chimica 2002, 92, 417–424.
- Nishikawa, Y. Supercritical fluid chromatographic separation of synthetic pyrethroids on packed columns and capillary columns. Analytical Sciences 1992, 8, 817–822.