Abstract
Yield, textural, proteolysis, melting, and sensory properties of exopolysaccharide-producing Lactobacillus paracasei on properties of half-fat (about 16 g fat/100 g cheese) Cheddar cheese during ripening at 8℃ for up to six months were investigated. The results revealed that B-3 cheese, made with 2.0% (v/v) high yield exopolysaccharide-producing L. paracasei in combination with 0.011% (w/w) commercial Cheddar culture (B-3 cheese), had a 10.15, 7.71, and 10.04% separately increase in moisture content and had a 7.70, 5.05, and 6.76% separately increase in yield compared with B-2, B-4, and B-5 cheese, texture and melting characteristics were significantly improved (P < 0.05), sensory score surpassed B-4 and B-5 cheese and was similar to the full-fat one. Any differences of B-3 cheese detected among half-fat Cheddar cheeses were attributed to the presence of high yield exopolysaccharide-producing L. paracasei.
INTRODUCTION
With the increasing demand of healthier food products, consumers begin to consume fat-reduced and high-protein foods. Fat-reduced cheese products are vigorously developed. However, fat-reduced cheeses are confronted with serious challenges relating to the hard and rubbery texture and undesirable flavors, which are the important attributes that help to determine the identity of a product and play determining roles in the consumer acceptance. Fat plays vital role in texture, fat reduction in cheese caused a lack of flavor precursors, and fat in cheeses contributes to flow by providing a lubricating effect when melted.[Citation1] It has been studied that fat is present in cheese or filled the voids in the cheese protein matrix.[Citation2] It has been reported that increasing moisture content to a certain level beyond that of full-fat cheese is regarded as an effective method to correct the textural defects associated with fat reduction.[Citation3] Strategies for increasing the moisture level of cheese were to modify cheese making process, such as increasing the time of rennet coagulation, lowering the cooking temperatures, draining and milling at a higher pH, and washing curd, etc.[Citation3] Another method is to add fat imitations or fat replacers, such as xanthan gum[Citation4] and carrageenan.[Citation5]
Lactic acid bacteria (LAB) are generally regarded as safe (GRAS), and exopolysaccharides (EPS) produced by these bacteria have received attention of many researchers,[Citation6] due to their potential application in improving the rheology, texture, and mouthfeel of fermented milk products including yogurt and cheese.[Citation7,Citation10−Citation13] It is thought to be mainly attributed to the much stronger moisture-holding capacity of EPS. An article related to this research was published by Hassan et al.[Citation7] who put forward that the presence of the EPS within the structure reduced protein-protein interactions and cheese rigidity. EPS can strengthen the water-binding capacity of casein within the cheese matrix and then influence the physical properties of cheese. Moreover, the addition of stabilizer that is the origin of animal or plant to the food manufacture is banned in many countries as is known, which makes the application of EPS produced by LAB much more popular. The application of EPS meets today’s demands that consumers prefer food products low in food additives. Therefore, the EPS produced by LAB have an economic importance[Citation8,Citation9] and have extensively been used as gels, emulsifiers, and stabilizing agents.
Recent researches have focused on soft cheeses, Mozzarella has been extensively studied in particular.[Citation10−Citation12] EPS-producing strains, which are almost completely Lactococcus lactis, have been used as starter strains to improve fat-reduced cheese physicochemical, textural, melting, and sensory qualities.[Citation13−Citation16] However, very limited work has been done in the application of EPS-producing Lactobacillus spp. to the hard cheese, which is necessary to be investigated. In addition, in a recent series of studies, Rynne et al.[Citation17] found that a ropy EPS-producing starter was able to significantly increase cheese yield, coagulation proprieties, and moisture content, and significantly improve functionality of half-fat Cheddar cheese; Costa et al.[Citation13,Citation16] found that cheeses made with the EPS-producing starter had increase in actual cheese yield, increase in moisture content, and a significant improvement in both textural and cooking properties of half-fat Cheddar cheese. The EPS-producing Lactobacillus spp. as adjunct in half-fat Cheddar cheese should be further studied, The high yield EPS-producing L. paracasei (EPS++) and non-EPS-producing L. paracasei (EPS−) were obtained by the method of mutagenesis which combined microwave and diethyl sulfate, and screened with EPS production, acid production as indicators. In this research the EPS++ strain was chosen to be used as adjunct to study the effect on the quality of half-fat Cheddar cheese, and cheeses made with EPS+ and EPS− strain as adjunct separately were control ones. All cheeses were ripened for six months and assessed for composition, texture, microbiology, melt property, proteolysis, and sensory property.
MATERIALS AND METHODS
Preparation of Starters and Bacterial Strains
Commercial Cheddar culture (Lactococcus lactis subsp. cremoris and Lactococcus lactis subsp. lactis) was obtained from Chr. Hansen. EPS-producing strain L. paracasei 20408 (EPS+) was obtained from China Center of Industrial Culture Collection. High yield EPS++ and EPS−, which were mutants of the EPS+ strain, were obtained by the strategies of microwave and diethyl sulfate mutagenesis. In addition, the test strains were screened to exhibit a low acidification activity.
Determination of Physiologic Properties of EPS++ Strain Grown in Skim Milks
Isolation and quantification of EPS
Fresh 10% skim milks were prepared. The EPS++ strain were incubated (1% v/v) at 37℃ for 24 h. The fermented milk sample was mixed with 12% (v/v) of 80% (w/v) trichloroacetic acid (TCA) and stored at 4℃ for 24 h, for the precipitation of proteins. The samples were centrifuged at 12,000 g for 30 min at 4℃. The supernatant was mixed with two volumes of chilled absolute ethanol, and stored at 4℃ for 24 h for the precipitation of EPS. The precipitate was separated by centrifugation at 12,000 g for 30 min at 4℃. The pellet containing the EPS was dissolved in ultrapure water and dialyzed against distilled water at 4℃ for 2 days, with two water changes per day, using a dialysis membrane tube (molecular mass cut-off value 8000 ˜ 10,000 D, Sigma, USA). And quantification of EPS was carried out using the phenol/sulfuric acid test[Citation18] and absorbance was measured at 490 nm.
Enumeration of EPS++ strain
The total counts of EPS++ strain were determined by dilution plating with MRS agar medium incubated at 37℃ for 48 h and the bacterial counts were reported as log10 cfu/mL.
Measurement of pH and titratable acidity
The pH of the skim milk was measured with a pH meter (model PHS-3C), and the titratable acidity was determined by the acid-base titration method of GB 5413.34–2010. All above measurement indexes were separately determined every 2 h during fermentation.
Selection of the Best Level of EPS++ Strain in Half-Fat Cheddar Cheese
Cheese manufacture with different level of EPS++ strain
Cheddar cheeses were manufactured from standardized (1.5% fat) pasteurized milk (63℃, 30 min), and cooled to 31℃. Mixed starter cultures were inoculated, and ripened until pH dropped to 6.5. Calcium chloride was added to cheese milk at 0.01% (w/v), and 0.4 mL/L of bovine rennet (120 international milk clotting units/mL, from Chr. Hansen) was added to cheese milk at 31℃ and it was incubated for 45 min. The coagulum was cut and raised the cooking temperature to 35℃ in 30 min and cooked for 45 min. Then the whey was drained and curds were subjected to the process of cheddaring for approximately 90 min at 35℃. The curd was milled and dry salted (2.0%, w/w) when the pH of whey reached 5.4, transferred into a round mold, and pressed (15 pa) overnight. Following pressing, the cheese was vacuum-packed in plastic film, and ripened at 8℃. Five batches of half-fat Cheddar cheeses were made with commercial Cheddar culture (0.011% w/w) plus different (1.0, 1.5, 2.0, 2.5, 3.0% v/v, corresponding to 5, 6, 7, 8, 9 log cfu/mL approximately) addition level of EPS++ strain. The commercial Cheddar culture and EPS++ strain were added at the same time as the starter culture. Each batch of cheeses was ripened for one month.
Sensory evaluation of cheeses after one month ripening
Cheddar cheeses were cut into 20 mm cubes and placed at room temperature for 1 h for sensory evaluation. A trained sensory panel (n = 20, 10 females, 10 males, ages 20–50) with more than 200 h of experience with the ability of analysis of Cheddar cheese sensory evaluated cheeses. The test was carried out in a well-ventilated, odorless, and quiet location. The cheeses were assessed for texture, (hand firmness, hand springiness, cohesiveness, and chewiness), flavor (sweet, sour, bitter, nutty, and cowy), and color. Assessment scores were used a 1 ˜ 5-point scale corresponding to very poor, poor, fair, good, very good. Each assessment by each member was individually assessed, there should be no exchange between each other. Using water and unsalted crackers to gargle during samples assessed.[Citation19]
Proteolysis of cheeses after one month ripening
pH 4.6-soluble nitrogen (pH4.6-SN). Blending 80 g of grated cheese with 160 mL of acetate buffer and pH value of 4.6, the mixture was insulated at 60℃ for 1 h. The supernatant was centrifuged at 4000 g for 20 min, took a middle solution to digestion bottle quantitatively, expressed as percentage of total nitrogen. 12% TCA-soluble nitrogen (12% TCA-SN). Blending 16 mL the middle solution with 4 mL 12% TCA. The supernatant was centrifuged at 4000 g for 20 min, took a middle solution to digestion bottle quantitatively, expressed as percentage of total nitrogen.
Cheese Manufacture with Different Kinds of EPS-Producing Strain
Half-fat Cheddar cheeses were made with commercial Cheddar culture (0.011% w/w) plus the suitable addition amount (which had determined above) of EPS-producing strain. Five batch cheeses were manufactured with the same addition amount of strains as follows: (1) B-1 = full-fat Cheddar cheese made using the commercial Cheddar culture; (2) B-2 = half-fat Cheddar cheese made using the commercial Cheddar culture; (3) B-3 = half-fat Cheddar cheese made with the high yield EPS++ plus the commercial Cheddar culture; (4) B-4 = half-fat Cheddar cheese made with EPS+ plus the commercial Cheddar culture; (5) B-5 = half-fat Cheddar cheese made with EPS− plus the commercial Cheddar culture. Then cheeses were vacuum-packed and ripened at 8℃ for six months.
Analysis of Cheeses Manufactured with Different Kinds of EPS-Producing Strain
Cheese composition analysis
Cheese composition was analyzed on the day of manufacture and all analyses were made in triplicate. Moisture content of the cheeses was determined by the method of an oven drying (GB 5009.3-2010), fat by the Gerber method (GB 5413.3-2010) and total protein by the macro-Kjeldahl procedure (GB 5009.5-2010), moisture in the non-fat substance (MNFS) and fat in dry matter (FDM) by the method in GB/T21375-2008. Cheese pH was measured with pH meter in slurry prepared by macerating 20 g of grated cheese in 20 mL of deionized water. Cheese yield was expressed as the ratio mass between the curd obtained after pressing stage and the weight of milk.
Microbiological analysis
Cheese samples (10 g) were shredded and homogenized with 90 mL of sterile distilled water. Appropriate dilutions were pour-plated in duplicate with ROGOSA agar.[Citation20] The counts of non-starter LAB in control and experimental Cheddar cheese were analyzed in month 0, 1, 2, 4, and 6 of ripening periods. Plates were incubated at 37℃ for 72 h in the electro-heating standing-temperature cultivator (model DHP-9162).
Instrumental texture profile analysis
Cheese samples were cut into cylinders (height 20 mm, diameter 20 mm) from the middle of the whole cheese in order to avoid surface effects. And then, cheese samples were stored at 4℃ overnight before analysis. Texture parameters including hardness, springiness, adhesiveness, and cohesiveness of Cheddar cheese during ripening were assessed on month 0, 1, 2, 4, and 6 by compression on a texture analyzer (model TA.XT plus). The speed of before test, on test, and after test were 5.0, 1.0, and 5.0 mm/s, respectively, compression ratio was 30%, the value of trigger was 5 g. The time between the first and the second press was 5.0 s, probe type was P/50. Data were the average of three independent assays. Five replicate samples from each cheese were compressed at each ripening time.
Melt analysis
Melt analysis was carried out using a covered Schreiber test[Citation21] with some modification. Each cheese cylinder (5 mm height, 35 mm diameter) was placed in a covered glass Petri dish and then placed in an oven at 232℃ for 5 min. These were then removed and cooled for 30 min at room temperature. Before and after melting, marking a line along the edge of each cheese sample and the midpoint on the back of the glass Petri dish with a marker. Measurements of the melt distance were made using ruler. The diameter of the melted sample was measured at five different points and an average diameter was determined. Results were expressed as percentage increase in cheese diameter. Analysis on each cheese sample was performed in triplicate.
Proteolysis analysis
Proteolysis analysis of five batch cheeses with different kinds of EPS-producing strains was determined at the ripening time 0, 1, 2, 4, and 6 month with the method mentioned above.
Sensory analysis
Sensory analysis of five batch cheeses with different kinds of EPS-producing strain was determined after six months ripening with the method mentioned previously.
Statistical Analysis
Data reported were the average of three measurements per replicate. Each treatment cheese was also made three times. And all cheese treatments were made from different batches of milk in different days. Analysis of variance (ANOVA) was carried out using SPSS software (version 19.0). The Duncan’s test was used to determine whether the averages of two sets of measurements were significantly different at P < 0.05. The data are presented as mean ± standard error of means.
RESULTS AND DISCUSSION
Determination of Physiologic Properties of EPS++ Strain
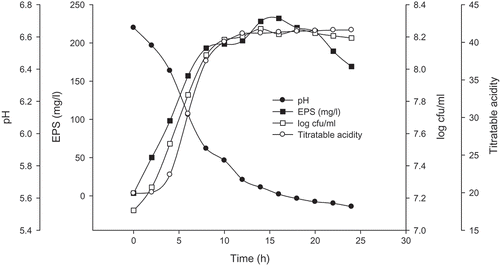
The growth, EPS-production properties, pH, and titratable acidity of the EPS++ strain grown in 10% skim milks at 37℃ were investigated (). It can be found that the growth of EPS++ strain had entered the logarithmic phase when fermented for just 2 h. The amount of EPS increased with increasing EPS++ strain counts. It was also seen that a large amount of EPS were accumulated in a short time which reached a maximum of 232.07 mg/L in the late logarithmic phase of growth, and decreased to 169.07 mg/L at 24 h. While the maximum amount of EPS produced by EPS+ strain was 73.38 mg/L. Usually, the pH and titratable acidity were negatively correlated, in this study, the pH and titratable acidity variation of the fermented skim milks were 1.4 and 40°T, respectively.
Selection of the Best Level of EPS++ Strain in Half-Fat Cheddar Cheese
Sensory evaluation
Sensory evaluation is one of the most important evaluation indexes of the food quality, so it can reflect the quality of the cheese. Moreover, it could also provide a basis for products development and quality control.[Citation22] It was seen from that the differences of sensory evaluation scores of flavor and texture were not significant (P ≥ 0.05) when the addition level of EPS++ strain was 1.5% (v/v) to 2.5% (v/v) in the manufacture of half-fat Cheddar cheese. And the sensory evaluation scores were lower significantly (P < 0.05) when the addition level of EPS++ strain was 1.0% (v/v) or 3.0% (v/v). As a result, 1.5% (v/v) to 2.0% (v/v) addition level of EPS++ strain were chosen first.
TABLE 1 Effect of EPS++ strain content on the sensory values of half-fat Cheddar cheeses
Proteolysis
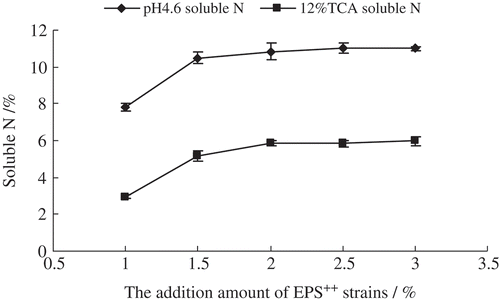
The extent of proteolysis increased with increasing addition level of EPS++ strain (). The vitality of EPS++ strain was not well when the addition level of EPS++ strain was 1.0% (v/v), so the extent of proteolysis appeared to be lower and the content of soluble N was lower significantly (P < 0.05). However, the differences of soluble N were not significant (P ≥ 0.05) when the addition level of EPS++ strain was 2.0% (v/v) to 3.0% (v/v). Considering the economical principle in commerce, the 2.0% (v/v) addition level of EPS++ strain was used in the manufacture of half-fat Cheddar cheese.
Analysis of Cheeses Manufactured with Different Kinds of EPS-Producing Strain
Cheese composition
The composition of five cheeses is shown in . In agreement with previous studies,[Citation14,Citation15] decreasing the fat content of cheese milk resulted in an increase in protein and moisture in cheese and a decrease in MNFS, level of FDM and cheese yield. Cheese (B-3) made with EPS++ strains showed higher (P < 0.05) moisture and yield than other half-fat cheeses. Yield of the B-3 cheese was 12.75, 9.98, and 11.76% higher than that of B-2, B-4, and B-5 cheese, respectively. MNFS and FDM were higher (P < 0.05) in B-3 cheese than in other half-fat cheeses. It may result from the trapping of water by EPS through hydrogen binding,[Citation17] which made the higher moisture level in B-3 cheese. The use of EPS++ strain made the B-3 cheese with similar content of MNFS to that of the full-fat control cheese without the need for modifying the cheese-making procedure. Moreover, pH of the B-3 cheese was significantly lower (P < 0.05) than that of other half-fat cheeses, it was because of the higher moisture content of B-3 cheese and also attributed that the EPS had a protective effect on the bacterial cells after salting so that the bacterial cells had a great tolerance of salt, which made the pH a further decrease during ripening. This is consistent with Rynne et al.[Citation23] and Costa et al.[Citation13]
TABLE 2 Chemical composition, pH, and actual yield of full- and half-fat Cheddar cheeses
Microbiological Analysis
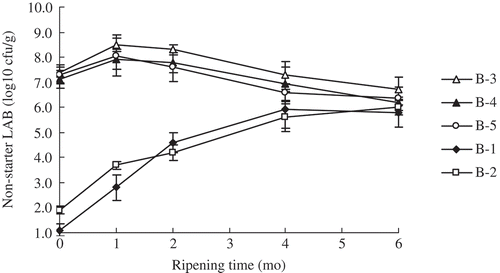
Mean counts of non-starter LAB of half- and full-fat Cheddar cheeses over ripening were shown in . The counts of initial inoculum of EPS++, EPS+, and EPS− strain were controlled, respectively, in B-3, B-4, and B-5 cheeses manufacture, and the counts were 107 cfu/mL or so. The counts of non-starter LAB were similar (P ≥ 0.05) at the day of manufacture in three treated half-fat Cheddar cheeses. After then the counts of non-starter LAB were higher (P < 0.05) in the B-3 cheese than those in B-4 cheese and B-5 cheese. Furthermore, it was significantly higher (P < 0.05) in the B-3 cheese over ripening. However, the counts of non-starter LAB in B-3, B-4, and B-5 cheeses decreased gradually which were thought to be autolysis. The counts of non-starter LAB in B-1 and B-2 control cheeses increased significantly over ripening, from 101 to 102 cfu/g of cheese at the day of manufacture (0 month) to 106 to 107 cfu/g of cheese (the sixth month), consistent with the similar trend as previously reported about half-fat Cheddar cheeses.[Citation13,Citation24]
Instrumental Textural Characteristics
Texture parameters including hardness, springiness, adhesiveness, and cohesiveness of Cheddar cheese during ripening are shown in .
TABLE 3 Texture parameters of half- and full-fat Cheddar cheeses over ripening
Hardness
shows the changes in hardness values during cheese ripening. Compared with all other half-fat Cheddar cheeses, hardness was significantly lower (P < 0.05) in the B-3 cheese which was similar (P ≥ 0.05) to that of the B-1 cheese throughout ripening in agreement with previous reports.[Citation25,Citation26] This might be attributed to the better water-holding capacity of EPS which made the half-fat cheese a higher content of moisture and MNFS () as well as proteolysis. Moreover, there had been an article[Citation25] reported that the reduced hardness of reduced-fat cheese was related not only to the high moisture and MNFS but also to the change of protein network caused by the presence of EPS. Just like the report from Ayala-Hernandez et al.[Citation27] pointed that the EPS within the cheese matrix may have helped to reduce the hardness by filling voids in the matrix and by interacting with the proteins.
Springiness
Reduction of fat increased the springiness of all half-fat Cheddar cheeses. A sharp decrease in springiness of the B-3 cheese appeared during ripening which was similar (P ≥ 0.05) to that of the B-1 cheese (). This might be due to the higher moisture content in the B-3 cheese. It might also be attributed to the reduction of intact casein content or the hydrolysis by the proteolytic activity of the residual chymosin and culture enzymes.[Citation28] Moreover, the decrease in springiness during ripening might be due to the release of calcium ions from monocalcium and dicalcium para κ-caseinate molecules and the hydrolysis of these molecules, which were responsible for the springiness of cheese curd.[Citation29,Citation30] Soluble nitrogen in the B-3 cheese was much higher () which might also explain the much lower springiness value of it.
TABLE 4 Levels of pH 4.6-SN and 12% TCA-SN in half- and full-fat Cheddar cheeses over ripening
Adhesiveness
The adhesiveness that indicates a reduction in elasticity and an increase in an interaction between water-protein matrix. All cheeses except the B-2 one showed a decrease in adhesiveness at the first month ripening (), but the adhesiveness of the B-3 cheese increased gradually and became much higher (P < 0.05) than other half-fat cheeses after four months ripening. The presence of EPS caused phase separation in the casein network owing to depletion flocculation.[Citation31] Since the EPS was responsible for the considerable water retention in cheese, there was an increase in an interaction between water-protein matrix, the adhesiveness of cheeses increased. Another reason was that the adhesiveness of cheese decreased with the fat content of cheese decreasing.[Citation32] Moreover, the adhesiveness was higher in the B-3 sample in this study, which was related to the higher levels of primary proteolysis () throughout ripening.
Cohesiveness
Reduction of fat increased the cohesiveness () of all half-fat Cheddar cheeses, so the cohesiveness of them were higher (P < 0.05) than that of the B-1 cheese in agreement with previous study.[Citation32] Lane et al.[Citation33] reported that the cohesiveness of the cheese was inversely related to cheese proteolysis, decreasing with the increasing proteolysis. In this study, the cohesiveness of the B-3 cheese was lower (P < 0.05) than that of the B-4 and B-5 cheese. The primary effect caused on the cohesiveness of cheese was thought to be attributed to the nature of protein network and the distribution of fat. Moreover, the interactions of EPS with the cheese protein matrix were related to the cheese proteolysis which influenced cheese cohesiveness further.[Citation34] In addition, the lower pH of the B-3 cheese might cause further reduction in cheese cohesiveness, since decrease in pH of the cheese curd was correlated with gradual dissociation of the casein micelles into small aggregates.[Citation35] When the pH was below 4.8, the integrity and cohesion of casein micelles were lost.[Citation36]
Meltability
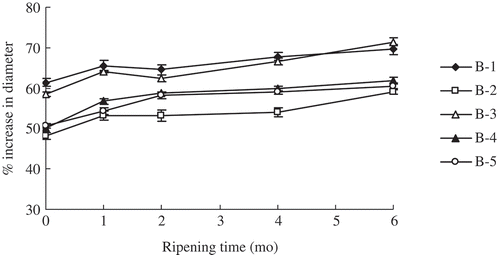
It can be seen that the percentage increase in diameter of five cheeses increased over ripening in this study (). It might be due to degradation of the para-casein matrix and/or changes to its calcium equilibrium,[Citation37] which led to the casein matrix in cheese softer and less elastic over ripening. Results from indicated that the B-1 cheese melted much more than B-2, B-4, and B-5 cheese, they were consistent with the study pointed that reducing the fat content of Cheddar cheese led to a decrease in meltability[Citation38] and an increase in the density of the protein matrix. Moreover, the lower level of MNFS in half-fat cheeses, except the B-3 cheese, could be responsible for the lower meltability. EPS in the B-3 cheese caused the more moisture in it, which was related to the higher meltability. No much higher percentage increase in diameter of the B-1 cheese was observed than that of the B-3 cheese before the first five months ripening, while the percentage increase in diameter of the B-3 cheese was a little higher (P < 0.05) than that of the B-1 cheese at the sixth month ripening. The similarity in pattern of these two types of cheeses might be due to the similar level of MNFS.
Proteolysis Analysis
pH4.6-SN
Levels of pH4.6-soluble nitrogen as a percentage of total nitrogen (pH4.6-SN) are shown in . It was considered to be the first hydrolytic breakdown of proteins into simpler, soluble substances. This primary proteolysis process occurred in all cheeses during ripening. Compared with other half-fat cheeses, the level of pH4.6-SN was the highest (P < 0.05) in the B-3 cheese which was higher (P < 0.05) than the B-1 cheese throughout ripening. It was thought to be attributed to the high MNFS as well as the ratio of the residual chymosin activity, which induced proteolysis.[Citation39] There was no significant difference (P ≥ 0.05) in levels of pH4.6-SN between the B-4 and B-5 cheese at the beginning of ripening. However, pH4.6-SN of the B-4 cheese was higher (P < 0.05) than that of the B-5 cheese after four months ripening.
12% trichloroacetic acid-soluble nitrogen
Levels of 12% trichloroacetic acid-soluble nitrogen as a percentage of total nitrogen (12% TCA-SN) are shown in . It was considered to be the secondary hydrolytic breakdown of proteins into simpler, soluble substances. Compared with other half-fat cheeses and the B-1 cheese, 12%TCA-SN was highest (P < 0.05) in the B-3 cheese over ripening. Similar conclusions were made that the higher content of EPS, the more level of 12%TCA-SN.[Citation40] Maybe the chymosin and the protease activity of the strains should be concerned, which were responsible for production of 12%TCA-SN. Furthermore, the B-3 cheese contained higher levels of 12%TCA-SN than the corresponding cheeses B-4 and B-5, indicating a better proteolytic system in the former strain.
Sensory Evaluation
shows the results of sensory assessments of full- and half-fat Cheddar cheeses at the sixth month ripening. The B-3 cheese received the highest scores of flavor and color. Furthermore, the score of texture was second only to the B-1 cheese. The B-1 and B-2 cheeses were the two treatments that made with commercial Cheddar starters alone. The reason B-3 cheese was accessible was that it showed higher moisture, higher levels of primary proteolysis. The finding of this survey was in agreement with the previous report[Citation39] that the EPS reduced the bitterness of cheese.
TABLE 5 Sensory values of full- and half-fat Cheddar cheeses at the sixth month ripening
The main purpose of this study was to improve the quality of half-fat Cheddar cheese by adding the EPS++ strain to the manufacture of the half-fat Cheddar cheese, and to make the textural and melt characteristics of half-fat Cheddar cheese be similar to its full-fat counterpart. The EPS-producing strain were used in conjunction with commercial Cheddar culture in this study, it promoted the flavor development. The quality of half-fat cheese had been improved indeed. This EPS++ strain were seen potential application in making half-fat and even low-fat Cheddar cheese.
CONCLUSION
This article studied a new measure of improving quality of half-fat Cheddar cheese based on using EPS++ strain, and using EPS+ and EPS− strain as control simultaneously. It had shown that the EPS++ strain had a positive impact on the textural, melt, proteolysis, and sensory characteristics of half-fat Cheddar cheese. The MNFS level, melt property, and textural properties of the B-3 cheese were similar (P ≥ 0.05) to those of the full-fat counterpart. It was due to the excellent water-binding properties and moisture-retention of EPS which took the place of milk fat and mimicked its characteristics in cheese. These properties showed that EPS had potential for the development of novel and improved dairy products with enhanced texture, mouthfeel, taste perception, and stability, representing potential sources for economic gains for the dairy industry.
FUNDING
This research was supported by Science and Technology Research Project of Heilongjiang Province Education Department (12541027), Synergetic Innovation Center of Food Safety and Nutrition, and Early Research and Development Training Programs of the University Scientific and Technological Achievements Industrialization of Heilongjiang Province in 2013.
Additional information
Funding
REFERENCES
- Totosaus, A.; Guemes-Vera, N. Effect of κ-and λ-carrageenans as fat-replacers in low-fat Oaxaca cheese. International Journal of Food Properties 2008, 11 (3), 656–668.
- Everett, D.W.; Olson, N.F. Free oil and rheology of Cheddar cheese containing fat globules stabilized with different proteins. Journal of Dairy Science 2003, 86, 755–763.
- Mistry, V.V. Low-fat cheese technology. International Dairy Journal 2001, 11, 413–422.
- Nateghi, L.; Roohinejad, S.; Totosaus, A.; Mirhosseini, H.; Shuhaimi, M.; Meimandipour, A.; Manap, M.Y.A. Optimization of textural properties and formulation of reduced fat Cheddar cheeses containing fat replacers. Journal of Food, Agriculture, and Environment 2012, 10 (2), 46–54.
- Xiao, Y.; Miao, J.L.; Zheng, Y.R.; Liu, Z.M. Effects of carrageenan and xanthan gum on texture of processed acid-coagulated cheese. Food Science 2012, 33 (23), 93–97.
- Costa, N.E.; O’Callaghan, D.J.; Mateo, M.J.; Chaurin, V.; Castillo, M.; Han-non, A.J.; McSweeney, P.L.H.; Beresford, T.P. Influence of an exopolysaccharides produced by a starter on milk coagulation and curd syneresis. International Dairy Journal 2012, 22, 48–57.
- Hassan, A.N. ADSA Foundation Scholar Award: Possibilities and challenges of exopolysaccharide-producing lactic cultures in dairy foods. Journal of Dairy Science 2008, 91, 1282–1298.
- Welman, A.D.; Maddox, I.S. Exopolysaccharides from lactic acid bacteria: Perspectives and challenges. Trends Biotechnology 2003, 6, 269–274.
- Soydemir, E. Determination of whey-based medium requirements and growth characteristics for the production of yoghurt starter cultures. Master Thesis, University of Pretoria: Pretoria, 2008.
- Perry, D.B.; McMahon, D.J.; Oberg, C.J. Manufacture of low fat Mozzarella cheese using exopolysaccharide-producing starter cultures. Journal of Dairy Science 1998, 81, 563–566.
- Low, D.; Ahlgren, J.A.; Horne, D.; McMahon, D.J.; Oberg, C.J.; Broadbent, J.R. Role of Streptococcus thermophilus MR-1C capsular exopolysaccharide in cheese moisture retention. Applied and Environmental Microbiology 1998, 64, 2147–2151.
- Petersen, B.L.; Dave, R.I.; McMahon, D.J.; Oberg, C.J.; Broadbent, J.R. Influence of capsular and ropy exopolysaccharide-producing Streptococcus thermophilus on Mozzarella cheese and cheese whey. Journal of Dairy Science 2000, 83, 1952–1956.
- Costa, N.E.; Hannon, J.A.; Guinee, T.P.; Auty, M.A.E.; McSweeney, P.L.H.; Beresford, T.P. Effect of exopolysaccharide produced by isogenic strains of Lactococcus lactis on half-fat Cheddar cheese. Journal of Dairy Science 2010, 93, 3469–3486.
- Awad, S.; Hassan, A.N.; Halaweish, F. Application of exopolysaccharide-producing cultures in reduced-fat Cheddar cheese: Composition and proteolysis. Journal of Dairy Science 2005, 88, 4195–4203.
- Dabour, N.; Kheadr, E.E.; Fliss, I.; LaPointe, G. Impact of ropy and capsular exopolysaccharide-producing strains of Lactococcus lactis ssp. cremoris on reduced-fat Cheddar cheese production and whey composition. International Dairy Journal 2005, 15, 459–471.
- Jiménez-Guzmán, J.; Flores-Nájera, A.; Cruz-Guerrero, A.E.; García-Garibay, M. Use of an exopolysaccharide-producing strain of Streptococcus thermophilus in the manufacture of Mexican Panela cheese. LWT–Food Science & Technology 2009, 42, 1508–1512.
- Kanmani, P.; Satish, K.R.; Yuvaraj, N.; Paari, K.A.; Pattukumar, V.; Arul, V. Production and purification of a novel exoploysaccharide from lactic acid bacterium Streptococcus phocae PI80 and its functional characteristics activity in vitro. Bioresource Technology 2011, 102, 4827–4833.
- Guo, Q.H.; Bai, X.; Sheng, Q.H.; Liu, W.X. The application of sensory evaluation method in Cheddar cheese. Dairy Science & Technology 2010, 5, 223–224.
- Rogosa, M.; Mitchell, J.A.; Wiseman, R. A selective medium for the isolation and enumeration of oral and faecal lactobacilli. Journal of Bacteriology 1951, 62, 132–133.
- Altan, A.; Turhan, M.; Gunasekaran, S. Comparison of covered and uncovered Schreiber test for cheese meltability evaluation. Journal of Dairy Science 2005, 88, 857–861.
- Zhang, A.X.; Sheng, Q.H. Elements analysis of sensory evaluation of food. Dairy Industry of China 2006, 34, 51–53.
- Rynne, N.M.; Beresford, T.P.; Kelly, A.L.; Tunick, M.H.; Malin, E.; Guinee, T.P. Effect of exopolysaccharide-producing adjunct starter cultures on the manufacture, composition and yield of half-fat Cheddar cheese. Australian Journal of Dairy Technology 2007, 62, 12–18.
- Rynne, N.M.; Beresford, T.P.; Kelly, A.L.; Guinee, T.P. Effect of milk pasteurisation temperature on age-related changes in lactose metabolism, pH, and the growth of non-starter lactic acid bacteria in half-fat Cheddar cheese. Food Chemistry. 2007, 100, 375–382.
- Fenelon, M.A.; Beresford, T.P.; Guinee, T.P. Comparison of different bacterial culture systems for the production of reduced-fat Cheddar cheese. International Journal of Dairy Science and Technology 2002, 55, 194–203.
- Awad, S.; Hassan, A.N.; Muthukumarappan, K. Application of exopolysaccharide-producing cultures in reduced-fat Cheddar cheese: Texture and melting properties. Journal of Dairy Science 2005, 88, 4204–4213.
- Dabour, N.; Kheadr, E.; Benhamou, N.; Fliss, I.; LaPointe, G. Improvement of texture and structure of reduced-fat Cheddar cheese by exopolysaccharide-producing lactococci. Journal of Dairy Science 2006, 89, 95–110.
- Ayala-Hernandez, I.; Goff, H.D.; Corredig, M. Interactions between milk proteins and exopolysaccharides produced by Lactococcus lactis observed by scanning electron microscopy. Journal of Dairy Science 2008, 91, 2583–2590.
- Gunasekaran, S.; Mehmet, M.A. Cheese texture. Cheese Rheology and Texture 2004, 299–324.
- Kanawjia, S.K.; Rajesh, P.; Sabikhi, L.; Singh, S. Flavor, chemical, and texture profile changes in accelerated ripened Gouda cheese. Food Science and Technology 1995, 28, 577–583.
- Maifreni, M.; Marino, M.; Pittia, P.; Rondinini, G. Textural and sensorial characterization of Montasio cheese produced using proteolytic starters. Milchwissenschaft 2002, 57, 23–26.
- Girard, M.; Schaffer-Lequart, C. Gelation and resistance to shearing of fermented milk: Role of exopolysaccharides. International Dairy Journal 2007, 17, 666–673.
- Bryant, A.; Ustunol, Z.; Steffe, J. Texture of Cheddar cheeses as influenced by fat reduction. Journal of Food Science 1995, 60, 1216–1219.
- Lane, C.N.; Fox, P.F.; Johnston, D.E.; McSweeney, P.L. Contribution of coagulant to proteolysis and textural changes in Cheddar cheese during ripening. International Dairy Journal 1997, 7, 453–464.
- Lluis-Arroyo, D.; Flores-Nájera, A.; Cruz-Guerrero, A.; Gallardo-Escamilla, F.; Lobato-Calleros, C.; Jiménez-Guzmán, J.; García-Garibay, M. Effect of an exopolysaccharide-producing strain of Streptococcus thermophilus on the yield and texture of Mexican manchego-type cheese. International Journal of Food Properties 2014, 17, 1680–1693.
- Roefs, S.P.; Walstra, P.; Dalgleish, D.G.; Horne, D.S. Preliminary note on the change in casein micelles caused by acidification. Netherlands Milk and Dairy Journal 1985, 39, 119–122.
- Lawrence, R.C.; Creamer, L.K.; Gilles, J. Texture development during cheese ripening. Journal of Dairy Science 1987, 70, 1748–1760.
- Cooke, D.R.; Khosrowshahi, A.; McSweeney, P.L.H. Effect of gum tragacanth on the rheological and functional properties of full-fat and half-fat Cheddar cheese. Dairy Science and Technology 2013, 93, 45–62.
- Konuklar, G.; Inglett, G.E.; Warner, K.; Carriere, C.J. Use of a beta-glucan hydrocolloidal suspension in the manufacture of low-fat Cheddar cheeses: Textural properties by instrumental methods and sensory panels. Food Hydrocoll 2004, 18, 535–545.
- Fenelon, M.A.; O’Connor, P.; Guinee, T.P. The effect of fat content on the microbiology and proteolysis in Cheddar cheese during ripening. Journal of Dairy Science 2000, 83, 2173–2183.
- Agrawal, P.; Hassan, A.N. Ultrafiltered milk reduces bitterness in reduced-fat Cheddar cheese made with an exopolysaccharide-producing culture. Journal of Dairy Science 2007, 90, 3110–3117.