Abstract
Concentrations and profiles of polycyclic aromatic hydrocarbons were determined in some popular commercial brands of tea-, coffee-, and cocoa-based food drinks in Nigeria by gas chromatograph-flame ionization after hexane/dichloromethane extraction and clean up. The concentrations of the ∑16 Polycyclic aromatic hydrocarbons in these products ranged from 5.2–913.1, 38.7–593.1, and 38.0–1406.4 μg kg−1 for tea-, coffee-, and cocoa-based food drinks, respectively. The polycyclic aromatic hydrocarbon profiles indicate the dominance of three and four rings PAHs in these food items. The concentrations of the European Food Safety Authority (EFSA) eight carcinogenic polycyclic aromatic hydrocarbons ranged from nd–218.9, nd–102.9, and nd–1248.5 μg kg−1 for tea-, coffee-, and cocoa-based food drinks, respectively.
INTRODUCTION
Polycyclic aromatic hydrocarbons (PAHs) are a large group of over 100 different organic compounds that consist of two or more fused aromatic rings. PAHs have relatively low solubility in water and they are very lipophilic. PAHs are products of pyrolitic processes such as the incomplete combustion of organic matter, coal, and oil[Citation1,Citation2] as well as natural processes such as volcanic eruption, forest fire, and carbonization.[Citation3−Citation5] PAHs occur in the environment as complex mixture of different compositions. PAHs are widespread in foodstuffs as a consequence of environmental contamination, physiological and ecological characteristics of the product as well as thermal processes to which foods are subjected to during preparation and manufacturing.[Citation2,Citation6] The concentrations and profiles of PAHs in foodstuffs depend on the temperature, treatment time, energy sources, distances from the heating source, oxygen availability, the fat and endogenous PAHs content of the foodstuff, and the kind of combustible material used.[Citation7] Food treatment processes such as smoking, grilling, roasting, drying, and baking have been recognized as the main sources of food contamination by PAH compounds.[Citation4,Citation6,Citation8,Citation9]
The main health concern on PAH is due to the fact that some of them have proven carcinogenic and mutagenic properties. However, those PAHs that have not been found to be carcinogenic may synergically enhance the carcinogenicity of other PAHs.[Citation10,Citation11] PAHs have been implicated for different types of human cancers (e.g., breast, lung, and colon) as a result of metabolic activation in mammalian cell to dioexpoxides causing errors in DNA replication and mutation (which initiate the processes of carcinogenesis).[Citation12,Citation13]
The European Food Safety Authority (EFSA)[Citation3] has classified benzo(a)anthracene, chrysene, benzo(a)pyrene, benzo(b)fluoranthrene, benzo(k)fluoranthrene, dibenz(a,h)anthracene, benzo(g,h,i)perelyne, and indeno(1,2,3-cd)pyrene as the most carcinogenic PAHs and suggested that these eight higher molecular weight (PAH8) are the most suitable indicator for occurrence and effect of PAH in food rather than using benzo(a)pyrene alone.
Tea-, coffee-, and cocoa-based food drinks are beverages commonly consumed all the strata of the society. They are consumed for their health promoting properties such as anti-oxidative and nutritional effects. The presence of PAHs in tea-, coffee-, and cocoa-based food drinks originates from different sources including atmospheric pollution and technological processes, such as roasting and drying used in preparing these food drinks. Studies on the concentrations of PAHs in tea and coffee have been reported for Asian and European countries in the literature.[Citation14−Citation22] However, information on the concentrations and profiles of PAHs in commercial brands of tea-, coffee-, and cocoa-based food drinks available in Nigeria is limited. The objective of this study was to determine the concentrations and profiles of 16 priority PAHs in the different commercial brands of tea-, coffee-, and cocoa-based food drinks in the Nigerian market.
MATERIALS AND METHODS
Sampling
A total of 20 commercial brands of tea-, coffee-, and cocoa-based food drinks were purchased from different sales outlets in Warri, Agbor, Abraka, and Asaba (Nigeria). Information with respect to the origins of the examined samples is displayed in . The choice of the samples was carefully made to reflect popular brands consumed by different income classes and the choice of samples was influenced by availability during the period of study. The samples were stored in a freezer at 4°C prior to analysis.
TABLE 1 Information on the commercial brands of tea-, coffee-, and cocoa-based food drink
Reagents
All chemicals and reagents used were of analytical grade. Dichloromethane (DCM; LC grade), alumina and silica gel were obtained from BDH (Poole, UK), while n-hexane was obtained from Aldrich (USA). A PAH standard mixture (NIST, Baltimore, MD) containing the 16 priority PAHs was used in this study.
Sample Preparation, Extraction, and Clean-Up
Five grams of the subsamples were mixed with equal amount of drying agent, sodium sulphate (Na2SO4). The resulting dried material was poured into 33 mL cell and extracted with hexane and DCM in accelerated solvent extraction (ASE, 200, Dionex Sunnyvale, CA). The extraction cells were filled with solvent, pressurized to 14 MPa, and heated to 120°C for 6 min. Pressure and temperature was held constant for extraction time of 5 min and cells were rinsed with cold solvent (60% of cell volumes) and purged with argon 150 s. The static extraction and purge steps were repeated three times for each sample and the extracts were combined.[Citation20] The extracts were evaporated to 1 mL by using a rotary evaporator (35°C; 250 mbar) and purified by solid phase extractions with 2 g of aluminium oxide (5% deactivated lower part). The PAHs were subsequently eluted with 15 mL of hexane, 15 mL hexane and DCM (9:1), and 20 mL of hexane and DCM (4:1). The eluted fractions were combined and evaporated to approximately 0.5 mL using a stream of nitrogen gas.
Chemical Analysis
The PAHs in the eluted fraction was determined by using gas chromatography (HP 6890 Palo Alto, CA) equipped with a HP5 (Cross Linked PHME Siloxane; 0.25 μm film thickness, 0.25 μm × 30 m) and flame ionization detector (FID). The carrier gas was helium with a flow rate of 30 cm/s. The initial column temperature was 100°C and it was increased at a rate of 4°C/min to a final temperature of 310°C. The injector temperature was maintained at 250°C and the injection volumes were 2.0 μL in the splitless mode. Quantification was carried out by external standard method.
Quality Control/Quality Assurance and Statistical Analysis
To evaluate the extraction efficiency for the Target compounds, spike recovery studies were carried out by spiking some selected samples with known concentrations of the individual PAH compounds at three concentration levels and reanalyzing the samples. The recoveries for the PAH compounds ranged from 69.4–99.2%. The relative standard deviations for replicate analysis were less than 7.8%. The detection and quantification limits (LODs and LOQs) were evaluated on the basis of noise obtained with analysis blank sample (n = 4). The LOD and LOQ were defined as the concentration of the analyte that produced signal-to-noise ratio of three and ten, respectively. The percent recoveries, R2 values for the calibration, LOD, and LOQ values for the PAH compounds are displayed in . The matrix effect was evaluated by spiking the tea, coffee, and cocoa food drink samples with a concentration range used for calibration, then comparing the correlation coefficient and the slope of spiked standard to the matrix with the original standard calibration curve. No matrix effect was observed for any of the PAHs in any type of the matrices. Analysis of variance and the Tukey multiple-comparison test were used to determine whether the concentrations of the metals varied significantly within and between the groups with values less than 0.05 (p < 0.05) considered to be statistically significant. The statistical calculations were performed with SPSS version 11.5 (SPSS Inc., Chicago, IL, USA).
TABLE 2 Percent spike recoveries, R2 values for calibration, limit of detection (LOD), and limits of quantification (LOQ)
TABLE 3 Concentrations of polycyclic aromatic hydrocarbons (μg kg−1) in tea-, coffee-, and cocoa-based food drinks
RESULTS AND DISCUSSION
Tea
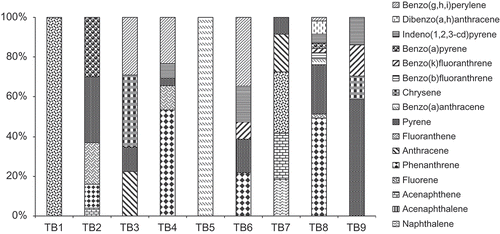
The concentrations of ∑16 PAHs of these brands of tea examined ranged between 5.2 and 913.1 μg kg−1 (). The lowest concentration of ∑16 PAHs was observed TB-2, No other PAH compound was detected in TB-1 except fluorene at concentration of 5.2 μg kg−1. Ziegenhals et al.[Citation20] report found concentration of US EPA 16 priority PAHs in 40 brands of tea in German’s market to range from 14 to 2662 μg kg−1. A wide concentration range of ∑16 PAHs have been reported in tea in the literature. For example, Bishnoi et al.[Citation19] reported PAH levels in the range of 18.79–13.37 μg/L. Ciemark and Mocek[Citation16] reported total PAHs concentration in tea as varying between 22.9 and 2945.5 μg kg−1. Lin[Citation17] found that ∑16 PAHs content in tea samples from China varied between 323 and 8880 μg kg−1. Ciecierska and Obiedzinski[Citation23] reported PAHs content of 8.64 to 12.43 μg kg−1 in fruit tea and herbal tea from Poland. Total PAHs concentration of 13.41 to 7536.33 μg kg−1 were found in tea.[Citation14] High levels of PAHs (536 ng/g to 2906 ng/g) have been reported in different brands of yerba mate leaves.[Citation24] The concentrations of PAHs in this study were within the concentration range previously reported in the literature.
The occurrence pattern and profiles of the PAHs in these brands differed significantly (). In these commercial brands of tea, the concentrations of the 3-ringed PAHs were higher than the 4-ringed PAHs except in brands TB-5 and TB-6. Fluorene, acenaphthene, and anthracene were detected in two brands out of the nine brands of tea examined at concentrations of 5.2–67.4, 18.4–50.5, and 33.7–41.5 μg kg−1, respectively. However, phenanthrene had the highest occurrence frequency and was detected in four out of the nine brands at concentrations of 55.0 and 450.5 μg kg−1 with brand TB-8 having the highest concentration. Three and four ringed PAHs were the dominant PAH compounds in these brands of tea. The dominance of three- and four-ring PAH compounds in tea have been reported in the literature.[Citation17,Citation23]
The four-ring PAHs (Flt+Pyr+BaA+Chy) concentrations were in the range of 18.5 to 250.1 μg kg−1 in these commercial brands of tea. Pyrene was found in seven out of the nine brands of tea studied at concentrations of 9.2–224 μg kg−1 which constituted 40.1 to 58.69% of the ∑16 PAHs in these samples. Fluoranthrene was detected in only three brands at concentrations in the range of 19.6 to 97 μg kg−1 and constituted up to 21.1% of the ∑16 PAHs in these commercial brands of tea (). Benzo(a)anthracene and chrysene were found in two brands of the tea at concentrations of 28.6 to 44 μg kg−1 and 18.7 to 56.6 μg kg−1, respectively.
Five ringed PAHs were found in only four brands at concentrations in the range of 25 to 224 μg kg−1. Benzo(k)fluoranthrene had the highest frequency of occurrence than benzo(b)fluoranthrene and benzo(a)pyrene in these brands of tea. Bkf concentrations of 22.4, 18.9, and 25.0 μg kg−1 were found TB-6, TB-8, and TB-9, respectively. BaP was detected at relatively high concentrations in two brands (TB-2 137.1 μg kg−1 and TB-8 26.8 μg kg−1). Ciemniak and Mocek[Citation16] reported BaP levels of 2.7 to 63.1 μg kg−1 in some brands of tea in Poland. BaP concentrations of 0.3 to 542.26 μg kg−1 have been reported in tea.[Citation14] Contrary to these findings, BaP concentration less than detection limit have also been reported in the literature.[Citation19,Citation23] Dibenz(a,h)anthracene was detected in a sample (TB-8) at concentration of 59.4 μg kg−1.
The six ringed PAHs, benzo(g,h,i)perylene and indeno(1,2,3-c,d)pyrene were found in four commercial brands of tea at levels of 16.7 to 88.2 μg kg−1 and 16.6 to 45.7 μg kg−1, respectively. In these brands of tea, BghiP and IndP constituted 1.83 to 34.64% and 4.52 to 13.95% of the ∑16 PAHs, respectively. The sum of the eight most carcinogenic PAHs (PAH8) according to EFSA[Citation3] in these brands of tea were in the range of 44 to 218.9 μg kg−1 with TB-8 having the highest concentration of the PAH8 compounds.
Coffee
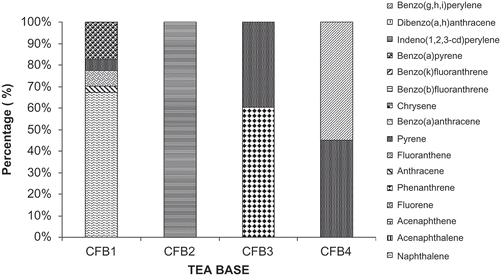
The concentrations of ∑16 PAHs in the different commercial brands of coffee ranged from 38.7–593.1 μg kg−1 (). Higher concentrations of ∑16 PAHs were found in CF-1 than in other brands. PAHs concentrations of 16.57 to 17.20 μg/L have been reported in some brands of coffee in India.[Citation19] Grover et al.[Citation22] reported concentrations of total PAHs in different brands of coffee as 831.7–1589.7 μg kg−1. Camargo and Toledo[Citation25] reported mean total PAH content in coffee in Brazil as 10.12 μg kg−1. The concentrations and profiles of the individual PAH compound varied from one brand to another (). The PAH contents of roasted coffee bean depend on the roasting temperature and roasting time. The concentrations of lower molecular weight PAHs such as phenathrene, fluoranthene, and pyrene increased during roasting process at temperatures above 220°C. Strong roasting conditions (260°C) could lead to significant amounts of pyrene, chrysene, and benzo(a)anthracene. Roasting at higher temperature results to possible transformation of lower molecular PAHs to higher molecular weight PAHs.[26] In these brand of coffee, naphthalene was detected only in brand CF-1 at concentration of 389.9 μg kg−1 constituting 67.26% of the ∑16 PAHs in that brand. The concentrations of three ring PAHs such as acenaphthylene, acenaphthene, and fluorene were below their respective limits of detection in these brands of coffee while phenanthrene and anthracene were found in brands CF-3 and CF-1, respectively. The concentrations of Phe and Ant in these two brands of coffee were 56.9 and 16.7 μg kg−1, respectively.
Pyrene was the dominant PAH compound in these commercial brands of coffee; its concentrations ranged from 30.1 to 44.0 μg kg−1. Fluoranthrene was found in sample CF-1 at concentration of 44.4 μg kg−1 while its concentrations in the other brands were less than detection limit. PAH compounds such as BaA, Chry, Bbf, Bkf, DahA were less than their respective limits of quantification in these samples of coffee, while BaP, IndP, and BghiP were found in samples CF1, CF-2, and CF-4 at concentrations of 102.9, 38.7, and 53.4 μg kg−1, respectively. The concentration of individual PAHs compounds such as BaP, Bbf, BaA, Chry, DahA, and BghiP in brew coffee have been reported to be less than 140 ng/L.[Citation26−Citation29] The concentrations of PAH8 were up to 102.9 μg kg−1 in these commercial brands of coffee.
Cocoa-Based Food Drinks
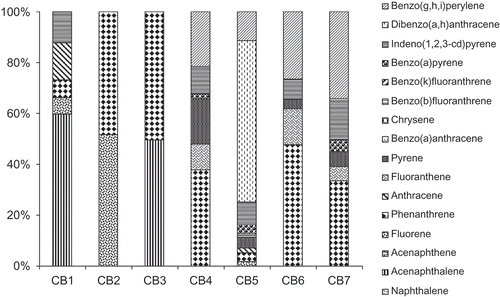
The concentrations of ∑16 PAHs in these cocoa based food drinks varied between 38.0 and 1406.4 μg kg−1 (). The highest concentration of the 16 PAHs was observed in the brand CB-5 while the lowest concentration was observed in CB-2. The major source of PAH in the cocoa-based food drinks is the cocoa powder and in addition to the thermal processes used during the manufacturing of these food drinks. The profiles of PAHs in these commercial brands of cocoa-based food drinks differ significantly (). Naphthalene and acenaphthene were not detected in these samples of cocoa-based food drinks, while fluorine and anthracene were detected in two brands at concentrations of 16.6 to 19.6 μg kg−1 and anthracene in three brands at concentration of 33.1 to 37.4 μg kg−1. Phenanthrene is the most dominant three-ring PAH compound in the cocoa-based food drinks. The concentration of phenanthrene ranged from 18.4 to 174.2 μg kg−1 which constituted 7.2 to 50.7% of the ∑16 PAHs. Fluoranthrene and pyrene were the only four-ring PAHs found in these commercial brands of cocoa-based food drinks. Pyrene was the most dominant of the four ringed PAH compounds with concentrations in the range of 12.5 to 59.6 μg kg−1. Benzo(a)anthracene and chrysene were not detected in the cocoa based food drinks.
The five ring PAHs (Bbf + BKf + BaP + DahA) were found in four samples at concentrations in the range of 59.6 to 107.5 μg kg−1. CB-4 had the highest concentrations of five ringed PAHs than other samples. Bbf was detected in CB-5 at concentration of 18.9 μg kg−1 while BaP was detected in CB-4, CB-5, and CB-7 at concentrations of 6.6, 53.2, and 24 μg kg−1, respectively. The European Union legislation has set 5 μg kg−1 as the maximum level of BaP in cocoa butter.[Citation30] BaP was found in these three samples of cocoa based food drinks (CB-4, CB-5, and CB-7) at concentrations above the permissible limits for cocoa butter. The concentration BaP found were higher than BaP concentrations reported in cocoa-based confectionaries reported previously in the literature.[Citation5,Citation20,Citation31] However, Kumari et al.[Citation32] found BaP concentrations in the range of 162 to 12.76 μg kg−1 in chocolate. Dibenz(a,h)anthracene was only detected in CB-5 at levels of 890.8 μg kg−1.
The six-ring PAHs were detected in five brands of the cocoa-based food drinks at concentrations ranging between 31.6 and 1176.5 μg kg−1. The highest concentrations of 6-ring PAHs were observed in CB-5. Indeno(1,2,3-c,d)pyrene was detected in five brands of cocoa-based food drink at concentrations between 28.5 and 123.8 μg kg−1 while BghiP occurred in four of the brands at levels of 82.4 to 177.4 μg kg−1. The highest concentration of BghiP was observed in CB7.
CONCLUSIONS
The results revealed that tea-, coffee-, and cocoa-based food drinks contained significant amounts of the eight carcinogenic PAHs which could pose a serious hazard to consumers who take these products on a regular basis. The PAH profiles indicate the dominance of 3-4-ring PAHs. The results of this study further stressed the need for good manufacturing practice among the processors of these foods. Also, relevant agencies such as Consumers Protect Rights, National Agency for Food, Drug Administration and Control (NAFDAC) and Standard Organization of Nigeria (SON) need to ensure and enforce strict compliance to the desired levels of these contaminants in foods and Hazard Analysis Critical Control Point (HACCP) in all food production.
ACKNOWLEDGMENTS
The authors are grateful to Miss Sunday Helen and Obiyan Peace for assistance with the collection of samples and to Dukoria (Nig.) Ltd. for chromatographic analysis.
REFERENCES
- Danyi, S.; Bose, F.; Brasseur, C.; Schneider, Y.J.; Larndelle, Y.; Pussemier, L.; Robbens, J.; De Saeger, S.; Maghuin-Rogister, G.; Scippo, M.L. Analysis of EU priority polycyclic aromatic hydrocarbons in food supplement using high performance liquid chromatography coupled to an ultraviolet, diode array, or fluorescence detector. Analytica Chemica. Acta. 2009, 633, 293–299.
- Martorell, I.; Perello, G.; Marti-cid, R.; Castell, V.; Llobet, J.M.; Domingo, J.L. Polycyclic aromatic hydrocarbons (PAH) in foods and estimated PAH intake by the population of Catalonia, Spain: Temporal trend. Environment International 2010, 36, 424–432.
- European Food Safety Authority (EFSA). Scientific opinion of the panel on contaminants in the food chain on a request from the European Commission on polycyclic aromatic hydrocarbons in food. The EFSA Journal 2008, 724, 1–114.
- European Commission (EC). Opinion on the Scientific Committee on Food on the Risk to Human Health of Polycyclic Aromatic Hydrocarbons in Food. Brussels, Belgium, 4 December, 2002.
- Food Safety Authority of Ireland (FSAI) 2006. Investigation into levels of polycyclic aromatic hydrocarbons (PAHs) in food on the Irish market. Ireland Food Safety Authority of Ireland. http://www.fsai.ie/surveillance/food_ safety/chemical/PAH-Levels.pdf (accessed November, 2012).
- Ciecierska, M.; Obiedzinski, M. Influence of smoking process on polycyclic aromatic hydrocarbons’ content in meat products. Acta Scientiarium Polonorum. Technologia Alimentaria 2007, 6 (4), 17–28.
- Visciano, P.; Perugini, M.; Amorena, M.; Janineri, A. Polycyclic aromatic hydrocarbons in fresh and cold smoked Atlantic salmon fillet. Journal of Food Protection 2006, 69, 1134–1138.
- Codex Committee on Food Additives and Contaminants (CCFAC). Discussion paper on polycyclic aromatic hydrocarbons contamination. 37th session. The Hague, The Netherlands, 2005.
- Moret, S.; Purcarco, G.; Conte, L.S. Polycyclic aromatic hydrocarbons in vegetable oils from canned foods. European. Journal of Lipid Science and Technology 2005, 107, 488–496.
- Wenzl, T.; Simon, R.; Anklan, E.; Kleiner, J. Analytical methods of polycyclic aromatic hydrocarbons (PAHs) in food and the environment needed for new food legislation in the European Union. Trends in Analytical Chemistry 2006, 26, 726–725.
- Al-Rashdan, A.; Murad, I.H.; Helaleh, N.A.; Ibtisam, A.; Al-Ballam, Z. Determination of the levels of polycyclic aromatic hydrocarbons in toasted bread using gas chromatography mass spectrometry. International Journal of Analytical Chemistry 2010. DOI:10.1155/2010/821216.
- USEPA (2002). Polycyclic organic matter. Environmental Protection Agency, Washington, DC. Available at: http://www.epa.gov/ttn/atw/hlthef/polycycl.html.
- Yoon, E.; Park, K.; Lee, H.; Yang, J.H.; Lee, C. Estimation of excess cancer risk on time-weighted life time average daily intake of PAHs from food ingestion. Human Ecological Risk Assessment 2007, 13, 669–680.
- Schlemitz, S.; Pfannhauser, W. Supercritical fluid extraction of monitored polycyclic aromatic hydrocarbons from tea—Correlation with the PAH concentration. Z. Lebensm Unters Forsch A 1997, 205, 305–310.
- Kayali-Sayadi, M.N.; Rubio-Barroso, S.; Cuesta-Jimenez, M.P.; Polo-Diez, L.M. Rapid determination of polycyclic aromatic hydrocarbons in tea infusion samples by high-performance liquid chromatography and fluorimetric detection based on solid-phase extraction. Analyst 1998, 123, 2145–2148.
- Ciemniak, A.; Mocek, K. Polycyclic aromatic hydrocarbons in tea and tea infusions. Roczniki Panstwowy Zaklad Higieny 2010, 61 (3), 243–248.
- Lin, D.; Yu, Y.; Zhu, L. Concentrations and health risk of polycyclic aromatic hydrocarbon in tea. Food Chemistry and Toxicology 2005, 43 (1), 41–48.
- Lin, D.; Zhu, L. Polycyclic aromatic hydrocarbons: Pollution and source analysis of a Black tea. Journal of Agriculture and Food Chemistry 2004, 52 (26), 8268–8271.
- Bishnoi, N.R.; Mehta, U.; Sain, U.; Pandit, G.G. Quantification of polycyclic aromatic hydrocarbons in tea and coffee samples of Mumbai City (India) by high performance liquid chromatography. Environmental Monitoring and Assessment 2005, 107, 399–406. DOI:10.1007/S 10661–05-3547-7.
- Ziegenhals, K.; Jira, W.; Speer, K. Polycyclic aromatic hydrocarbons (PAHs) in various types of tea. European Food Research and Technology 2008, 228, 83–91. DOI:10.1007/s00217-008-099-8.
- Orecchio, S.; Ciotti, V.P.; Culotta, L. Polycyclic aromatic hydrocarbons (PAHs) in coffee brew samples: Analytical method by GC-MS, profile, levels, and sources. Food and Chemical Toxicology 2009, 47, 819–826. DOI:10.1016/j.fct.2009.01.011.
- Grover, I.S.; Sharma, R.; Singh, S.; Pal, B. Polycyclic aromatic hydrocarbons in some grounded coffee brands. Environmental Monitoring and Assessment 2013, 185, 6459–6463.
- Ciecierska, M.; Obiedzinski, M. The GC-MS analysis of polycyclic aromatic hydrocarbons content in selected fruit and herbal teas. Herba Polonica 2009, 55 (2), 18–24.
- Kamangar, F.; Schantz, M.M.; Abnet, C.C.; Fugundes, R.B.; Dawsey, S.M. High level of carcinogenic polycyclic aromatic hydrocarbons in mate drinks. Cancer Epidemiology, Biomarker, and Prevention 2008, 17 (5), 1262–1268. DOI:10.1158/1055-9965.EPI-08-0025.
- Camargo, M.C.R.; Toledo, M.C.F. Coffee and mate tea as a dietary source of polycyclic aromatic hydrocarbons (PAHs) in Campinas. Ciencias Technologia Alimentaria Campinas 2002, 22 (1), 49–53.
- Housessou, J.K.; Maloung, S.; Leveque, A.S.; Delteil, C.; Heyd, B.; Camel, V. Effect of roasting conditions on the polycyclic aromatic hydrocarbons content in ground Arabica Coffee and Coffee Brew. Journal of Agriculture and Food Chemistry 2007, 55, 9719–9726.
- Hischenhuber, C.; Stijve, T. Determination of benzo(a)pyrene in roasted coffe and coffee brews by HPLC with fluorescence detection. Deursche Lebensmittel-Rundschau 1987, 83, 1–4.
- Kayali-Sayadi, M.N.; Rubio-Barroso, S.; Cuesta-Jimenez, M.P.; Polo-Diez, L.M. A new method for the determination of selected PAHs in coffee brew samples by HPLC with fluorimetric detection and solid phase extraction. Journal of Liquid Chromatography and Related Technology 1999, 22 (4), 615–627.
- Houessou, J.K.; Benac, C.; Delteil, C.; Camel, V. Determination of polycyclic aromatic hydrocarbons in coffee brew using solid-phase extraction. Journal of Agriculture Food Chemistry 2005, 53, 871–879.
- European Commission. Commission Regulation (EC) No. 1881/2006 of 19 December, 2006, setting maximum levels for certain contaminants in food stuffs. Official Journal of European Union 2006, L364, 5–24.
- Kazerouni, N.; Sinha, R.; Hsu, C.H.; Greenberg, A.; Rothman, N. Analysis of 200 food items for benzo(a)pyrene and estimation of its intake in an epidemiological study. Food and Chemical Toxicology 2001, 39, 423–436.
- Kumari, R.; Chaturvedi, P.; Ansari, N.G.; Murthy, R.C.; Patel, D.K. Optimization and validation of extraction method for analysis of polycyclic aromatic hydrocarbons in chocolate candies. Journal of Food Science 2012, 21 (1), T34–T40. DOI: 10.11.