Abstract
The aim of this study was to evaluate the changes in the antioxidant capacity of fresh and frozen kale during domestic cooking methods (e.g., boiling, microwaving, and steaming). The antioxidant activities of the samples were measured using three in vitro assays (1,1-diphenyl-2-picrylhydrazyl assay, 2, 2’-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid assay, and ferric reducing antioxidant power assay). The steaming treatment was found to be the best cooking method to retain antioxidant compounds, followed by the microwaving and boiling treatments. The frozen uncooked samples exhibited a higher antioxidant capacity than the fresh, uncooked samples, indicating that the freezing process could enhance the antioxidant capacity of the kale.
INTRODUCTION
Epidemiological studies have shown that a high intake of vegetables is inversely correlated with the risk of chronic diseases such as cancer and cardiovascular diseases. Beneficial effects of vegetables on human diseases have been attributed to the presence of bioactive compounds such as vitamins C and E, carotenoids, and phenolic compounds. Oxidative stress is involved in a number of chronic diseases including cardiovascular diseases and cancers.[Citation1] Antioxidant compounds are capable of inhibiting the biomolecular oxidation by scavenging reactive oxygen species or chelating metal ions.[Citation2] Vegetables are a good source of bioactive compounds acting as antioxidants, which are believed to lower the risk of heart diseases and several types of cancers.[Citation3]
Brassica vegetables including cabbage, cauliflower, Brussels sprouts, broccoli, and kale have been reported to possess antioxidant properties.[Citation4] Kale, a leafy green vegetable, belongs to the Brassicaceae family and is known to be a good source of bioactive compounds such as vitamin C, carotenoids, and phenolic compounds.[Citation5] Kale was found to have the second strongest antioxidant activity against peroxy radicals among 22 common vegetables.[Citation6] Sikora et al.[Citation5] reported that kale showed the greatest antioxidant activity measured by the ABTS assay among five Brassica vegetables.
Antioxidant activity of vegetables is influenced by many factors: variety, maturity at harvest, the environment, and post-harvest storage.[Citation4] Vegetables are frozen to increase their shelf life. The consumption of frozen vegetables has increased, since they can be rapidly prepared. The freezing process affects the antioxidant activity of vegetables.[Citation7] Kale is generally cooked before being consumed. Domestic cooking affects the content and bioavailability of antioxidants. The effect of domestic cooking on the antioxidant activity of Brassica vegetables has been extensively studied, especially on broccoli.[Citation8−Citation12] However, very little information is available on kale.[Citation5] Information on the antioxidant activity of fresh and frozen kale and its changes during the cooking process is limited. Therefore, the aim of the study was to evaluate the effect of domestic cooking methods (e.g., boiling, microwaving, and steaming) on the antioxidant activity of fresh and frozen kale.
MATERIALS AND METHODS
Samples
Fresh kale was purchased from a local market. The kale leaves were washed and dried with a hand towel after the inedible parts were removed. The leaves were chopped into strips 2–3 cm in width. The leaves (1:4, leaves/water) were then blanched in a stainless steel vessel for 2 min in boiling water. The blanched leaves were cooled in cold water and drained. The efficiency of the blanching was controlled via a peroxidase test. The peroxidase test, using guaiacol as a substrate, was carried out in accordance with the method of the Food and Agriculture Organization (FAO).[Citation13] The leaves were frozen in a home freezer (Altus AL 309, Arçelik Co., Istanbul, Turkey) at –18°C, and stored for 6 months.
Chemicals
All reagents and chemicals used were of analytical or HPLC grade obtained from Merck (Darmstad, Germany). ABTS, DPPH, TPTZ, trolox, and gallic acid were obtained from Sigma-Aldrich (Sigma Chemical Co., St. Louis, MO).
Cooking Methods
A 500 g batch was prepared and divided into five equal portions (raw, blanching, and three cooking methods). All cooking methods were performed in triplicate.
A preliminary test was conducted to determine the ratio of water to sample for the cooking treatments. The boiling treatment (6:1) required more water than the microwaving treatment (4:1). A least ratio was selected in accordance with the surface area of the test equipment and the degree of water evaporation. A description of the three cooking methods used is provided here:
Boiling: Leaves (20 g) were added to the boiling water (120 mL) in a stainless steel vessel and cooked over a moderate flame. Cooked leaves were cooled in cold water and drained after 10 min of the cooking treatment.
Steaming: Leaves (20 g) were put in a steam basket (Fakir Tolero, Fakir Electrical Domestic Appliances Foreign Trade Co. Inc., Istanbul, Turkey). The cooking treatment lasted 20 min. Cooked leaves were cooled in cold water and drained.
Microwaving: Leaves (20 g) were put in a glass beaker and 80 mL water was added. The cooking treatment lasted 7 min at 900 W in a microwave oven (ICF475, ICF Kitchen Appliances Man. Co., Eskisehir, Turkey). Cooked leaves were cooled in cold water and drained.
Dry Matter Determination
A total of 3–4 g of the homogenized samples was dried in a convection oven at 105°C until a constant weight was reached.
Extraction of Antioxidant Compounds
The raw, blanched, and cooked samples were homogenized in a high-speed blender (Waring Laboratory Blender, Conair Corporation, Stamford, USA). One gram of the homogenized sample was extracted with 10 mL of water in an orbital shaker (Daihan Scientific, Seoul, South Korean) at room temperature, 250 rpm for 1 h. The supernatant was filtered. The extraction procedure was repeated and two filtrates were combined (water extract). The residue was extracted with 10 mL of acetone under agitation for 1 h at room temperature. The extraction was repeated and two filtrates were combined (acetone extract). The extracts were stored at –18°C until analysis.
Determination of Total Phenolic Content
Total phenolic content was estimated using the Folin-Ciocalteu method. A total of 0.1 mL of the extract solution was mixed with 0.50 mL of diluted Folin-Ciocalteu reagent, 0.4 mL of sodium carbonate (1 M), and 4 mL of distilled water. The absorbance of the mixture was measured at 765 nm after 1 h. The calibration curve was prepared with gallic acid standard, ranging from 0 to 200 mg/mL. Total phenolic content was expressed as mg of gallic acid equivalents (GAEs) per kg of dry sample.
Determination of Antioxidant Activity
The antioxidant activities of the raw, blanched, and cooked samples were determined by applying three in vitro assays: 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay,[Citation14] ferric reducing antioxidant power (FRAP) assay,[Citation15] and 2, 2’-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) assay.[Citation16] Each assay is discussed here:
DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging assay: 50 μL of the extract was mixed with 1500 μL of DPPH radical solution (6.10−5 M). The absorbance was measured at 515 nm after 60 min at room temperature. The results were expressed as millimoles of trolox per 100 g of dry sample. The DPPH values were calculated by summing the antioxidant capacity of the acetone and water extracts.
ABTS (2, 2’-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) radical scavenging assay:
First, ABTS stock solution was prepared by reacting 7 mM ABTS (ABTS•+) radical cation solution with 2.45 mM potassium persulfate solution. The stock solution was left in the dark at room temperature for 16 h. The stock solution was diluted with ethanol to reach an absorbance of 0.70 (± 0.02) AU at 734 nm. A total of 50 μL of the extract was mixed with 1000 μL of ABTS•+ solution, and the absorbance was measured at 734 nm after 10 min. The results were expressed as millimoles of trolox per 100 g of dry sample. The ABTS values were calculated by summing the antioxidant capacity of the acetone and water extracts.
FRAP ferric reducing antioxidant power assay: First, fresh FRAP reagent was prepared by mixing the following solutions (10:1:1): acetate buffer solution (pH = 3.6), TPTZ solution in 40 mM HCI (10 mM) and FeCI3 (20 mM) solution, respectively. A total of 50 μL of the extract was mixed with 1500 μL of FRAP reagent and the absorbance was measured at 595 nm after 20 min. The results were expressed as millimoles of trolox per 100 g of dry sample. The FRAP values were calculated by summing the antioxidant capacity of the acetone and water extracts.
Statistical Analysis
The Student’s t-test was applied to evaluate the effect of cooking treatments (boiling, microwaving, and steaming) on the studied parameters of kale. The values of the cooked samples were compared with the fresh uncooked sample. ANOVA was performed to determine significant differences among the cooking treatments, and a least significant difference (LSD) test was applied as well. The SPSS 17.0 software (IBM, New York, USA) was used for data analysis.
RESULTS
The TPC and antioxidant activity of the fresh and frozen kale samples are presented in . The frozen kale samples exhibited a higher TPC and antioxidant activity compared to the fresh ones. The findings showed that the freezing process enhanced the antioxidant activity of the kale.
TABLE 1 The total phenolic content (mg gallic acid/kg dry matter), and antioxidant activity (mmol trolox/100 g dry matter) of the fresh and frozen kale samples
The TPC and antioxidant activity of the blanched sample were determined, since the blanched kale samples were frozen. The changes in the TPC, DPPH radical scavenging activity, ABTS radical scavenging activity, and FRAP ferric reducing antioxidant power of the fresh and frozen samples during the blanching are presented in , , , and , respectively. The results of the Student’s t-test showed that the blanching treatment had no significant effect on the TPC of the fresh sample (p > 0.05), whereas it enhanced the TPC of the frozen sample (p < 0.05). The blanching treatment had a positive impact on the antioxidant activity of the fresh and frozen samples (p < 0.05).
FIGURE 1 (a) Changes in the total phenolic content (TPC) of the fresh and frozen kale samples after blanching and cooking treatments. The TPC of the fresh uncooked sample was set as 100, and changes due to treatments are indicated as increase or decrease with respect to 100. (b) Changes in the DPPH radical scavenging activity of the fresh and frozen kale samples after blanching and cooking treatments. The antioxidant activity of the fresh uncooked sample was set as 100, and changes due to treatments are indicated as increase or decrease with respect to 100. (c) Changes in the ABTS radical scavenging activity of the fresh and frozen kale samples after blanching and cooking treatments. The antioxidant activity of the fresh uncooked sample was set as 100, and changes due to treatments are indicated as increase or decrease with respect to 100. (d) Changes in the FRAP ferric reducing antioxidant power of the fresh and frozen kale samples after blanching and cooking treatments. The antioxidant activity of the fresh uncooked sample was set as 100, and changes due to treatments are indicated as increase or decrease with respect to 100.
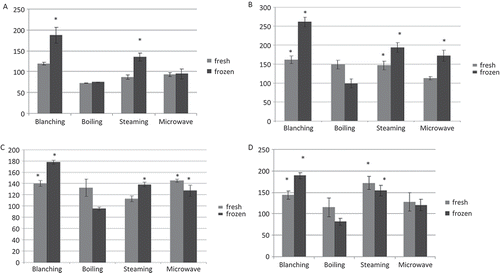
The changes in the TPC (), DPPH value (), ABTS value (), and FRAP value () during the cooking treatments were determined. A statistical evaluation was carried out by the Student’s t-test, comparing the value of the cooked sample with the fresh uncooked sample. The boiling and microwaving treatments had no significant effect on the TPC of the fresh and frozen samples (p > 0.05). The steaming treatment exhibited no significant effect on the TPC of the fresh sample (p > 0.05), whereas it enhanced the TPC of the frozen sample (p < 0.05). The boiling treatment had no significant effect on the DPPH, ABTS, and FRAP values of the fresh and frozen samples (p > 0.05). The steaming treatment showed a positive impact on the DPPH and FRAP values of the fresh and frozen samples (p < 0.05). Moreover, the steaming treatment enhanced the ABTS value of the frozen sample (p < 0.05). However, it had no significant effect on the ABTS value of the fresh sample (p > 0.05). The microwaving treatment showed no significant effect on the DPPH value of the fresh sample (p > 0.05), whereas it enhanced the DPPH value of the frozen sample (p < 0.05). The microwaving treatment exhibited a positive impact on the ABTS values of the fresh and frozen samples (p < 0.05). However, it showed no significant effect on the FRAP values of the fresh and frozen samples (p > 0.05).
The ANOVA analysis results (data not shown) revealed that the TPC and antioxidant activity of the samples presented significant difference with respect to the cooking treatments (p < 0.05). The steaming treatment showed the highest TPC (p < 0.05) for both fresh and frozen samples, whereas the boiling treatment had the lowest value (p < 0.05). For fresh samples, the microwaving treatment exhibited the lowest DPPH value (p < 0.05), and the boiling and steaming treatments did not show significant difference with respect to the DPPH value (p > 0.05). The steaming treatment had the lowest ABTS value (p < 0.05), and the microwaving and boiling treatments presented no significant difference with respect to the ABTS value (p > 0.05). No significant difference was observed among the cooking treatments with respect to the FRAP values (p > 0.05). For frozen samples, the boiling treatment showed the lowest value (p < 0.05), and there was no significant difference in the DPPH value between the steaming and microwaving treatments (p > 0.05). The steaming treatment exhibited the highest ABTS and FRAP values, followed by the microwaving and boiling treatments (p < 0.05).
DISCUSSION
The blanching treatment enhanced the antioxidant activity of the fresh and frozen samples. The positive impact of the blanching treatment on the antioxidant activity may be related to the destruction of cell walls and subcellular compartments of vegetables, making easier the release of antioxidant compounds.[Citation17] In our study, the blanched samples exhibited a higher TPC compared to the fresh uncooked sample, which may support this idea. Our results were comparable with the literature. The blanched vegetables (broccoli, carrot, and beans) were reported to have better retention of antiradical powers.[Citation18]
In this study, the antioxidant activity of the kale samples generally seemed to increase after domestic cooking procedures. An increase in the antioxidant activity of vegetables after cooking was previously attributed to four reasons:[Citation19] (1) Thermal treatment disrupts the cell walls and subcellular compartments that enhance the release of antioxidant compounds; (2) New antioxidant compounds possessing strong antioxidant activity may be formed during thermal chemical reactions; (3) Oxidation of antioxidant compounds may be suppressed via thermal inactivation of oxidative enzymes; and (4) The novel compounds possessing antioxidant activity may be formed during a Maillard reaction. Kale contains antioxidant compounds such as ascorbic acid, carotenoids, and phenolic compounds.[Citation5] Ascorbic acid is the most labile compound in these environmental conditions. The inactivation of ascorbate oxidase may help retain ascorbic acid during the cooking procedures.[Citation19] Bernhardt and Schlich[Citation20] reported that the cooking process promoted the release of carotenoids from green vegetables. The disruption of the carotenoid-protein complex allows for a better extraction of the carotenoids. Polyphenols are found in vegetables as free or bound form. Cooking procedures soften the vegetable matrix, which may enhance the release of phenolic compounds.[Citation21] Moreover, the cooking process might promote the formation of new antioxidant compounds possessing strong antioxidant activity.[Citation22] It is not easy to evaluate the contribution of each possible factor to total antioxidant activity.
Phenolic compounds are known as antioxidant compounds. Antioxidants are capable of inhibiting the oxidative mechanism, leading to degenerative diseases. Therefore, the measurements of the TPC and antioxidant activity have gained importance in exploring the reactive values of vegetables. In this study, we evaluated the antioxidant activity of the kale samples measured by three in vitro assays (DPPH, ABTS, and FRAP). The ABTS and DPPH assays measure the ability of antioxidants to quench a radical cation. The FRAP assay measures the chain-breaking antioxidant potential and the reducing power of the sample, respectively.[Citation23] Conflicting results have been reported on the effect of cooking on the antioxidant activity of vegetables. Several studies have shown that domestic cooking procedures enhanced the antioxidant activity of vegetables.[Citation8,Citation10,Citation21,Citation24] However, several studies indicated that the antioxidant activity of vegetables was decreased during the cooking procedures.[Citation11,Citation25] When comparing different studies, it is worth remembering that the effect of cooking likely depends on the vegetable structure, cooking procedures, extraction solvents, and antioxidant assays.[Citation26] Our results revealed that the type of vegetables (fresh or frozen) should be taken into consideration.
The steaming treatment generally enhanced the antioxidant activity of the fresh and frozen samples, indicating that it was the best cooking procedure to retain antioxidant compounds. The steaming treatment was reported to induce significant increases in the total antioxidant capacity of cooked vegetables.[Citation8,Citation9,Citation17] An increase in the total antioxidant capacity was previously attributed to the non-direct contact with water, which helps retain water soluble compounds.[Citation17] The microwaving treatment appeared to be a better cooking procedure as compared to the boiling treatment. A lower antioxidant capacity of the boiled samples may be related to the loss of antioxidant compounds, since the water soluble compounds could leach into the boiling water.[Citation17] In our study, the boiled samples had a lower TPC than the microwave cooked and steamed samples, which may be related to the loss of phenolic compounds by leaching into the boiling water.
The freezing process is generally regarded as destructive to antioxidant compounds. Frozen samples are assumed to have a lower antioxidant capacity compared to fresh ones. Our findings indicated that this was not universal truth. The frozen uncooked kale samples showed a higher antioxidant capacity than the fresh ones. Our findings were comparable with Danesi and Bordoni,[Citation27] who reported that home freezing increased the antioxidant activity of green vegetables. The frozen steamed samples exhibited a higher radical scavenging activity than the fresh steamed samples. The frozen microwave cooked sample had a higher DPPH radical scavenging activity than the fresh cooked sample. However, the frozen boiled sample showed a lower radical scavenging activity than the fresh boiled sample. Moreover, the frozen cooked samples had a lower FRAP value than the fresh cooked ones. These findings indicated that the cooking process may have more of an impact on the antioxidant activity than the freezing process. Our results agreed with Danesi and Bordoni,[Citation27] who reported that cooking had a negative impact on the antioxidant activity of green vegetables as compared to freezing.
CONCLUSION
The changes in the antioxidant activity of vegetables during cooking should be evaluated to calculate the dietary intake of antioxidants via foods. The assessment of the effect of domestic cooking on the antioxidant activity of vegetables helps in dietary survey evaluating, and planning intake. Our findings indicated that the steaming treatment was the best treatment to retain antioxidant compounds of the fresh and frozen kale followed by the microwaving and boiling treatments.
FUNDING
This research was supported by Gumushane University Scientific Research Council (GÜBAP Project No. 2012.02.Y102.1).
Additional information
Funding
REFERENCES
- Kaurl, C.; Kapoor, H.C. Antioxidants in fruits and vegetables: The millennium’s health. International Journal of Food Science and Technology 2001, 36, 703–725.
- Borguini, R.G.; Ferraz Da Silva Torres, E.A. Tomatoes and tomato products as dietary sources of antioxidants. Food Review International 2009, 25, 313–325.
- Poole, N.D.; Martinez-Carrasco, M.L.; Gimenez, F.V. Quality perceptions under evolving information conditions: Implications for diet, health, and consumer satisfaction. Food Policy 2007, 32, 175–188.
- Podsedek, A. Natural antioxidants and antioxidant activity of Brassica vegetables. LWT Food Science and Technology 2007, 40, 1–11.
- Sikora, E.; Cieslik, E.; Leszczynska, T.; Filipiak-Florkiewicz, A.; Pisulewski, P.M. The antioxidant activity of selected cruciferous vegetables subjected to aquathermal processing. Food Chemistry 2008, 107, 55–59.
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant activity of tea and common vegetables. Journal of Agricultural and Food Chemistry 1996, 44, 3426–3431.
- Murcia, M.A.; M Jimenez, A.; Martinez-Tome, M. Vegetable antioxidant losses during industrial processing and refrigerated storage. Food Research International 2009, 42, 1046–1052.
- Miglio, C.; Chiavaro, E.; Visconti, A.; Fogliano, V.; Pellegrini, N. Effects of different cooking methods on nutritional and physicochemical characteristics of selected vegetables. Journal of Agricultural and Food Chemistry 2008, 56, 139–147.
- Pellegrini, N.; Chiavaro, E.; Gardana, C.; Mazzeo, T.; Contino, D.; Gallo, M.; Riso, P.; Fogliano, V.; Porrini, M. Effect of different cooking methods on color, phytochemical concentration, and antioxidant activity of raw and frozen Brassica vegetables. Journal of Agricultural and Food Chemistry 2010, 58, 4310–4321.
- Turkmen, N.; Sarı, F.; Velioğlu, Y.S. The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chemistry 2005, 3, 713–718.
- Zhang, D.; Hamauzu, Y. Phenolics, ascorbic acid, carotenoids, and antioxidant activity of broccoli and their changes during conventional and microwave cooking. Food Chemistry 2004, 88, 503–509.
- Wachtel-Galor, S.; Wong, K.W.; Benzie, I.F.F. The effect of cooking on Brassica vegetables. Food Chemistry 2008, 110, 706–710.
- FAO Agricultural Service Bulletin No. 119. Fruit and Vegetables Processing (Chapter 9), Food and Agriculture Organization of United Nations, Rome, 1995.
- Brands-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant activity. Lebensmittel-Wissenshaft und Technologie 1995, 28, 25–30.
- Benzie, I.F.F.; Strain, J.J. Ferric reducing antioxidant power assay, direct measure of total antioxidant activity of biological fluids, and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymology 1999, 299, 15–19.
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology & Medicine 1999, 26, 1231–1237.
- Mazzeo, T.; N’Dri, D.; Chiavaro, E.; Visconti, A.; Fogliano, V.; Pellegrini, N. Effect of two cooking procedures on phytochemical compounds, total antioxidant activity, and colour of selected frozen vegetables. Food Chemistry 2011, 128, 627–633.
- Patras, A.; Tiwari, B.K.; Brunton, N.P. Influence of blanching and low temperature preservation strategies on antioxidant activity and phytochemical content of carrots, green beans, and broccoli. LWT Food Science and Technology 2011, 44, 299–306.
- Jimenez-Monreal, A.M.; Garcia-Diaz, L.; Martinez-Tome, M.; Mariscal, M.; Murcia, M.A. Influence of cooking methods on antioxidant activity of vegetables. Journal of Food Science 2009, 74, H97–H103.
- Bernhardt, S.; Schlich, E. Impact of different cooking methods on food quality: Retention of lipophilic vitamins in fresh and frozen vegetables. Journal of Food Engineering 2006, 77, 327–333.
- Roy, M.K.; Juneja, L.R.; Isobe, S.; Tsushida, T. Steam processed broccoli (Brassica oleracea) has higher antioxidant activity in chemical and cellular assay systems. Food Chemistry 2009, 114, 263–269.
- Ferracane, R.; Pellegrini, N.; Visconti, A.; Graziani, G.; Chiavaro, E.; Miglio, C.; Fogliano, V. Effects of different cooking methods on antioxidant profile, antioxidant capacity, and physical characteristics of artichoke. Journal of Agricultural and Food Chemistry 2008, 56, 8601–8608.
- Karadag, A.; Ozcelik, B.; Saner, S. Review of methods to determine antioxidant capacities. Food Analytical Methods 2009, 2, 41–60.
- Ng, Z-X.; Chai, J-W.; Kuppusamy, U.R. Customized cooking method improves total antioxidant activity in selected vegetables. International Journal of Food Science and Nutrition 2011, 62, 158–163.
- Chuah, A.M.; Lee, Y.-C.; Yamaguchi, T.; Takamura, H.; Yin, L.-J.; Matoba, T. Effect of cooking on the antioxidant properties of coloured peppers. Food Chemistry 2008, 111, 20–28.
- Ozyurt, D.; Goc, B.; Demirata, B.; Apak, R. Effect of oven and microwave heating on the total antioxidant capacity of dietary onions grown in Turkey. International of Journal of Properties 2013, 16, 536–548.
- Danesi, F.; Bordoni, A. Effect of home freezing and Italian style of cooking on antioxidant activity of edible vegetables. Journal of Food Science 2008, 73, H109–H112.
