Abstract
The properties of soaking water of some cereals under high temperature were determined, but limited information is available on chickpeas in the literature. Change in electrical conductivity, turbidity, color, and soluble solids content of soaking water of chickpeas were studied at 87, 92, and 97°C without ultrasound, and with 25 kHz frequency and different ultrasound powers (100–300 W) for 260 min. Water absorption and color values of chickpea seeds were also studied during soaking. All the properties of soaking water and chickpea seeds were significantly (P < 0.05) affected by temperature, ultrasound treatment, and power of ultrasounds indicating leaching of some nutritional and anti-nutritional compounds. The moisture absorption rate of chickpeas increased with the increase of temperature and power of ultrasound, while color of chickpeas changed inversely with soaking water. Results showed that leaching and reabsorption of some nutritional compounds occur simultaneously and ultrasounds can be used to enhance cooking operations.
INTRODUCTION
The chickpea (Cicer arietinum L.) is one of the oldest and most widely consumed legumes in the world, particularly in tropical and subtropical areas. It constitutes an important part of human and livestock diets in many parts of the world due to desirable nutritional properties (protein, fat, carbohydrate, calcium, phosphorus, magnesium, iron, copper, zinc, sodium, potassium, folic acid, B vitamins, and ascorbic acid).[Citation1,Citation2] The chickpea, like other legumes, is considered to be good for health due to its mutual compatibility for its properties in disease prevention, including cardiovascular diseases, diabetes, obesity, and possibly colon cancer.[Citation1]
Legumes are usually cooked before being used in the human diet to improve the protein quality by destruction or inactivation of the heat labile anti-nutritional factors.[Citation3] Also, germination and pressure cooking processes decreased in their total phenolic content and antioxidant activity of pulses.[Citation4] However, cooking causes considerable losses in soluble solids, especially vitamins and minerals.[Citation5] Probably due to combination of leaching and chemical destruction, riboflavin, thiamin, niacin, and pyridoxine content of chickpea seeds are reduced by cooking treatments.[Citation6] An increase in cooking temperature increased loss of total solids such as nitrogenous compounds, sugars, oligosaccharides, Ca, Mg, and water soluble vitamins (thiamine, riboflavin, and niacin).[Citation7−Citation9]
Ultrasound is a form of energy generated by sound waves of frequencies that are too high to be detected by human ear, i.e., above 16 kHz.[Citation10] It is a specialized and versatile technology with numerous applications in food processing, such as enhancing heat and mass transfer,[Citation11] reduced rice parboiling time.[Citation12] Han and Baik[Citation13] reported that ultrasound application on chickpeas during cooking promoted the leaching of oligosaccharides. Also, Yıldırım et al.[Citation14−Citation16] researced that thermosonication was used to increase the water absorption during soaking operation of chickpea. The drying time of habanero chili pepper following the ultrasound treatment was reduced from a 25 to a 50% compared to the results obtained in untreated samples.[Citation17]
The effect of cooking temperature on the hydration rate of legumes is well-researched.[Citation14,Citation15,Citation18,] Turbidity (as absorbance at 500 nm, for the starch and proteins), electrical conductivity (for the minerals and ionic compounds), and color (for the soluble pigments, vitamins, and coloring compounds) can be used to follow dissolved substances in the soaking water of chickpeas during cooking. The properties of the soaking water of some cereals on temperature were already determined,[Citation19] but limited information is available on the chickpea, including the ultrasound treatment on the properties of this legume. Also, effects of ultrasound treatment on the properties of soaking process have not been studied before. The objective of this study was to determine the effects of temperature, ultrasound treatment, and power of ultrasounds on the propeties of soaking water and chickpea seeds during soaking, which can be used to understand the cooking process.
MATERIALS AND METHODS
Certified chickpeas (inci) with initial moisture content of 11.58 % (d.b.) were obtained from Çukurova Agricultural Research Institute (Adana, Turkey) for this study. After removing foreign materials and damaged seeds, they were sieved to standardize the sizes, 7.5 to 9 mm. The average diameter was found to be 8.00 (±0.27) mm using digital micrometer (Mutitoyo No. 505–633, Japan).
Soaking/Cooking Operation
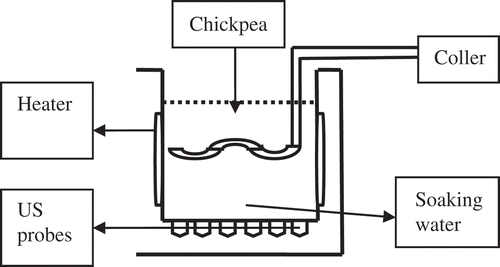
Chickpeas were soaked/cooked at 87, 92, 97°C and atmospheric pressure without pre-soaking. When the temperature of the soaking water (2400 mL) reached these values, 400 g of chickpeas were placed in soaking chamber (ultrasonic [US] tank, Intersonik, Turkey) with and without 25 kHz 100–300 W ultrasounds. Twenty grams of chickpea (to keep 1:6 chickpea/water ratio) and 120 mL of soaking water samples were collected within 20 min intervals during the total 260 min of cooking operations. Soaking water samples were used to determine color (L*, a*, and b* values), turbidity (absorbance at 500 nm), soluble solids content (brix, g/g%), and electrical conductivity (mS/cm) at 25°C. The chickpea seed samples were also used for color (L*, a*, and b* values) and water absorption (moisture content) determination. The procedure was repeated with 25 kHz 100 W (4 L, with acoustic energy densities [AED] of 0.025 W cm−3) and 25 kHz 300 W, 18 L (with AED of 0.017 W cm−3) ultrasonic applications. The temperature was kept constant by using a tubular cooler inserted into ultrasound tank. Diamentions of 4 L (25 kHz 100 W) and 18 L (25 kHz 300 W) volume capacity ultrasound tanks were 240 × 140 × 150 and 330 × 300 × 200 mm, respectively. Four and 18 L capacity ultrasonic tanks had 6, 12 probes (lead titanate) and 3, 6 transuders (piezoelectric crystals), respectively. Each ultrasonic probe was 60 mm in diameter and 75 mm in length. The ultrasonic tanks were operated intermittently ().
Electrical Conductivity and Turbidity of Soaking Water
The electrical conductivity (mS cm−1) of the soaking water was measured using conductometer (WTW, LF-330, Germany) at 25°C. Absorbances of the soaking water samples at 500 nm was measured using spectrophotometer (Pharmacia LKB-Novaspec II, UK) with deionized water as standard at 25°C.
Colors of Soaking Water and Chickpea Seeds
Color (L*, a*, and b*) of soaking water samples were measured using a Hunter Lab colorimeter (A60–1010–615, Colorflex, USA) at room temperature. The instrument was calibrated with a white standard tile (Lo* = 93.01, ao* = –1.11 and bo* = 1.30). L*, a*, and b*, or CIELab, color space is an international standard for color measurements, adopted by the Commission Internationale d’Eclairage (CIE) in 1976. L* is the luminance or lightness component, which ranges from 0 to 100, a* (from green –120 to red +120), and b* (from blue –120 to yellow +120).
Soluble Solids Content of Soaking Water
Soluble solids content of soaking water samples were measured using Abbe-refractometer (Opton-F.G. Bode and Co., Germany) at room temperature (25°C).
Moisture Content of Chickpea Seeds
The moisture contents of chickpea grains were determined in dry basis at 105°C for 48 h using oven-drying method.[Citation20] Average moisture content was subsequently calculated on a percentage dry basis (% d.b.).
Statistical Analysis
ANOVA and DUNCAN Multiple Range Tests, using SPSS version 16 (SPSS Inc., USA), at P < 0.05 were performed to determine effect of processing parameters. Graphs were plotted using Sigma PLOT 10 (Jandel Scientific, San Francisco, USA). The experiments were conducted in triplicate.
RESULTS AND DISCUSSION
Change in Electrical Conductivity of Soaking Water of Chickpeas with Temperature and Ultrasound Treatment
The presence of inorganic dissolved solids (electrolytes) such as nitrate, chloride, phosphate, and sulfate anions, or sodium, magnesium, calcium, iron, and aluminum cations affects on electrical conductivity of water. The major mineral components of chickpea are potassium, sodium, calcium, magnesium, sulfur, phosphorus, iron, cupper, and zinc.[Citation21] Increase in cooking temperature (87 to 97°C) of chickpea significantly (P < 0.05) increased electrical conductivity due to dissolved solids and electrolytes (). As the cooking temperature was increased from 87 to 97°C for 20 min and 120 min cooking, the electrical conductivity of soaking water increased from 1.66 (±0.08) to 2.78 (±0.08) and from 3.97 (±0.06) to 4.32 (±0.11) mS cm−1, respectively. Similar trends observed for all other cooking times. Furthermore, increase in cooking time increased electrical conductivity of soaking water of chickpea due to leached materials into soaking water such as from 1.66 (±0.08) to 4.77 (±0.04) mS cm−1 at 87°C for 20 and 260 min of cooking ().
FIGURE 2 Average change in electrical conductivity values of soaking water of chickpea with different temperatures and ultrasound treatments.
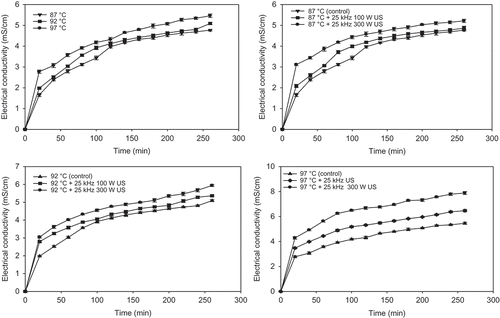
When ultrasound treatments, 25 kHz 100 W and 25 kHz 300 W were applied to chickpeas during 80 min of cooking at 87°C, the electrical conductivity increased significantly (P < 0.05) from 3.12 (±0.06) to 3.72 (±0.08) and 3.12 (±0.06) to 4.19 (±0.05) mS cm−1, respectively, when compared to cooking without ultrasound treatment (). Twenty-five kHz 100 W and 25 kHz 300 W ultrasounds at 87°C exhibited 1.92–25.90% and 6.91–63.86% increase in the electrical conductivities of soaking water of chickpeas compared with and without ultrasound samples for 20–260 min, respectively. Increase in power of ultrasound (from 100 to 300 W) also exhibited a 4.87–30.14% increase in the electrical conductivity of soaking water of chickpea. Similar increase observed at other temperatures (92 and 97°C). Results indicated that significant increase in leaching of electrolytes occurred with ultrasound treatment (25 kHz) and increase in the applied ultrasound power (100–300 W). Ultrasound may promote the leaching of electrolytes and soluble solids to soaking water due to cavitation and sponge effects on the chickpea. Ultrasound also could break cell structure,[Citation22] and broken cells may provide more pathways for soluble solids and other soluble materials to leach out from chickpea.
Change in Turbidity of Soaking Water of Chickpeas with Temperature and Ultrasound Treatment
The degree of turbidity of the soaking water may be used to decide the re-use of the water for next soaking operation and to prevent environmental problems caused by discharging the water. Measured absorbances at 500 nm were used to determine the turbidity level. Turbidity (absorbance at 500 nm) of soaking water, that is an important parameter to follow cooking operations, caused by leaching of organic compounds, pigments, proteins, sugars, starch, and vitamins into the water during cooking. The turbidity of blank (deionized water) used in cooking operation was 0.019 (±0.002; absorbance at 500 nm).
Absorbance of soaking water was affected significantly by temperature (P < 0.05) during soaking (). Absorbance values were found to be 0.53 (±0.02), 0.68 (±0.02), and 0.72 (±0.01) at 87, 92, and 97°C, respectively. The change in absorbance at low temperature (87°C) was less than at 92 and 97°C and this was due to the lower temperature effect on leaching.
FIGURE 3 Average change in turbidity values of soaking water of chickpea with different temperatures and ultrasound treatments.
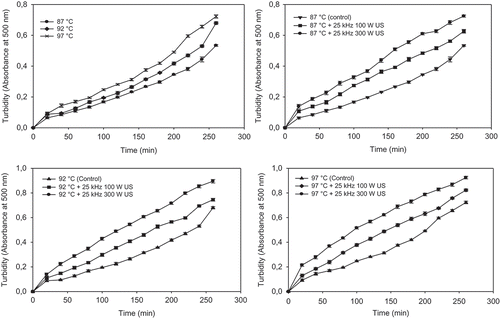
When ultrasound treatments (25 kHz 100 W and 25 kHz 300 W) were applied to chickpeas during 260 min cooking at all temperatures (87, 92, and 97°C) the turbidity of soaking water increased significantly (P < 0.05; 0.63 ± 0.01, 0.73 ± 0.02, 0.75 ± 0.01, 0.90 ± 0.03, 0.82 ± 0.04, 0.93 ± 0.01) compared the results of cooking without ultrasound treatments (0.53 ± 0.02, 0.68 ± 0.02, and 0.72 ± 0.02; ). Twenty-five kHz 100 W and 25 kHz 300 W ultrasounds at 87, 92, and 97°C exhibited 17.42–66.15% and 35.77–120.00%, 9.56–57.78% and 31.62–138.30%, 13.83–61.54% and 27.94–133.7% increase in the electrical conductivities of soaking water of chickpeas compared with without ultrasound samples for 20–260 min, respectively. Also, increase in power of ultrasounds (from 100 to 300 W) increased the turbidity of soaking water at 87°C for 20–260 min cooking of chickpea (15.63–36.75%). Ultrasound treatment and power of ultrasound increased in the absorbance or turbidity of soaking water due to more cavitaion and sponge effect of sonication. The increase in turbidity value of soaking water of chickpea may be attributed to leaching of amylase, amylopectin,[Citation23] and phosphate monoester derivatives,[Citation24] and other soluble matererials. Effect of high-power ultrasound to enhance protein and sugar release from defatted soybean flakes was studied by Karki et al.[Citation25] The study reported the increase in sugar and protein yield by 50 and 46%, respectively, when sonicated for 2 min at a high power level, i.e., 2.56 W/ml. The increase in yield was attributed to the cavitation phenomenon and resulting effect such as size reduction, increased mass, and heat transfer. An ultrasonic method for polysaccharide extraction from the herbal plant Salvia officinalis L. has been developed and increased yield by 18% relative to conventional polysaccharide extraction method.[Citation26]
Change in Soluble Solids (Brix) Content of Soaking Water of Chickpea with Temperature and Ultrasound Treatment
Leached solid for different grain products have been reported to be phytic acid, non-protein nitrogeneous compounds, sugars, minerals (Fe, Cu, Zn, Mn, P, Ca, Mg), pigments, starch, protein, water-soluble vitamins such as thiamine, riboflavin, and niacin.[Citation6,Citation27,Citation28] The temperature, time, and ultrasound powers significantly affect the soluble solids content of the soaking water (P < 0.05; ). Soluble solids content increased steadily until 260 min for 87, 92, and 97°C cooking. The rate of the change of soluble solids content increased with the increase of cooking temperature. Soluble solids contens of soaking water were measured as 1.98 ± 0.06, 2.09 ± 0.10 and 2.21 ± 0.06 at 87, 92, and 97°C for 120 min cooking, respectively. Similarly, at the end of cooking operation (i.e., 260 min), soluble solids contents were determined as 3.21 ± 0.06, 3.36 ± 0.07, and 3.41 ± 0.08 for 87, 92, and 97°C, respectively. A 6.35% higher soluble solids content was obtained at 97°C, when compared with 87°C of soaking. These results parallel the results of Wang et al.[Citation28] where cooking of soybean for 2 h resulted in a loss of 0.7–1.25% (total solids) as the temperature increased from 20 to 37°C, the results of Kar et al.[Citation29] on parboiling of dehusked rice where leaching losses ranged from 0.5 to 1.0% and the results of Bayram et al.[Citation17] for 120 min soaking of soybean where soluble solids content changed from 0.50 to 2.30% when the temperature increased from 30 to 70°C. The reported results were lower than the present study might be because of bran layers of grains.
FIGURE 4 Average change in soluble solids content of soaking water of chickpea with different temperatures and ultrasound treatments.
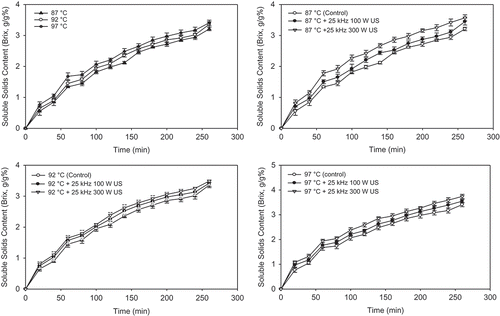
Ultrasound treatment also affected on soluble solids content of soaking water of chickpea at 87, 92, and 97°C during cooking. Soluble solids content and rate of change increased significantly (P < 0.05) with 25 kHz ultrasound treatment and ultrasound power (100 to 300 W) due to cavitaion and sponge effect of sonication on chickpea seeds (). This sponge effect may be attributed to leaching of more soluble solids to the soaking water. When compared with temperatures at 87, 92, and 97°C without ultrasound for 20–260 cooking, 25 kHz 100 W and 25 kHz 300 ultrasound treatments exhibited 2.85–26.79 and 11.21–46.43%; 3.57–30.77 and 7.74–43.07%; 4.11–25.97 and 9.68–40.26% increase in soluble solids contents of soaking water. Power of ultrasound (100–300 W) also exhibited a 3.48–18.56% increase in soluble solids of soaking water at 87°C during a 20–260 min soaking time range. Many studies on the effect of ultrasounds have been reported in the literature. An extraction yield of 34% of proteins from soybean was obtained when high-power ultrasound was applied prior to protein extraction from defatted soybean flakes.[Citation30] The energy released due to the collapse of cavitation bubbles promotes a higher penetration of the solvent into the cellular material and improves the mass transfer to and from the interfaces.[Citation31−Citation33] The benefit of using ultrasonic pretreatment before extracting oil from soybean and extracting carvone and limonene from caraway seeds have been reported.[Citation34,Citation35] These improvements were again attributed mainly to destruction of the cell wall and increased cell permeability along with the effects of solvent vapor pressure and surface tension on cavitation intensity.
Change in Color Values (L*, a*, and b*) of Soaking Water and Chickpea Seeds with Temperature and Ultrasound Treatment
The color (L*, a*, and b* values) of soaking water can be used to follow leaching of pigments and water-soluble vitamins. The main pigments of chickpea fall into the carotenoids class (β-carotene, cryptoxanthin, lutein, and zeaxanthin)[Citation36] although often small amounts of chlorophyll are also present. Water-soluble vitamins in chickpea are thiamine, riboflavin, niacin, panthothenic acid, vitamin B6, folate, ascorbic acid.[Citation37] More recent studies have shown that the ultrasonic disruption of buckwheat hulls for hemicelluloses components[Citation38] and sugarcane bagasse for celluloses[Citation39] accelerated the extraction process. The increase in hemi-cellulose extraction from sonicated buckwheat hull was attributed to the destruction of the cell wall and breakage of the links between lignin and hemicelluloses.[Citation40] Ultrasound has also been used for the extraction of proteins and enzymes from the cells.[Citation41−Citation43]
Increase in cooking temperature and time significantly (P < 0.05) increased the L* (lightness) value of soaking water due to leached materials especially chickpea starch (). Starch content of chickpea reported in the studies of Chavan et al.[Citation2] and Jood et al.[Citation44] were 55.3–58.1 and 48–58%, respectively. L* values of 0.1, 1, and 2% both raw and cooked chickpea flour suspensions in present study were found as 14.67 (±1.12), 46.14 (±1.03), 61.16 (±2.05); and 8.17 (±1.23), 42.44 (±1.07), 55.48 (±2.27), respectively. Although slightly lower values of L* were obtained from cooked chickpea solutions, results clearly shows that increased leaching increase L* values. Therefore, during cooking of chickpea starch might be leached to soaking water and enhanced to increase in L* value.
FIGURE 5 Average change in L* values of soaking water and chickpeas with different temperatures and ultrasound treatments.
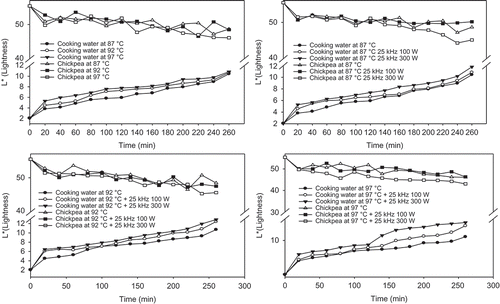
Increase in cooking temperature from 87 to 97°C at constant 120 min increased in L* values (lightness) of soaking water from 5.94 ± 0.08 to 7.78 ± 0.12 (). The L* value at 87°C increased from 2.06 ± 0.09 to 10.44 ± 0.08 during 260 min cooking. Same trend was observed for 92 and 97°C that it reached to 10.70 ± 0.12 and 10.85 ± 0.13 during 260 min cooking. But, increase in cooking time (0 to 260 min) and temperature (87 to 97°C) decreased (P < 0.05) L* value of chickpea seeds in contrast to L* value of soaking water (). Increase in cooking time from 0 to 260 min decreased lightness (L*) of chickpea from 55.43 ± 0.05 (L* value of uncooked chickpea) to 46.72 ± 0.17 at 97°C.
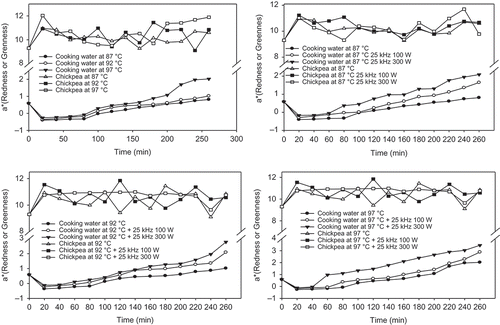
The change in a* value of soaking water and chickpea seeds for different time and temperatures are illustrated in . Increase in cooking time and temperature affected to a* value of soaking water and chickpea seeds. Increase in cooking time of chickpea from 20 to 260 min at 87°C changed a* value of soaking water from –0.40 ± 0.14 to 0.81 ± 0.08.This means that color of soaking water was changed from green to red color during soaking. Green color of soaking water resulted from disolving of chlorophyll from chickpea seed to water phase. After 220 min cooking at 87°C, the color of soaking water was changed from green to red (–0.40 ± 0.14 to 0.61 ± 0.10), likely due to more carotenoids destruction. Similarly, the values of a* value of soaking water were changed from –0.36 ± 0.10 to 0.48 ± 0.11 in 160 min at 92°C and from –0.26 ± 0.12 to 0.55 ± 0.14 in 140 min at 97°C, respectively. The color of soaking water was green (–a*) and this was due to leaching of chlorophyll pigments at the beginning of cooking operation, while it changed to red (+a*) at the end of cooking due to destruction of carotenoids. Increase in cooking time (20 to 260 min) and temperature (87 to 97°C) also increased a* value from 10.06 ± 0.07 to 11.49 ± 0.32 and from 11.03 ± 0.05 to 11.89 ± 0.09 of chickpea seed due to the browning and caramelization reactions that occurred in chickpea.
FIGURE 6 Average change in a* values of soaking water and chickpeas with different temperatures and ultrasound treatments.
The b* value (yellowness) of soaking water significantly (P < 0.05) increased with the increase in both cooking time and temperature (). At the end of cooking process, the yellowness (b*) of soaking water was 7.42 (±0.08), 8.43 (±0.06), and 8.97 (±0.02) at 87, 92, and 97°C, respectively. This increase in the yellowness can be explained as the leaching of yellow color pigments and vitamins by the effect of temperature in addition to loss of the greenness in soaking water. Futhermore, carotenoids (nearly red pigments which are non-polar and water-insoluble) that are found in chickpeas were not solubilized in soaking water at the early cooking times. Noisuwan et al.[Citation45] showed that only a very small amount of starch (1.2%) leached during the initial stages of gelatinization, and that the leached starch includes both amylose and amylopectin.
FIGURE 7 Average change in b* values of soaking water and chickpeas with different temperatures and ultrasound treatments.
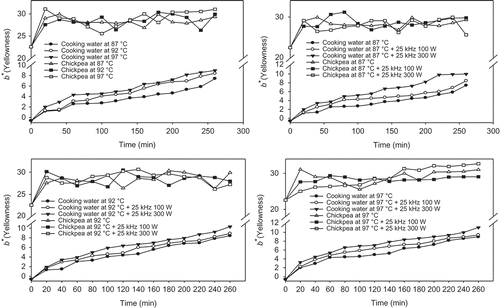
The b* value (yellowness) of chickpea seed increased slightly during cooking and was affected by cooking time and temperature, significantly (P < 0.05; ). Increase in b* value might be explained as the degradation of red pigments into yellow color and yellowish pigments and vitamins participate in chickpea. b* value of chickpea seed had a maximum value (31.0331.03 ± 0.08) at 97°C for 260 min cooking. This can be explained as the effect of temperature on the gelation of the protein at 87, 92, and 97°C and it might affect the appearance and firmness or existence of the degradation of yellowish pigments at this temperature, proportionally.
The color (L*, a*, and b* values) of soaking water of chickpea were changed with ultrasound treatment and power of ultrasounds (100 to 300 W; –). L* value of soaking water during 260 min at 87°C was increased from 10.44 (±0.04) to 10.84 (±0.02) for 25 kHz 100 W and 11.79 (±0.05) for 25 kHz 300 W. Similar increase in L* values were observed at 92 and 97°C cooking temperatures. The a* value of soaking water of chickpea without ultrasound, with 25 kHz 100 W and 25 kHz 300 W ultrasound treatments increased from 0.81 (±0.03) to 1.65 (±0.02) and 2.09 (±0.06) during 260 min at 87°C cooking. Trends of changes in a* values were similar at other cooking temperatures (92 and 97°C). The a* values of ultrasound treated chickpeas during cooking were red (+a*) and increased as the cooking time was increased at all cooking temperatures probably due to the browning reactions and starch gelatinization.
Thermosonication significantly (P < 0.05) affected the b* value of soaking water. During 260 min of cooking, b* values (yellowness) of soaking water increased from 7.42 (±0.08; blueness) to 8.44 (±0.06) and 9.97 (±0.04) for both 25 kHz 100 W and 25 kHz 300 W ultrasound treated samples at 87°C, respectively. Similarly, b* values of soaking water increased at 92 and 97°C cooking operations with ultrasounds treatment. Han and Baik,[Citation13] Akinyele and Akinlosotu,[Citation46] and Somiari and Balogh[Citation47] reported that change in color value (L*, a*, and b*) of legumes/chickpea during soaking and cooking was due to dissolved solids and oligosaccharides such as verbascose, raffinose, and stachyose. Change of color values might be due to mass transfer (leaching)[Citation12,Citation13,Citation48−Citation51] and some reactions such as browning. If the particles subjected to sonication are grains in water then some destruction of surface “shell” and grain fragmentation would be anticipated. Both of these effects would result in a faster release of starch during soaking/cooking.[Citation11] Thermosonication increased the water absorption during soaking operation of chickpea,[Citation14,Citation15,Citation17] changed in both color of soaking water and chickpea, leaching and reabsorption of some nutritional compounds might be occur simultaneously due to sponge effect and cavitation of ultrasounic pressure.
Change in Water Absorption of Chickpea with Temperature and Ultrasound Treatment
Seeds are usually soaked before cooking. The most important property for soaking of chickpeas is the moisture content to achieve the proper cooking operation. Understanding water absorption in legumes during soaking is of practical importance since it affects subsequent processing operations and the quality of the final product.
Acoustic energy causes oscillating velocities and micro streaming at the interfaces which may affect the diffusion boundary layer. Ultrasonic waves also produce rapid series of alternative contractions and expansions (sponge effect) of the material in which they are traveling; this alternating stress creates microscopic channels, which may make the moisture gain easier. In addition, acoustic waves may produce cavitations of water molecules inside the solid matrix, which may be beneficial for the gain of strongly attached moisture.[Citation52,Citation53]
The moisture content (% g/g, d.b.) of chickpea during soaking were significantly (P < 0.05) increased as the temperature and power of ultrasounds increased due to water absorption (). Rate of increase in moisture content was higher during the early stage of soaking, whereas lower in the late soaking periods because of approaching the saturation. Chickpea’s water adsorption curves were illustrated in , and are characterized by an initial phase of rapid water pickup followed by an equilibrium phase, during which the chickpea approaches its full soaking capacity. Results indicated that increasing soaking temperature enhanced water pickup in the initial phase, increasing the slope of the water absorption curve, thereby leading to faster attainment of the equilibrium phase, and this was in agreement with previously published data.[Citation54] The behavior of material during moisture absorption depends on the heat and mass transfer characteristics of the product.[Citation55]
FIGURE 8 Average change in hydration values (moisture content, % g/g, d.b.) of chickpeas with different temperatures and ultrasound treatments.
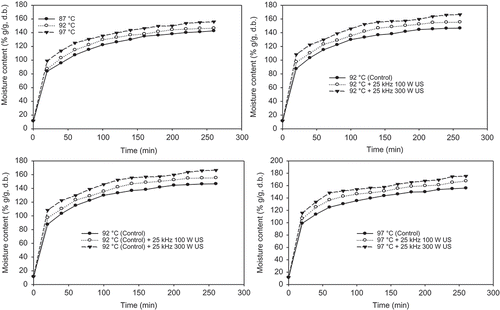
The effects of ultrasounds on water absorption of chickpeas were illustrated in . Application of 25 kHz 100 W ultrasound significantly (P < 0.05) increased the water absorption of chickpea for all temperatures (87–97°C) due to sponge effect of sonication, and to promote more mass transfer from soaking water to chickpea. The moisture content (%, d.b.) values of chickpea were found to be increased from 126.4 ± 0.35 to 139.2 ± 0.11% (d.b) (a 10.13% increase), when the 25 kHz 100 W ultrasound was applied at 87°C and 120 min soaking. Increase in power of ultrasounds (from 100 to 300 W) also further significantly (P < 0.05) increased the moisture content (from 139.2 ± 0.11 to 148.9 ± 0.26 % d.b.; a 7% increase) of chickpeas during 87°C and 120 min soaking. Twenty-five kHz 100 W ultrasound at 92°C exhibited a 4.54–11.54% increase in moisture content of chickpeas compared with and without ultrasound samples for 20–260 min. Also, the power of ultrasound (100–300 W) exhibited a 3.48–18.56% increase in moisture content of samples for the same soaking time and temperature. Similar increase in the rate of moisture content was found at the other temperatures (87 and 97°C) for the same period of soaking. Water absorption rates of chickpea significantly increased (P < 0.05) with increasing of soaking time, temperature, and power of ultrasound (100–300 W). Ultrasound treatment and power of ultrasound increased the water absorption during the soaking operation of the chickpeas.[Citation14,Citation15,Citation17] The mechanical effects of ultrasound provide a greater penetration of water into cellular materials and improve mass transfer.
CONCLUSIONS
Soaking temperature, ultrasound, and power of ultrasounds (100 to 300 W) significantly (P < 0.05) affected the electrical conductivity, turbidity, and soluble solids content of soaking water of chickpeas during cooking due to dissolved solids, electrolytes such as organic compounds, pigments, protein, sugars, starch, vitamins, etc., leaching into the water. Soluble solids content might be used to correlate the overall soluble solids content (organic substances, pigment, minerals, vitamins, etc.). During cooking, color of soaking water, and chickpea seeds changed at each temperature with ultrasound treatment and increase in power of ultrasound due to the coloring compounds and starch leached into water. Hydration rate of chickpea significantly increased (P < 0.05) with increasing of temperature and power of ultrasound (100–300 W). Leaching and reabsorption of some nutritional compounds might be occur simultaneously. Chickpea soaking water properties that measured with simple techniques may be used to follow cooking process. Soaking water of chickpea which contain nutritional compounds may be used in food formulas such as biscuits, crackers, etc. Also, this study showed that ultrasounds can be used as a treatment to decrease the cooking time of chickpeas and to increase mass transfer due to sponge effect of sonication.
REFERENCES
- Salunkhe, D.K.; Kadam, S.S.; Chavan, J.K. Postharvest Biotechnology of Food Legumes, CRC Press: Boca Raton, Florida, 1985.
- Chavan, J.K; Kadam, S.S.; Salunkhe, D.K. Biochemistry and technology of chickpea (Cicer arietinum L.) seeds. Critical Reviews in Food Science and Nutrition 1986, 25, 107–132.
- Wang, N.; Lewis, M.J.; Brennan, J.G.; Westby, A. Effect of processing methods on nutrients and anti-nutritional factors in cowpea. Food Chemistry 1997, 58, 59–68.
- Gujral, H.S.; Angurala, M.; Sharma, P.; Singh, J. Phenolic content and antioxidant activity of germinated and cooked pulses. International Journal of Food Properties 2011, 14, 1366–1374.
- Barampama, Z.; Simard, R.E. Effect of soaking, cooking, and fermentation on composition, in vitro starch digestibility and nutritive value of common beans. Plant Foods for Human Nutrition 1995, 48, 349–365.
- Alajaji, S.A.; El-Adawy, T.A. Nutritional composition of chickpea (Cicer arietinum L.) as affected by microwave cooking and other traditional cooking methods. Journal of Food Composition and Analysis 2006, 19, 806–812.
- Kon, S. Effect of soaking temperature on cooking and nutritional quality of beans. Journal of Food Science 1979, 44, 1329–1340.
- Abdel-Hamid, Y.A.R. Effect of cooking on triptophan, basic amino acids, protein solubility, and retention of some vitamins in two varieties of chickpea. Food Chemistry 1983, 11, 139–143.
- El-Adawy, T.A. Nutritional composition and antinutritional factors of chickpeas (Cicer arietinum L.) undergoing different cooking methods and germination. Plant Foods Human Nutrition 2002, 57, 83–97.
- Jayasooriya, S.D.; Bhandari, B.R.; Torley, P.; D’arcy, B.R. Effect of high power ultrasound waves on properties of meat: A review. International Journal of Food Properties 2004, 7, 301–319.
- Mason, T.J.; Paniwnyk, L.; Lorimer, J.P. The uses of ultrasound in food technology. Sonochemistry 1996, 3, 253–260.
- Wambura, P.; Yang, W.; Wang, Y. Power ultrasound enhanced one-step soaking and gelatinization for rough rice parboiling. International Journal of Food Engineering 2008, 4, 1–12.
- Han, I.H.; Baik, B.K. Oligosaccharide content and composition of legumes and their reduction by soaking, cooking, ultrasound, and high hydrostatic pressure. Cereal Chemistry 2006, 83, 428–433.
- Yıldırım, A.; Oner, M.D.; Bayram M. Modeling of water absorption of ultrasound applied chickpeas (Cicer aritenium L.) using Peleg’s equation. Journal of Agricultural Sciences 2010, 16, 278–286.
- Yıldırım, A.; Oner, M.D.; Bayram M. Fitting Fick’s model to analyze water diffusion into chickpeas during soaking with ultrasound treatment. Journal of Food Engineering 2011, 104, 134–142.
- Lucio-Juárez, J.S.; Moscosa-Santillán, M.; González-García, R.; Grajales-Lagunes, A.; Ruiz-Cabrera, M.A. Ultrasonic assisted pre-treatment method for enhancing mass transfer during the air-drying of habanero chili pepper (Capsicum chinense). International Journal of Food Properties 2013, 16, 867–881.
- Yıldırım, A.; Oner, M.D.; Bayram M. Effect of soaking and ultrasound treatments on texture of chickpea. Journal of Food Science and Technology 2013, 50, 455–465.
- Abu-Ghannam, N.; Mckenna, B. Hydration kinetics of red kidney beans (Phaseolus vulgaris L). Journal of Food Science 1997, 62, 520–523.
- Bayram, M.; Kaya, A.; Oner, M.D. Changes in properties of soaking water during production of soy-bulgur. Journal of Food Engineering 2004a, 61, 221–230.
- AOAC. Official methods of analysis of AOAC International, 17th Ed; Revision I, AOAC International: Gaithersburg, MD, 2002.
- Singh, U.; Subrahmanyam, N.; Kumar, J. Cooking quality and nutritional attributes of some newly developed cultivars of chickpea (Cicer arietinum L). Journal of Science of Food and Agriculture 1991, 55, 37–46.
- Burits, M.; Bucar, F. Antioxidant activity of Nigella sativa essential oil. Phytotherapy Research 2000, 14, 323–328.
- Perera, C.; Hoover, R. Influence of hydroxypropylation on retrogradation properties of native, defatted, and heat-moisture treated potato starches. Food Chemistry 1999, 64, 361–375.
- Jane, J.; Kasemsuwan, T.; Chen, J.F. Phosphorous in rice and other starches. Cereal Foods World 1996, 41, 827–838.
- Karki, B.; Lamsal, B.P.; Jung, S.; van Leeuwen, J.; Grewell, D.; Pometto III, A.L.; Khanal, S.K. Enhancing protein and sugar release from defatted soybean flakes using ultrasound technology. Journal of Food Engineering 2010, 96, 270–278.
- Hromadkova, Z.; Ebringerova, A.; Valachovic, P. Comparison of classical and ultrasound-assisted extraction of polysaccharides from Salvia officinalis L. Ultrasonics Sonochemistry 1999, 5, 163–168.
- Synder, H.E.; Kwon, T.W. Soybean Utilization, Van Nostrand Reinhold Company Pres.: New York, NY: 1987; 31–70, 163–178, 243–289.
- Wang, H.L.; Swain, E.W.; Hesseltine, C.W.; Heath, H.D. Hydration of whole soybeans affects solids losses and cooking quality. Journal of Food Science 1979, 44, 1510–1513.
- Kar, N.; Jain, R.K.; Srivastav, P.P. Parboiling of dehusked rice. Journal of Food Engineering 1999, 39, 17–22.
- Karki, B.; Lamsal, B.P.; Grewell, D.; Pometto III, A.L.; van Leeuwen, J.; Khanal, S.K.; Jung, S. Functional properties of soy protein isolates produced from ultrasonicated defatted soybean flakes. Journal of American Oil Chemists Society 2009, 86, 1021–1028.
- Knorr, D. Impact of non-thermal processing on plant metabolites. Journal of Food Engineering 2003, 56, 131–134.
- Li, H.; Pordesimo, L.; Weiss, J. High intensity ultrasound-assisted extraction of oil from soybeans. Food Research International 2004, 37, 731–738.
- Vinatoru, M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrasonic Sonochemistry 2001, 8, 303–313.
- Chemat, S.; Lagha, A.; AitAmar, H.; Bartels, V.; Chemat, F. Comparison of conventional and ultrasound-assisted extraction of carvone and limonene from caraway seeds. Flavor and Fragrance Journal 2004, 19, 188–195.
- Haizhou, L.; Pordesimo, L.; Weiss, J. High intensity ultrasound-assisted extraction of oil from soybeans. Food Research International 2004, 37, 731–738.
- Abbo, S.; Gopher, A.; Rubin, B.; Lev-Yadun, S. Quantitative trait loci governing carotenoid concentration and weight in seeds of chickpea (Cicer arietinum L.). Genetic Resources and Crop Evolution 2005, 52, 491–495.
- U.S.D.A. Nutrient database for standard reference. 2001. http://www.nal.usda.gov/fnic/foodcomp
- Hromádková, Z.; Ebringerová, A. Ultrasonic extraction of plant materials—Investigation of hemicelluloses release from buckwheat hulls. Ultrasonics Sonochemistry 2003, 10, 127–133.
- Sun, X.; Sun, F.; Zhao, H.; Sun, C. Isolation and characterization of cellulose from sugarcane bagasse. Polymer Degradation and Stability 2004, 84, 331–339.
- Jing, S.; RunCang, S.; Xiao, S.; YinQuan, S. Fractional and physicochemical characterization of hemicelluloses from ultrasonic irradiated sugarcane bagasse. Carbohydrate Research 2004, 339, 291–300.
- Kim, S.M.; Zayas, J.F. Processing parameters of chymosin extraction by ultrasound. Journal of Food Science 1989, 54, 700–703.
- Zayas, J.F. Effect of ultrasound treatment on the extraction of insulin. Biotechnology and Bioengineering 1985, 27, 1223–1228.
- Zayas, J.F. Effects of ultrasonic treatment on the extraction of chymosin. Journal of Dairy Science 1986, 69, 1767–1775
- Jood, S.; Bishnoi, S.; Sharma, A. Chemical analysis and physico-chemical properties of chickpea and lentil cultivars. Nahrung 1998, 42, 71–74.
- Noisuwan, A.; Hemarb, Y.; Bronlund, J.E.; Wilkinson, A.B.; Williams, M.A.K. Viscosity, swelling, and starch leaching during the early stages of pasting of normal and waxy rice starch suspensions containing different milk protein ingredients. Starch 2007, 59, 379–387.
- Akinyele, I.O.; Akinlosotu, A. Effect of soaking, dehulling, and fermentation on the oligosaccharides and nutrients of cowpeas. Food Chemistry 1991, 41, 43–53.
- Somiari, R.J.; Balogh, E. Effects of soaking, cooking, and crude α-galactosidase content of cowpea flours. Journal of Food Science 1993, 61, 339–343.
- Romdhane, M.; Gourdon, C. Investigations in solid-liquid extraction: influence of ultrasound. Journal of Chemical Engineering 2002, 87, 11–19.
- Fuente, D.L.S; Riera, E.; Gallego, J.A. Effect of power ultrasound on mass transfer in food processing. In: Proceedings of the 18th International Congress on Acoustics (ICA), Acoustical Science and Technology for Quality of Life; Kyoto Japan, 2004; 1853–1854.
- Riera, E.; Golas, Y.; Blanco, A.; Gallego, J.A.; Blasco, M.; Mulet, A. Mass transfer enhancement in supercritical fluids extraction by means of power ultrasound. Sonochemistry 2004, 11, 241–244.
- Carcel, J.; Benedito, J.; Rossello, C.; Mulet, A. Influence of ultrasound intensity on mass transfer in apple immersed in a sucrose solution. Journal of Food Engineering 2007b, 78, 472–479.
- Gallego-Juarez, J.A. Some applications of airborne power ultrasound to food processing. In: Ultrasound in Food Processing; Povey, M.J.W.; Mason, T.J.; Eds.; Chapman & Hall: London, UK, 1998.
- Mulet, A.; Carcel, J.A.; Sanjuan, N.; Bon, J. New food drying. Technologies use of ultrasound. Food Science and Technology International 2003, 9, 215–221.
- Turhan, M.; Sayar, S.; Gunasekaran, S. Application of Peleg model to study water absorption in chickpea during soaking. Journal of Food Engineering 2002, 53, 153–159.
- Fasina, O.O.; Irudhayaraj, J.; Sokhansanj, S. Three-dimensional finite elements analysis of moisture absorption in expanding alfalfa cubes. Journal of Agricultural Engineering Research 1993, 56, 337–352.