Abstract
Biogenic amines content of four types of Tuscan ewes’ milk “pecorino” cheese was evaluated using HPLC-UV analysis. All cheeses were manufactured in the same dairy factory with different combinations of milk (raw or pasteurized) and type of ripening. Total biogenic amines and tyramine levels of a raw milk “pecorino” ripened five months, partly in a traditional cave, were significantly higher than those of a pasteurized milk “pecorino” with a similar ripening; and of a two months raw milk “pecorino” ripened in the dairy plant. No statistical significant difference was found when comparing total biogenic amines and tyramine contents of the same five month ripened raw milk “pecorino” with a pasteurized milk “pecorino” ripened six months, partly in a traditional “fossa.” In raw milk cave-ripened and “fossa”-ripened cheeses, total biogenic amines exceeded 1000 mg/kg. In cheeses manufactured with raw milk and/or in particular ripening environments, specific hygienic cares are needed to limit biogenic amines formation.
INTRODUCTION
Biogenic amines (BAs) are low molecular weight compounds naturally occurring in many foods and beverages, especially fermented ones, in which they are mainly the result of amino acids decarboxylation by microorganisms. The most abundant BAs in food are histamine (HIS), tyramine (TYR), putrescine (PUT), cadaverine (CAD) and 2-phenylethylamine (2PHE). The presence of high amounts of amines in food can pose a health risk due to their toxicity. HIS is responsible for “scombroid fish poisoning,” TYR is the main cause of the so-called “cheese reaction,” while other BAs mostly act as a potentiating factor. Where nitrites are present, the presence of amines can also be a source of worry due to the possible formation of carcinogenic nitrosamines. The identification of BA safety levels in food is difficult, because the toxicity varies depending on the single and total amines content. Moreover, the effect on the consumer is related to the efficiency of the detoxification system which may be greatly different among various individuals and can be influenced by specific drugs (mono amine oxidases inhibitors, MAOI), smoking, or alcohol consumption.[Citation1] Some BAs are also considered a quality index for food and high doses have often been associated with poor manufacturing practices.[Citation2]
The availability of free amino acids, due to the proteolysis, the environmental conditions that allow the growth of the decarboxylase-positive microorganisms, and the presence of the pyridoxal phosphate cofactor make cheese an ideal matrix for amine production.[Citation3,Citation4]
Several studies on the presence of BAs in cheeses have been conducted, showing a great variability among different types of cheese.[Citation4] The aim of this study was to evaluate the content of BAs (single and total values) in four different types of ewes’ milk semi-hard cheese (“pecorino” cheese) manufactured in the same dairy factory in Tuscany, Italy. Specifically, we analyzed cheeses manufactured with raw and pasteurized milk and with a ripening period of two to six months carried out both in common ripening rooms located in the dairy plant, and in particular ripening environments, such as “grotte” (caves traditionally used as cellars) and “fosse” (pits dug in tuff), to gather data on whether those factors can have a role in affecting BAs accumulation in cheese.
MATERIALS AND METHODS
Reagents and Standard Solutions
2PHE hydrochloride, CAD dihydrochloride, HIS dihydrochloride, PUT hydrochloride, spermidine (SPD) trihydrochloride, spermine (SPM) tetrahydrochloride, tryptamine (TRP) hydrochloride, TYR hydrochloride, 1,7-diaminoheptane (internal standard, IS), dansyl-chloride (DCl), and L-proline were purchased from Sigma-Aldrich Inc. (Saint Louis, MO, USA); hydrochloric acid (HCl), sodium hydrogen carbonate (NaHCO3), sodium hydroxide (NaOH), acetone, and diethyl ether were acquired from Carlo Erba (Milan, Italy). HPLC-grade acetonitrile (ACN) was purchased from Panreac Quimica S.A.U. (Barcellona, Spain). Ultra pure water from a Millipore Milli-Q system (Millipore, Milan, Italy) was used for all the solutions. Stock solutions (10,000 μg/ml) in HCl 0.1M of each BA and of the IS were stored at –20°C and used to prepare working solutions in HCl 0.1 M (ranging from 1 to 100 μg/ml) that were utilized for chromatographic peaks identification and to calculate the calibration curves.
Cheese Sampling
Four types of semi-hard “pecorino” cheeses manufactured in the same dairy factory in the province of Pisa were analyzed. Each type was sampled twice, collecting three samples from the same batch each time. All types of cheese were made in batches of approximately 1500 L of milk, acidification was achieved with the addition of commercial mesophilic starter cultures, and the coagulation was obtained using a commercial calf powder rennet (1:125,000; chimosin 96%, pepsin 4%; Caglificio Clerici, Cadorago, Como, Italy). The curd was cut into hazelnut-sized grains and put into plastic molds; after draining by steaming, the cheeses were dry salted and then sent to the ripening room (temperature: 7°C, relative humidity: 92%).
The first type of cheese (Type 1) was manufactured with pasteurized milk and a commercial starter culture of mesophilic lactococci and homofermentative lactobacilli (Fiore Sardo, Centro Sperimentale del Latte SpA, Zelo Buon Persico, Lodi, Italy), and underwent a first ripening phase of 60 days in the ripening room and a subsequent 90-day period of ripening in a natural cave (temperature and humidity not controlled, approximate temperature: 7–8 °C in winter, 20–22 °C in summer, approximate relative humidity: 85–90%) in the region of Garfagnana, Tuscany; the final product weighed approximately 2.5 kg. For the second type of cheese (Type 2) raw milk and a commercial starter culture of Lactococcus lactis subsp. lactis and L. lactis subsp. cremoris (Lyofast MO 0.31, Sacco s.r.l., Cadorago, Como, Italy) were used. At the end of a 60 days long ripening period in the ripening room, Type 2 cheeses weighed approximately 2.2 kg. The third type of cheese (Type 3) was made from pasteurized milk with the same starter culture as Type 1 cheese. After 90 days of storage in the ripening room, Type 3 cheeses were ripened for 90 more days in a traditional “fossa” (temperature and humidity not controlled, approximate temperature and relative humidity values: 16°C and 85–90%, respectively) in Sogliano al Rubicone, in the region of Emilia-Romagna, Italy, where “fosse” have been used for centuries for the ripening of cheese. Type 3 cheeses weighed approximately 1 kg. The fourth type of “pecorino” cheese (Type 4) was produced with raw milk and the same starter culture as Type 2 cheese, and underwent an initial ripening period of 60 days in the ripening room. Type 4 cheeses were then covered with straw and ripened for 90 more days in a 18th century tuff cave (temperature and humidity not controlled, approximate values: temperature: 13–14°C in winter, 17–18°C in summer; relative humidity higher than 90%) in the province of Pisa. Type 4 cheeses weighed approximately 2.4 kg.
BAs Extraction and Derivatization
Preparation of samples for the chromatographic analysis followed the procedure previously described by Innocente et al.,[Citation5] with some modifications. For each sample, 10 g of ground cheese were added with 20 ml of HCl 0.1 M and 100 μl of IS (10 mg/ml) and homogenized with a Lab-Blender 80 Stomacher (PbI, Milan, Italy) for two min. The samples were then centrifuged at 12,000 g for 20 min at 4°C; the aqueous layer was collected and the pellet re-extracted using the same procedure. The two aqueous extracts were combined and brought up to a final volume of 50 ml with HCl 0.1 M. For the derivatization, 2 ml of filtered extract or of the standard solution were added with 1 ml of saturated NaHCO3 solution, and the final pH value was adjusted to 11.5, as indicated by Moret et al.[Citation6] using NaOH 5 M and a GLP 21 pH meter (Crison Instruments S.A., Barcelona, Spain). Then, 2 ml of the derivatizating agent DCl (5 mg/ml in acetone) were added to the mixture that was incubated for 60 min at 40°C with occasional shaking. Afterwards, 400 μl of L-proline (100 mg/ml) were added to the sample to remove the excess of DCl, and left to react 15 min at room temperature in the dark. The sample was then extracted twice with 2 ml of diethyl ether; the organic layers were combined and dried under nitrogen flow at room temperature. The residue was re-dissolved in 1 ml of ACN and was then ready for the HPLC analysis.
HPLC Apparatus and Chromatographic Conditions
HPLC analyses were performed with a Jasco HPLC apparatus (Jasco Corporation, Japan) equipped with two gradient pumps (PU-1580), a mixer unit (HG-2080-03), and a UV detector (870-UV). The stationary phase was a RP Gemini C18 column (250 mm × 4.60 mm × 5 μm; Phenomenex, Torrance, CA, U.S.A.). The mobile phase contained water (A) and ACN (B) and was eluted at a flow rate of 0.8 ml/min using the following gradient: time = 0 min, 65% B; time = 1 min, 65% B; time = 10 min, 80% B; time = 14 min, 80% B; time = 21 min, 100% B; time = 30 min, 100% B. The injection volume was 20 μl and the UV detector was set at 254 nm. All samples were extracted and analyzed twice and only the results with a coefficient of variation lower than 10% were considered.
Statistical Analysis
Statistical analysis was performed using the R v. 3.0.0 software (R Foundation for Statistical Computing, Vienna, Austria). The statistical significance of differences in single and total BAs concentrations among the four types of cheese was tested with the non-parametric Kruskal-Wallis test, followed by Tukey HSD post-hoc comparisons on the ranks of data. Results were considered significant if associated with a p value lower than 0.05.
RESULTS AND DISCUSSION
Single and total BAs concentrations of each of the four types of cheese are detailed in , and shows the chromatogram of a standard solution and the chromatogram of a cheese sample. In all cases TYR was the most abundant BA representing the 82.03% of the total BAs content in Type 1 cheese, the 53.79% in Type 2, the 81.00% in Type 3, and the 52.37% in Type 4. PUT, CAD, 2PHE, and TRP were also present in different degrees. HIS could only be detected in Type 4 cheeses while SPD and SPM were only present in traces or not detected. These data on the most abundant BAs are in agreement with previous studies on similar cheeses.[Citation7−Citation11] The results were almost invariably associated with a high standard deviation, showing a great variability in BAs content even among the same type of cheese; a similar variability has been reported by other authors in Italian traditional ripened “pecorino” cheeses.[Citation7,Citation8,Citation12]
TABLE 1 Concentrations (average ± standard deviation) and percentage composition of single and total biogenic amines in the four types of “pecorino” cheese under analysis
FIGURE 1 HPLC chromatograms of a standard solution (A) and of a cheese sample (B): 1, tryptamine; 2, 2-phenylethylamine; 3, putrescine; 4, cadaverine; 5, histamine; 6, 1,7-diaminoheptane (internal standard); 7, tyramine; 8, spermidine; 9, spermine.
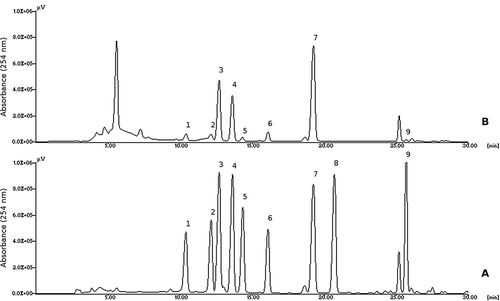
The four types of cheese had very different total BA concentrations. In particular, Type 3 and Type 4 cheeses showed significantly higher total BAs and TYR concentrations; in both types, TYR and 2PHE concentrations were higher than the safety levels (100–800 mg/kg for TYR and 30 mg/kg for 2PHE) proposed by some authors[Citation1] and the total amount of amines was higher than 1000 mg/kg, indicated as a health risk level.[Citation13] Such relevant levels of BAs have been previously found mainly in blue cheeses,[Citation14−Citation17] but have also been recently reported in not molded ripened cheeses. In fact, total BAs values higher than 1000 mg/kg have been found in four different batches of Pecorino di Farindola, two of which had also more than 800 mg/kg of TYR[Citation8] in Formaggio di Fossa cheese[Citation9] in an experimental Pecorino Abruzzese cheese[Citation12] in goats’ cheese[Citation18] and in samples of herby cheese (otlu peynir) from Turkey[Citation19] two of which had more than 1000 mg/kg of TYR. A high total BA content, but lower than 1000 mg/kg has also been reported in a raw ewes’ milk Terrincho cheese.[Citation11]
Although some data indicate a lowering effect of pasteurization on BAs production[Citation15,Citation17,Citation20,Citation21] some authors report no effect or an opposite effect of thermal treatments.[Citation10,Citation12] In this study, Type 1 cheeses made from pasteurized milk had significantly lower levels of BAs compared to Type 4 cheeses manufactured with raw milk and with a similar ripening process. However, the effect of other variables, such as different starter cultures or variations of the temperature of the two caves, cannot be ruled out. On the other hand, Type 3 cheeses, manufactured with pasteurized milk, had a high BA content; this could be due to the particular ripening conditions inside the “fossa.” Such conditions have been reported as a contributing factor to BAs formation in cheese,[Citation9] thanks to the anaerobic environment that promotes fermentative, proteolytic, and lipolytic processes and also the availability of free amino acids. Moreover, since the decarboxylase activity can be independent of the microbial cell viability and integrity[Citation22] the microbial population of raw milk could have influenced the BAs presence in this type of pasteurized milk cheese.[Citation10] Besides, Ladero et al.[Citation23] showed that some BAs producing microorganisms can survive pasteurization conditions, although the thermal treatment greatly reduces the load of decarboxylase-positive microorganisms. In this regard, it is noteworthy that in this study TYR made up more than 80% of the total BAs content in pasteurized milk cheeses (82.03% in Type 1 and 81.00% in Type 3), whereas the BAs composition of raw milk cheeses was more varied and TYR represented only the 53.79% of total BAs in Type 2 cheese and 52.37% in Type 4. This difference in the relative presence of BAs could be the result of the selective effect of the pasteurization on the microbial population of milk with a reduced variability of decarboxylase-positive microorganisms.
The lower concentration of BAs in Type 2 cheeses, although manufactured with raw milk, could be explained with the short ripening period (60 days), whereas all the three other types of cheese underwent a ripening period at least double that length. Fernández-García et al.[Citation24] reported for raw ewes’ milk Manchego cheese at 60 days of ripening very similar values of TYR which almost doubled after 30 more days of ripening. Several authors have indeed reported that the length of the ripening process is an important factor influencing the final BA content of cheeses.[Citation11,Citation15,Citation17,Citation25]
CONCLUSIONS
Several examined samples of “pecorino” cheese had a high total content of BAs and of TYR in particular. These preliminary data show that in semi-hard “pecorino” high values of BAs could be less uncommon than they are usually thought to be. Although pasteurization is a limiting factor for the accumulation of BAs in cheese, it may not be sufficient to guarantee a low BAs concentration in the final product, since the length and type of ripening are important factors too. When cheeses are manufactured with raw milk and/or with a particular ripening, special care must be applied to the microbial quality of the milk used in the cheese-making process, and to the respect of the good manufacturing practices, in order to limit BAs formation and accumulation.
REFERENCES
- ten Brink, B.; Damink, C.; Joosten, H.M.L.J.; Huis in ’t Veld, J.H.J. Occurrence and formation of biologically active amines in foods. International Journal of Food Microbiology 1990, 11 (1), 73–84.
- Nout, M.J.R. Fermented foods and food safety. Food Research International 1994, 27 (3), 291–298.
- Stratton, J.E.; Hutkins, R.W.; Taylor, S.L. Biogenic amines in cheese and other fermented foods: A review. Journal of Food Protection 1991, 54 (6), 460–470.
- Loizzo, M.R.; Menichini, F.; Picci, N.; Puoci, F.; Spizzirri, U.G.; Restuccia, D. Technological aspects and analytical determination of biogenic amines in cheese. Trends in Food Science & Technology 2013, 30, 38–55.
- Innocente, N.; Biasutti, M.; Padovese, M.; Moret, S. Determination of biogenic amines in cheese using HPLC technique and direct derivatization of acid extract. Food Chemistry 2007, 101 (3), 1285–1289.
- Moret, S.; Conte, L.; Spoto, E. Presenza delle amine biogene nei formaggi: Fattori che ne influenzano le determinazioni analitiche [Presence of biogenic amines in cheeses: Factors influencing their analytical determination]. Industrie Alimentari Italy 1996, 35, 788–792.
- Forzale, F.; Nuvoloni, R.; Pedonese, F.; D’Ascenzi, C.; Giorgi, M. Biogenic amines content in Tuscan traditional products of animal origin. Medycyna Weterynaryina 2011, 67 (2), 110–114.
- Schirone, M.; Tofalo, R.; Mazzone, G.; Corsetti, A.; Suzzi, G. Biogenic amine content and microbiological profile of Pecorino di Farindola cheese. Food Microbiology 2011, 28 (1), 128–136.
- Mascaro, N.; Stocchi, R.; Ricciutelli, M.; Cammertoni, N.; Renzi, F.; Cecchini, S.; Loschi, A.R.; Rea, S. Biogenic amine content and chemical and physical features of Italian Formaggio di Fossa [Article in Italian with English abstract]. Rivista dell’Associazione Italiana Veterinari Igienisti 2010, 8, 49–53.
- Lanciotti, R.; Patrignani, F.; Iucci, L.; Guerzoni, M.E.; Suzzi, G.; Belletti, N.; Gardini, F. Effects of milk high pressure homogenization on biogenic amine accumulation during ripening of ovine and bovine Italian cheeses. Food Chemistry 2007, 104 (2), 693–701.
- Pinho, O.; Pintado, A.I.E.; Gomes, A.M.P.; Pintado, M.M.E.; Malcata, F.X.; Ferreira, I.M.P.L.V.O. Interrelationship among microbiological, physicochemical, and biochemical properties of Terrincho cheese, with emphasis on biogenic amines. Journal of Food Protection 2004, 67 (12), 2779–2785.
- Martuscelli, M.; Gardini, F.; Torriani, S.; Mastrocola, D.; Serio, A.; Chaves-López, C.; Schirone, M.; Suzzi, G. Production of biogenic amines during the ripening of Pecorino Abruzzese cheese. International Dairy Journal 2005, 15, 571–578.
- Silla-Santos, M.H. Biogenic amines: Their importance in foods. International Journal of Food Microbiology 1996, 29 (2), 213–231.
- Mayer, H.K.; Fiechter, G.; Fischer, E. A new ultra-pressure liquid chromatography method for the determination of biogenic amines in cheese. Journal of Chromatography A 2010, 1217 (19), 3251–3257.
- Fernández, M.; Linares, D.M.; Del Rio, B.; Ladero, V.; Alvarez, M.A. HPLC quantification of biogenic amines in cheeses: Correlation with PCR-detection of tyramine-producing microorganisms. Journal of Dairy Research 2007, 74 (3), 276–282.
- Gosetti, F.; Mazzucco, E.; Gianotti, V.; Polati, S.; Gennaro, M.C. High performance liquid chromatography/tandem mass-spectrometry determination of biogenic amines in typical Piedmont cheeses. Journal of Chromatography A 2007, 1149 (2), 151–157.
- Novella-Rodríguez, S.; Veciana-Nogués, M.T.; Izquierdo-Pulido, M.; Vidal-Carou, M.C. Distribution of biogenic amines and polyamines in cheese. Journal of Food Science 2003, 68 (3), 750–756.
- Galgano, F.; Suzzi, G.; Favati, F.; Caruso, M.; Martuscelli, M.; Gardini, F.; Salzano, G. Biogenic amines during ripening in “Semicotto Caprino” cheese: Role of enterococci. International Journal of Food Science & Technology 2001, 36 (2), 153–160.
- Andic, S.; Genccelep, H.; Kose, S. Determination of biogenic amines in herby cheese. International Journal of Food Properties 2010, 13 (6), 1300–1314.
- Novella-Rodríguez, S.; Veciana-Nogués, M.T.; Roig-Sagués, A.X.; Trujillo-Mesa, A.J.; Vidal-Carou, M.C. Evaluation of biogenic amines and microbial counts throughout the ripening of goat cheeses from pasteurized and raw milk. Journal of Dairy Research 2004, 71, 245–252.
- Pattono, D.; Bottero, M.T.; Civera, T.; Grassi, A.; Turi, R.M. Frazioni azotate e amine biogene nel formaggio—Influenza di alcune variabili introdotte nel processo di caseificazione [Nitrogen fractions and biogenic amines in cheese - Effect of some variables introduced in the cheese-making process]. Industrie Alimentari Italy 2002, 41 (11), 1186–1190.
- Moreno-Arribas, V.; Lonvaud-Funel, A. Tyrosine decarboxylase activity of Lactobacillus brevis IOEB 9809 isolated from wine and L. brevis ATCC 367. FEMS Microbiology Letters 1999, 180 (1), 55–60.
- Ladero, V.; Sánchez-Llana, E.; Fernández, M.; Alvarez, M.F. Survival of biogenic amine-producing dairy LAB strains at pasteurization conditions. International Journal of Food Science & Technology 2011, 46, 516–521.
- Fernández-García, E.; Tomillo, J.; Núñez, M. Effect of added proteinases and level of starter culture on the formation of biogenic amines in raw milk Manchego cheese. International Journal of Food Microbiology 1999, 52, 189–196.
- Komprda, T.; Burdychová, R.; Dohnal, V.; Cwiková, O.; Sládková, P.; Dvořáčková, H. Tyramine production in Dutch-type semi-hard cheese from two different producers. Food Microbiology 2008, 25, 219–227.
