Abstract
This study analyzed the increment kinetics of acidity in French bread during baking and storage and discussed some critical control points based on the acidity of French bread. The reactions of acidity increments in baking and storage were first order reactions and the relationship between reaction rate and temperature fitted the Arrhenius equation. The activation energy of acidity increment during baking was 16.80 kJ/mol. Furthermore, by adding antioxidants, the activation energy of acidity increment in the storage increased from 14.50 to 19.25 kJ/mol. Antioxidants, such as 3-tert-butyl-4-hydroxyanisole, could be used to control the acidity increment in French bread. Finally, some critical control points in the production of French bread, which are important to the property of bread, include adjusting fermentation temperature, checking oil quality, adding appropriate amount of salt, and checking the cleanness of baking molds.
INTRODUCTION
French bread (“baguette” or other shapes) which was developed in France is favored by the people around the world now.[Citation1] Quality is an important concern in the production and storage of food. The factors used to evaluate the quality of French bread include the bread crumb rheological properties, bread cohesiveness and hardness, color of bread crust, and so on.[Citation2,Citation3] In order to improve the flavor and quality of French bread, people use different kinds of fat, such as butter, egg yolks, and shortening, as the ingredients.[Citation1] The fat in bread can act as a lubricant which contributes to a greater volume, smoother texture, and finer crumb structure. As a result, French bread becomes much more tender, chewy, and buttery.[Citation4] Furthermore, fat can retard the firming rate of bread during storage and extend the shelf life of bread.[Citation4] Therefore, fat plays an important role in the production of French bread.
Acidity is an important factor reflecting the amount of carboxylic acid groups in food.[Citation5] To improve the food quality, the acidity should be controlled at a low level. In food processing and storage, factors impacting the acidity include processing temperature, growth of microorganisms, properties of raw materials, and so forth.[Citation6] In the production and storage of edible oil, high temperature and growth of microorganisms can cause the deterioration of fatty acids and improve the acid value.[Citation7] Oil with high acidity always has bad odor and flavor. Therefore, in the food industry, acidity is used as a significant factor to evaluate the quality of oil and food stuffs containing oil.[Citation5]
Due to the high content of oil in French bread, oil deterioration can cause bad flavor and negatively impact the consumers’ health.[Citation8,Citation9] For instance, the intake of deep-fried oil can increase the risks of cancer and high blood pressure.[Citation10] So the quality of French bread is largely influenced by the quality of oil. To our knowledge, acidity, which is used to evaluate the fat and oil quality, has not been widely used in the quality evaluation of French bread. Therefore, using acidity to evaluate the quality of French bread can help people further improve the processing technology and storage conditions.
The primary objectives of this study included analyzing the changes of acidity of bread using a kinetics model and improving the processing technologies of French bread to prevent the rapid increment of acidity during baking and storage. We focused on the increment kinetics of acidity during baking and storage and analyzed some critical control points (CCPs) which can impact the acidity of final products. In the first part, the increment kinetics of acidity in French bread in baking conditions was analyzed and the activation energies of acidity increment were calculated. In the second part, increment kinetics of acidity in different storage conditions was analyzed. In the third part, impacts of some CCPs, including quality of oil, cleanliness of baking molds, and fermentation temperature, on the acidity of French bread were evaluated.
MATERIALS AND METHODS
Materials
Wheat flour, the brand of which was Xiangxue, was purchased from market of COFCO Group (Zhengzhou, P.R. China). Dry yeast was obtained from Angel Yeast Co., Ltd (Zhengzhou, P.R. China). BHA (3-tert-butyl-4-hydroxyanisole) was obtained from Sigma Co., Ltd (USA). Chicken eggs, salt, butter, and sugar were obtained from local markets in Zhengzhou City, P.R. China.
Baking Process and Storage Condition
The standard making process of French bread was described as follows: wheat flour (200 g), salt (1 g), butter (4 g), sugar (16 g), yeast (1.6 g), BHA (0.04 g), and water were mixed and kneaded by hand for 20 min. The dough was covered by a piece of plastic wrap and proofed at 38°C with 90% for 60 min. Dough was proofed sufficiently when it had doubled in size. The proofed wheat dough was deflated gently and baked in oven at 190°C for 12 min. Finally, the bread was put at room temperature to cool down.[Citation11] To evaluate the changes of acidity under different conditions, relevant factors, such as fermentation time, baking time, and salt content were adjusted in this study. After baking process, bread was covered by the plastic wrap and stored at 10, 20, and 30°C for 2, 4, 6, 8, and 10 days.
Analysis of Acidity
According to the definition of the World Health Organization (WHO), the acidity is the mass of potassium hydroxide (in milligrams) required to neutralize the free acid in 1 g sample. The analysis method used in this study was described in The International Pharmacopoeia (4th Ed.) from the WHO. The acidity was expressed as mg potassium hydroxide (KOH). To reduce the impact of environment on the final result, the French bread was analyzed immediately after baking or storage.
Increment Kinetics of Acidity
The proofed dough was baked at 160, 180, 200, 220, 240, and 260°C for different periods of time and the French bread was stored at 10, 20, and 30°C for 2, 4, 6, 8, and 10 days. The first order reaction model was employed to analyze the changes of acidity in baking and storage. The first order reaction equation could be expressed as Eq. 1.
where, t is time of baking in minutes or the storage time in days; k is the parameter of changes; C0 is initial value of acidity; C is the actual value of acidity at a certain baking or storage time. The values of k and k0 could be calculated depending on Eq. 1. Arrhenius equation was expressed as Eq. 2.
where, Ea is the activation energy; T is the absolute temperature (K); k is the parameter of acidity; R is the universal gas constant (8.318 kJmol–1K–1); and k0 is a constant.[Citation12] The curve of Arrhenius model was plotted by liner regression of Napierian logarithm of reaction rate constant (y) versus reciprocal temperature (x).[Citation13]
CCPs
Impacts of fermentation temperature, fermentation time, acidity of oil, addition of salt, and cleanness of baking molds on the acidity of French bread were analyzed. In the first part, the fermentation time ranged from 60 to 210 min and the fermentation temperature ranged from 20 to 40°C. In the second part, the edible oil with different acidities (0.15, 2.40, and 4.30 mg KOH) was added into dough before baking. In the third part, different amounts of salt were added into dough making the salt concentration to be 6, 8, 10, 12, and 14 mg/g dough. In the fourth part, French bread was baked in two kinds of molds, one with residuals and rust and one without residuals or rust. The acidities of French bread made in different conditions were analyzed and compared.
Statistical Analysis
All tests in this study were performed in triplicate. The average results were shown as means ± SD. Excel® (Microsoft) was used to analyze the variance of these results. Differences in means and F-tests were considered only when p < 0.05.
RESULTS AND DISCUSSION
Impact of Baking Temperature and Time on Acidity
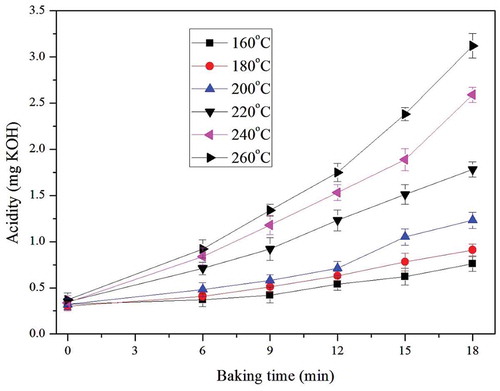
The acidity of the dough before being baked was 0.33 ± 0.02 mg KOH. The acidity increment of French bread was shown in . During baking process, the acidity increased with baking time. When the baking temperature was 200°C, the acidity was 0.56 mg KOH at 6 min and then increased to 1.23 mg KOH at 18 min. In addition, acidity increment rate was impacted by baking temperature. After being baked for 18 min, the acidity of the French bread increased by 0.44 mg KOH at 160°C while it increased by 2.75 mg KOH at 260°C. The average increment rate of acidity increased from 0.0244 mg KOH/min at 160°C to 0.1528 mg KOH/min at 260°C. Therefore, with the increase of temperature and baking time, the increment rate of acidity increased greatly. The main reason for the increment of acidity during baking is the deterioration of oil in bread. In the baking process, a high temperature could break down the compounds in oil and improve the content of free fatty acids in dough.[Citation14,Citation15] A higher baking temperature improved the reaction rate of oil deterioration. So the rate of acidity increment at higher baking temperature was higher than that at lower baking temperature.
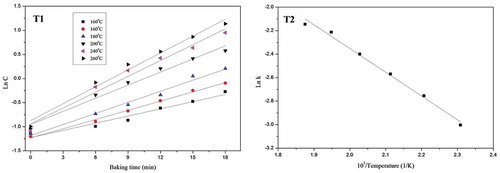
Numerical values for the change of acidity were shown in and . The parameters showed that acidity increment during baking was a first order reaction. (T2) indicated that acidity increment during baking could be fitted the Arrhenius model. The linear regression equation was Lnk = –2.0199/T + 1.6872 and R2 was 0.9915. So the activation energy of the acidity increment of the French bread during baking was 16.80 kJ/mol and the value of k0 was 5.40.
TABLE 1 Numerical values for the change of acidity during baking
Impact of Storage on Acidity
Currently, antioxidants are widely used to prevent fat deterioration and extend the shelf life of bread.[Citation16,Citation17] These antioxidants, which are usually used in food, could be divided into two categories, natural antioxidants and synthetic antioxidants. Natural antioxidants include astaxanthin, vitamin E, and polyphenols. However, the high price of natural antioxidants limits their wide use in the food industry. Therefore, synthetic antioxidants with low price are still mainly used in the food industry. BHA, one type of synthetic antioxidant, which consists of a mixture of 2-tert-butyl-4-hydroxyanisole and 3-tert-butyl-4-hydroxyanisole, is widely used in food protection. In this study, BHA, which is regarded as Generally Recognized As Safe (GRAS) substance by FDA, was used as antioxidant added in wheat dough.
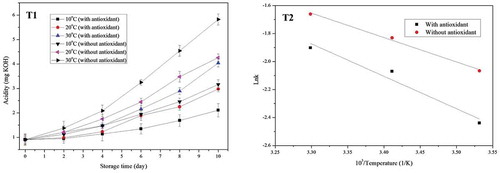
Two storage methods, storage with antioxidant and storage without antioxidant, were analyzed and compared. Changes of acidity in the storage without BHA and in the storage with BHA were shown in (T1). Under the same storage condition, the acidity increment in the storage without BHA was much larger than that in the storage with BHA. Numerical values for the change of acidity were shown in . The acidity increment in French bread was the first order reaction. The data in (T2) indicated that acidity increment during storage could be fitted the Arrhenius model. The linear regression equations for storage without BHA and the storage with BHA were Lnk = –1.742/T + 4.0967 and Lnk = –2.314/T + 5.762, respectively. The activation energy for the acidity increment in storage without BHA, which was 14.50 kJ/mol, was much lower than that for the acidity increment in storage with BHA, which was 19.25 kJ/mol. So the addition of antioxidant can effectively prevent the acidity increment and fat deterioration in French bread. However, many countries have strict regulations on the use of synthetic antioxidants in food.[Citation18,Citation19] Therefore, in the selection of antioxidants, both the antioxidant activity and the regulation of food agency should be evaluated.
TABLE 2 Numerical values for the change of acidity during storage
FIGURE 3 T1: Acidity changes during storage; T2: Arrhenius plot of increase rate constant versus reciprocal temperature (K) for acidity in storage.
The mechanism for the increment of acidity during storage is the auto-oxidation of oil in bread. Oil after auto-oxidation has more free fatty acids and higher acid value.[Citation20,Citation21] During storage, French bread is exposed to the air if it is not packed by the vacuum bag. Although the auto-oxidation of oil is slow, it could still improve the acidity of bread obviously in a long period of time. Therefore, how to control the rapid increment of acidity during storage is a critical concern. BHA, which is more likely to be oxidized than oil, will react with oxygen in air on priority. As a result, the auto-oxidation of oil in bread and the increment rate of acidity would be reduced to a low level.
Impact of Fermentation on Acidity
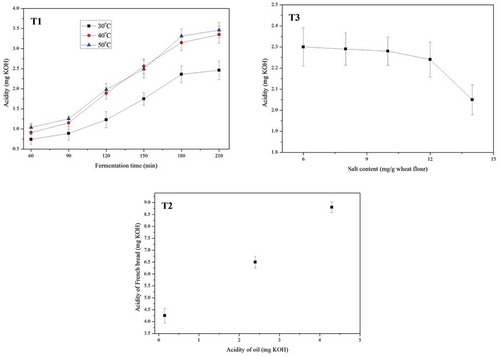
In the fermentation, growth of microorganisms can determine the physical properties of French bread, such as volume, density, and flavor.[Citation22] However, the growth of some microorganisms can cause acids, such as lactic acid, in dough.[Citation23] The impact of different fermentation temperatures and time duration on the acidity of French bread was shown in . The acidity of French bread increased with the extension of fermentation time. In this study, during the same fermentation time period, higher fermentation temperature improved the growth rate of microorganisms and the increment rate of acidity in dough. Therefore, fermentation temperature and time should be considered as a CCP in the production of French bread. Lower fermentation temperature and shorter fermentation time could not get the dough to be fermented fully. As a result, the sensory properties, including taste, shape, and flavor, of French bread would be negatively influenced. Therefore, as a CCP in French bread production, fermentation temperature and time should be controlled accurately.
Impact of Oil Quality on Acidity
To improve the flavor of French bread, before baking, oil was added into dough or on the surface of dough.[Citation24] Impacts of oil with different acidities on the acidity of French bread were shown in . The data in our work indicated that bread added with deteriorated oil had higher acid value. The mechanism for this phenomenon is that oil with high acid value is more likely to be further deteriorated in baking and storage.[Citation15] Some research indicated that the oil with poor quality was more likely to deteriorate in thermal treatment.[Citation25] So the highly deteriorated oil improved the increment rate of acidity of French bread in baking and storage. To reduce the negative impact of oil with poor quality on the French bread, checking the oil quality should be considered as an essential CCP in the production of French bread.
Impact of Salt on Acidity
indicated that acidity of French bread decreased with the addition of salt. Acidity was reduced to a low level when the amount of salt added in the wheat flour exceeded 14 mg/g wheat flour. Therefore, salt is an ingredient which can control acidity increment in French bread during baking. The main reason is that higher content of salt can prevent the growth of some microorganisms in dough during fermentation.[Citation26] In this way, acids in dough produced by microorganism activities during fermentation could be reduced to a low level. On the contrary, when the salt content in dough is low, the growth of most microorganisms could not be retarded and the preventive effect would not be obvious.[Citation26] So acidity of bread was still high after baking when the salt content was less than 12 mg/g wheat flour. When salt content increased from 12 mg/g wheat flour to 14 mg/g wheat flour, the growth of some microorganisms was retarded. Therefore, adding salt should be considered as a CCP which prevents the acidity increment in French bread during baking. In the commercial production of French bread, to meet different expectations of consumers, producers always add different amounts of salt to modify the taste and flavor of bread. Therefore, to optimize the salt content in wheat dough, both the acidity and flavor of French bread should be evaluated.
Impact of Cleanness of Baking Molds on Acidity
This study showed that the acidity of French bread baked in the mold without rust or residual was 1.32 mg KOH while that of French bread baked in the mold with rust and residual was 1.98 mg KOH. It indicated that the mold with rust can increase the acidity greatly. After the use of baking molds, oil and metallic oxides left on the surface of baking molds may not be cleaned by the users in a timely fashion. Both metallic oxide and oil on the surface of baking molds are regarded as rusts. The first reason for the increment of acidity was that in the baking process residual and metallic oxide on the mold served as catalysts to promote the deterioration of fat in bread. In most cases, baking molds, containing the wheat dough during baking process to control the shape of bread, was made of iron. After being used for a long time, iron may be oxidized and the metallic oxide may be left on the surface of baking mold. The second reason is that the oil residual on the mold, which has been heated for several times, can move into the French bread during baking. The highly deteriorated oil residual increases the acidity of French bread.
Furthermore, it was reported that the metallic oxide could serve as catalyst in the oxidation of organic components.[Citation27] Furthermore, deteriorated oil left on the surface of baking molds could release free radicals which could cause the auto-oxidation of vitamins, fatty acids and other components in the bread.[Citation28] Therefore, both metallic oxide and deteriorated oil left on baking molds pose serious threats to the quality of bread.
Therefore, the cleanness of baking molds is an important CCP in the production of French bread. To prevent the failure in this step, residual or rust on the baking mold should be cleaned and checked before baking. Furthermore, the baking mold could be made of stainless metal or the rust on the mold should be removed before baking. The mechanisms for the impact of different metallic oxides on the oxidation rate are very different.[Citation29] Therefore, to optimize the bread production process, further research on the impacts of different kinds of metallic oxide on the acidity change of French bread is needed.
CONCLUSIONS
In this study, increment kinetics of acidity in French bread during baking and storage was analyzed. Acidity increment in baking and storage followed the first-order reaction. The activation energy of acidity increment during bake was 16.80 kJ/mol. In addition, by adding antioxidant, activation energy of acidity increment in the storage increased from 14.50 kJ/mol to 19.25 kJ/mol. This result theoretically supported the opinion that adding antioxidant could extend the shelf life of food, including French bread. Finally, acidity is an important factor which could determine the taste and sensory property of bread. Therefore, to prevent the rapid increment of acidity, CCPs, which are important to the acidity, include adjusting fermentation temperature, checking oil quality, adding appropriate amount of salt, and checking the cleanness of baking molds, should be applied in the production of French bread.
REFERENCES
- Della Valle, G.; Chiron, H.; Jury, V.; Raitiere, M.; Reguerre, A.L.; Della Valle, G.; Chiron, H.; Jury, V.; Raitiere, M.; Reguerre, A.L. Kinetics of Crust Formation During Conventional French Bread Baking. Journal of Cereal Science 2012, 56, 440–444.
- Rouille, J.; Chiron, H.; Colonna, P.; Della Valle, G.; Lourdin, D.; Rouille, J.; Chiron, H.; Colonna, P.; Della Valle, G.; Lourdin, D. Dough/Crumb Transition During French Bread Baking. Journal of Cereal Science 2010, 52, 161–169.
- Quílez, J.; Ruiz, J.; Romero, M. Relationships Between Sensory Flavor Evaluation and Volatile and Nonvolatile Compounds in Commercial Wheat Bread Type Baguette. Journal of Food Science 2006, 71, S423–S427.
- Pareyt, B.; Finnie, S.M.; Putseys, J.A.; Delcour, J.A. Lipids in Bread Making: Sources, Interactions, and Impact on Bread Quality. Journal of Cereal Science 2011, 54, 266–279.
- Gutierrez, F.; Varona, I.; Albi, M.A.; Gutierrez, F.; Varona, I.; Albi, M.A. Relation of Acidity and Sensory Quality with Sterol Content of Olive Oil from Stored Fruit. Journal of Agricultural and Food Chemistry 2000, 48, 1106–1110.
- Hakoda, A.; Sakaida, K.; Suzuki, T.; Yasui, A.; Hakoda, A.; Sakaida, K.; Suzuki, T.; Yasui, A. Determination of the Acid Value of Instant Noodles: Interlaboratory Study. Journal of AOAC International 2006, 89, 1341.
- Armenta, S.; Garrigues, S.; de la Guardia, M.; Armenta, S.; Garrigues, S.; de la Guardia, M. Determination of Edible Oil Parameters by Near Infrared Spectrometry. Analytica Chimica Acta 2007, 596, 330–337.
- Xu, T.-T.; Li, J.; Fan, Y.-W.; Zheng, T.-W.; Deng, Z.-Y. Comparison of Oxidative Stability among Edible Oils under Continuous Frying Conditions. International Journal of Food Properties 2015, 18, 1478–1490.
- Ko, Y.-C.; Cheng, L.S.-C.; Lee, C.-H.; Huang, J.-J.; Huang, M.-S.; Kao, E.-L.; Wang, H.-Z.; Lin, H.-J. Chinese Food Cooking and Lung Cancer in Women Nonsmokers. American Journal of Epidemiology 2000, 151, 140–147.
- Chen, M.J.; Hsu, H.T.; Lin, C.L.; Ju, W.Y.; Chen, M.J.; Hsu, H.T.; Lin, C.L.; Ju, W.Y. A Statistical Regression Model for the Estimation of Acrylamide Concentrations in French Fries for Excess Lifetime Cancer Risk Assessment. Food and Chemil Toxicology 2012, 50, 3867–3876.
- Baardseth, P.; Kvaal, K.; Lea, P.; Ellekjær, M.; Færgestad, E. The Effects of Bread Making Process and Wheat Quality on French Baguettes. Journal of Cereal Science 2000, 32, 73–87.
- Cosgrove, J.P.; Church, D.F.; Pryor, W.A. The Kinetics of the Autoxidation of Polyunsaturated Fatty Acids. Lipids 1987, 22, 299–304.
- Brimberg, U.I. On the Kinetics of the Autoxidation of Fats. Journal of the American Oil Chemists’ Society 1993, 70, 249–254.
- Mohamed, K.M.; Elsanhoty, R.M.; Hassanien, M.F. Improving Thermal Stability of High Linoleic Corn Oil by Blending with Black Cumin and Coriander Oils. International Journal of Food Properties 2014, 17, 500–510.
- Makni, M.; Haddar, A.; Fraj, A.B.; Zeghal, N. Physico-Chemical Properties, Composition, and Oxidative Stability of Olive and Soybean Oils under Different Conditions. International Journal of Food Properties 2015, 18, 194–204.
- Tomaino, A.; Cimino, F.; Zimbalatti, V.; Venuti, V.; Sulfaro, V.; De Pasquale, A.; Saija, A.; Tomaino, A.; Cimino, F.; Zimbalatti, V.; Venuti, V.; Sulfaro, V.; De Pasquale, A.; Saija, A. Influence of Heating on Antioxidant Activity and the Chemical Composition of Some Spice Essential Oils. Food Chemistry 2005, 89, 549–554.
- Gougoulias, N.; Papachatzis, A.; Kalorizou, H.; Vagelas, I.; Giurgiulescu, L.; Chouliaras, N. Total Phenolics, Lycopene, and Antioxidant Activity of Hydroponically Cultured Tomato Sandin F1. Carpathian Journal of Food Science & Technology 2012, 4, 46–51.
- Lu, X.; Rasco, B.; Lu, X.; Rasco, B. Determination of Antioxidant Content and Antioxidant Activity in Foods using Infrared Spectroscopy and Chemometrics: A Review. Critical Reviews in Food Science and Nutrition 2012, 52, 853–875.
- Chikku Meera, C.; Estherlydia, D., Sensory, Physicochemical, and Antimicrobial Evaluation of Jams Made from Indigenous Fruit Peels. Carpathian Journal of Food Science & Technology 2013, 5, 69–75.
- Rohman, A.; Che Man, Y. Application of FTIR Spectroscopy for Monitoring the Stabilities of Selected Vegetable Oils During Thermal Oxidation. International Journal of Food Properties 2013, 16, 1594–1603.
- Kiokias, S.; Varzakas, T. Activity of Flavonoids and β-Carotene During the Auto-Oxidative Deterioration of Model Food Oil-in-Water Emulsions. Food Chemistry 2014, 150, 280–286.
- Bahal, G.; Sudha, M.; Ramasarma, P. Wheat Germ Lipoxygenase: Its Effect on Dough Rheology, Microstructure, and Bread Making Quality. International Journal of Food Properties 2013, 16, 1730–1739.
- Plessas, S.; Alexopoulos, A.; Bekatorou, A.; Bezirtzoglou, E.; Plessas, S.; Alexopoulos, A.; Bekatorou, A.; Bezirtzoglou, E. Kefir Immobilized on Corn Grains as Biocatalyst for Lactic Acid Fermentation and Sourdough Bread Making. Journal of Food Science 2012, 77, C1256–C1262.
- de Aguiar, A.C.; Boroski, M.; Monteiro, A.R.G.; de Souza, N.E.; Visentainer, J.V.; de Aguiar, A.C.; Boroski, M.; Monteiro, A.R.G.; de Souza, N.E.; Visentainer, J.V. Enrichment of Whole Wheat Flaxseed Bread with Flaxseed Oil. Journal of Food Processing and Preservation 2011, 35, 605–609.
- Tena, N.; Aparicio, R.; García-González, D.L.; Tena, N.; Aparicio, R.; García-González, D.L. Thermal Deterioration of Virgin Olive Oil Monitored by ATR-FTIR Analysis of Trans Content. Journal of Agricultural and Food Chemistry 2009, 57, 9997.
- Betts, R.; Roy Betts, D. Reducing Salt Levels in Food: Preliminary Observations on the Effect of Salt on the Predicted Growth of Selected Microorganisms in Meat Products; Campden & Chorleywood Food Research Association: Chipping Campden, United Kingdom, 2005.
- Xu, X.; Randorn, C.; Efstathiou, P.; Irvine, J.T. A Red Metallic Oxide Photocatalyst. Nature Materials 2012, 11, 595–598.
- Zhang, H.; Ji, Z.; Xia, T.; Meng, H.; Low-Kam, C.; Liu, R.; Pokhrel, S.; Lin, S.; Wang, X.; Liao, Y.-P. Use of metal Oxide Nanoparticle Band Gap to Develop a Predictive Paradigm for Oxidative Stress and Acute Pulmonary Inflammation. Acs Nano 2012, 6, 4349–4368.
- Utrera, M.; Estévez, M.; Utrera, M.; Estévez, M. Analysis of tryptophan Oxidation by Fluorescence Spectroscopy: Effect of Metal-Catalyzed Oxidation and Selected Phenolic Compounds. Food Chemistry 2012, 135, 88–93.