Abstract
Effect of pressure level (60–740 MPa), process dwell time (3–40 min), and pH (2.32–5.68) on rheological properties, pectinmethylesterase (PME) enzyme activity and microbiological characteristics of aloe vera juice was studied. A quadratic model was developed for rheological parameters and it was found that pressure level had the most significant effect on all the responses (p < 0.05) followed by pH and dwell time. Pectinmethylesterase activity was evaluated and a maximum of up to 30% inactivation was obtained. Microbial analysis of high pressure treated samples revealed that samples treated at 400 MPa for 20 min at pH 4 reduced microbial counts to <10 cfu mL−1 achieving up to 5.66 log cycle reduction.
INTRODUCTION
Aloe vera (Aloe barbadensis Miller), a medicinal plant, belongs to the Aloe genus and Liliaceae family. All aloe species are perennial leaf succulent xerophytes; they have thick and fleshy leaves arranged in rosette pattern, enlarged to accommodate the aqueous tissue.[Citation1] Aloe vera is widely used in preparation of medicines, cosmetics, health drinks, and currently, is increasingly being incorporated into beverage formulations and dairy products. Some of the health benefits from aloe vera plant include immunomodulatory activity, digestive aid, anti-diabetic activity, oral/gum health, anti-inflammatory, cholesterol/triglyceride reduction, antioxidant protection, and detoxification.[Citation2,Citation3] Aloe vera juice contains more than 98% of water and remaining solid portion comprises of polysaccharides, vitamins, minerals, enzymes, and organic acids. High molecular weight polysaccharides from the aloe vera are mainly responsible for its biological activities.[Citation3] The most important polysaccharide of these is glucomannan composed of glucose and mannose; however, it is highly unstable and undergoes rapid degradation due to the action of hydrolyzing enzymes and microbial growth, if not preserved immediately.[Citation4] Further, the viscous behavior of aloe vera gel is mainly due to the presence of high molecular weight polysaccharides composed of acetylated glucomannans.[Citation5−Citation7] Various technologies employed during the processing of aloe vera juice play a vital role in preserving the bioactive ingredients and preventing degradation. Therefore, it is essential to select appropriate technology to maximize the retention of health promoting compounds. Application of non-thermal processing technologies singly or in combination as a hurdle approach is now an emerging area of research for preparing shelf-stable products.
Among the non-thermal methods of food processing, high pressure processing (HPP) also known as high hydrostatic pressure (HHP) processing has been one of the most successful one in retaining quality attributes and enhancing shelf life of various food products. HPP has recently gained huge importance and is being commercialized in many parts of the world.[Citation8−Citation10] Some of the advantages of HPP include minimal heat damage problems, retention of freshness, flavor, texture, color, and decrease in functionality changes and reduced processing time compared to thermal counterparts.[Citation11,Citation12] High pressure can inactivate, stabilize, or enhance the activity of enzymes, which is dependent on the pressure level, temperature, source, and substrate.[Citation13] High pressure inactivates the microorganisms by altering their cell permeability and biochemical reactions. However, in case of its effect on enzymes, cell permeability may facilitate the enzyme to come in contact with substrate, so it may enhance or reduce the activity depending on the type of microorganisms present, processing conditions (pressure, hold duration, temperature, etc.) and substrate composition.[Citation14] Aloe vera juice is low acid (pH < 4.5) product and highly susceptible to microbial spoilage. Microbiological stabilization of aloe vera using HPP has been studied by Reyes et al.[Citation15] and they reported complete inactivation of microorganisms in samples treated at or over 400 MPa for 3 min. Similarly, Vega-Galvez et al. reported beneficial effects of HPP on aloe vera; like retention of texture, increase in antioxidant activity, retention of vitamins and minerals.[Citation16] However there is no information available on effect of HPP on enzymes related aloe vera.
Rheological properties of foods are widely studied to understand the flow characteristics, structural properties applicable to food processing, equipment design, and quality control.[Citation17,Citation18] Rheological properties of foods are dependent upon temperature, concentration and composition,[Citation19] which makes it an important parameter to be studied while development of food products. Several rheological models are used to describe the flow characteristics of fluid foods such as Newtonian, Power Law (Ostwald-de-Waele), Herschel-Bulkley, and Casson model.[Citation20] Rheological models provide an advantage of representing large quantity of experimental data in simple mathematical form and are widely preferred. HPP induced changes in rheological properties have been reported for mango pulp,[Citation21] tomato puree,[Citation22] and for gum arabic.[Citation23]
Changes in the rheological properties of fruit-based products during processing are mainly attributed to changes in the structural properties of pectic substances due to action of enzyme like pectinmethylesterase (PME).[Citation8] There are many reports available on loss of textural properties, cloud loss, and settling due to PME enzymes like carrot juice,[Citation24] grape juic,[Citation25] orange juice,[Citation26] watermelon juice,[Citation27] and mango juice.[Citation28] Study of interaction between HPP conditions and their effect on enzyme activity and rheological properties is necessary to understand the stability of fruit and vegetable based products for determining the optimum processing conditions for HPP. Therefore, the objective of the present study was to evaluate the effect of pressure level, dwell time, and pH on the rheological properties, PME activity, and microbial characteristics of aloe vera juice.
MATERIAL AND METHODS
Sample Preparation
Fresh aloe vera leaves were harvested from the research farm of Agricultural and Food Engineering Department, Indian Institute of Technology, Kharagpur, India. Leaves were approximately three years old and selected according to uniform shape, size, and color. They were washed and disinfected with 50 ppm sodium hypochlorite solution. Yellow colored sap from aloe vera leaves contains anthraquinones and is not desirable in juice. Hence, it was drained by cutting the base of the leaves and allowing them to stand vertically for an hour. Hand filleting was done to separate fillet and rind portion; the fillet portion was then cut into small pieces and blended using domestic blender (Phillips HL 1631, India) and the juice obtained was subsequently filtered through muslin cloth. Citric acid was added to adjust the pH of juice samples and double packed in 50 mL low density polyethylene (LDPE) pouches (thickness 80 μm). Packed juice samples were kept in the refrigerator at 4°C until processing.
HHP Treatment
A laboratory scale HPP unit (Model: S-IL-100-250-09-W; Make: Stansted Fluid Power Ltd., UK) with 2 L capacity and maximum working pressure of 900 MPa was used for processing. Packed samples were inserted into cylindrical high pressure vessel and pressure treated between 60 to 740 MPa for dwell time of 3 to 30 min. The rate of pressurization was 300 MPa/min and depressurization was instantaneous. Aqueous monopropylene glycol (30% v/v) was used as a pressurization medium. Pressurization was carried out at ambient temperature conditions (25 ± 2°C). The temperature increase the pressurization fluid due to adiabatic heating was 2.4 ± 0.5°C per 100 MPa increase in pressure. Each treatment was conducted in duplicate and samples were stored in the refrigerator (4°C) for further analysis.
Rheological Analysis
Rheological characterization of aloe vera juice was carried out using a rate controlled rheometer (DV-III Ultra Rheometer, Brookfield Engineering, MA, USA) with a small sample adapter (spindle, SC4-21). Coaxial cylinder (cup and bob) geometry was chosen for its greater sensitivity with low viscosity materials and fluid suspensions. The diameter of the cup was in proportion to the bob size as described by the DIN Standard.[Citation29] Each treatment was conducted twice, and rheological measurements were duplicated (N = 4). For each measurement, a sample volume of 10 mL of aloe vera juice was loaded into the cylinder and was allowed to equilibrate at 25°C before measurement. The measurement device was equipped with a temperature control unit with accuracy of ± 0.2°C.
For steady shear measurements, rheometer was programmed within shear rate range from 0.1 and 250 s−1 and time interval for each measurement was 30 s. The parameters such as shear stress, shear rate, and viscosity were obtained from the instrument using the Rheocalc software (V3.2 Build 47-0; Brookfield Engineering Labs, MA, USA) provided. The experimental data so obtained was fitted to different mathematical models to evaluate rheological model parameters. The best fit model was selected based on standard error as given in Eq. (1):[Citation30]
PME Activity
PME activity was assayed using titration method as described by Basak and Ramaswamy.[Citation26] Briefly, 2 mL of aloe juice sample was added to 50 mL of 1% pectin solution containing 0.3 M NaCl and adjusted to pH 7.5 with 0.2 N NaOH at 30°C using digital pH meter (Model: 877 Titrino Plus, Make: Metrohm, Switzerland). The sample was then stirred using a magnetic stirrer and continuously titrated with 0.02 N NaOH to maintain pH 7.5 for a reaction period of 30 min. The PME unit was expressed in micro-equivalents of ester hydrolyzed per minute per mL of juice sample at pH 7.5 and 30°C. The units were multiplied by 1000 for easy interpretation and were calculated according to Eq. (2):
The percent of inactivation of PME was calculated according to Eq. (3):[Citation31]
Microbiological Analysis
Microbiological analysis of HPP aloe vera juice was performed using serial dilution-pour plate method as per APHA method.[Citation32] Briefly, 10 mL of sample mixed with 90 mL sterile peptone solution (0.1%), and serial dilutions were prepared. One mL of the diluted inoculum was pour plated in duplicates in different media plates. For aerobic mesophiles count (AMC), the diluted samples were pour plated on nutrient agar and incubated at 37°C for 24 h. Lactic acid bacteria (LAB) were plated on MRS agar and incubated at 30°C for 5 days. For total coliforms (TC), violet red bile agar was used, and plates were incubated at 37°C for 18 to 24 h. Yeasts and Molds (Y&M) were plated on potato dextrose agar and incubated for 5 days at 30°C. After incubation, colony forming units (CFU) were counted and the results were expressed as log (base 10) CFU mL−1. The detection limit (DL) of the analysis was 10 CFU mL−1 of sample.
The percent of reduction of microbial counts was calculated according to following Eq. (4):
Experimental Design and Statistical Analysis
Response surface methodology (RSM) was used to evaluate the effect on response of interest by a number of independent variables. The experimental design used was a central composite rotatable design (CCRD) for three independent variables each at five levels, as detailed in . The most important characteristic of CCRD is that all the axial and factorial design points are on the sphere of radius, which allows accurate predictions throughout the region of interest.[Citation33,Citation34] A quadratic polynomial regression model was selected for predicting the response described as:
TABLE 1 Independent variables and levels of a rotatable central composite design (RCCD) used in the present study for aloe vera juice
TABLE 2 The matrix of RCCD followed in this study and experimental data obtained of rheological properties and PME activity of aloe vera juice
Data from the physical experiments were analyzed using regression analysis and analysis of variance (ANOVA) using a statistical package software, Design-Expert version 6.0.0 (Stat-Ease Inc., Minneapolis, MN, USA). Response surface plots were obtained from the software and for statistical tests significance was calculated at p ≤ 0.05 using Fisher’s least significant difference (LSD) test.
RESULTS AND DISCUSSION
Flow Characteristics of Aloe Vera Juice
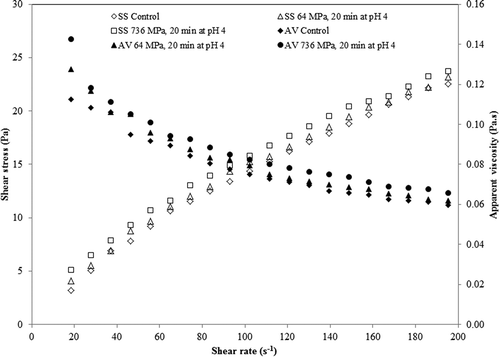
A typical flow curve showing shear stress and apparent viscosity versus shear rate of aloe vera juice at the lowest (64 MPa) and highest pressure (736 MPa) level is presented in . Aloe vera juice showed shear thinning behavior for all the treatments. This type of behavior is common for fruit- and vegetable-based products like mango juice,[Citation35] tomato juice,[Citation36] and apple juice.[Citation37] Shear rate-shear stress data was tested for various mathematical models (Newtonian, Power, Herchel-Bulkley, and Casson), and it was found that Power law (Eq. 6) and Casson model (Eq. 7) fit adequately,
Verification of the Fitted Model
Response surface analysis was used to develop a correlation between pressure level, dwell time, and pH as independent variables on different responses. Results of effect of processing parameters on different responses of processed aloe vera juice are given in . ANOVA revealed that quadratic model well fitted to experimental data, indicating that the model is sufficiently accurate for predicting the response variables at any combination of independent variables studied within the range. Coefficient of determination varied between 0.819 and 0.907 for all the response variables indicating the good acceptability of fitted models. The developed regression models in terms of coded variables describing the effect of pressure (X1), dwell time (X2), and pH (X3) on consistency index (Y1), flow behavior index (Y2), yield stress (Y3), and PME activity (Y4) are given in Eqs. (8), (9), (10), and (11), respectively. The highlighted bold terms in the equations represents that their effect on the response is statistically significant (p < 0.05).
Effect of Pressure, Dwell Time, and pH on Consistency Index (K)
The shear stress-shear rate data obtained was fitted to Power law model to evaluate the consistency index. represents the response surface plot for the effect of pressure and dwell time on the consistency index at fixed pH of 4. It clearly indicated an increase in the consistency index with increase in pressure level. shows the effect of pH and pressure on the consistency at a fixed dwell time of 20 min, it was observed that the magnitude of change in consistency index with pH and dwell time was small compared to pressure. The obtained quadratic regression equation for the consistency index having R2 = 0.819 is given in Eq. (8):
FIGURE 2 Response surface plots showing the effect of pressure level, dwell time, and pH on consistency index (a and b), flow behavior index (c and d), and yield stress (e and f) of aloe vera juice.
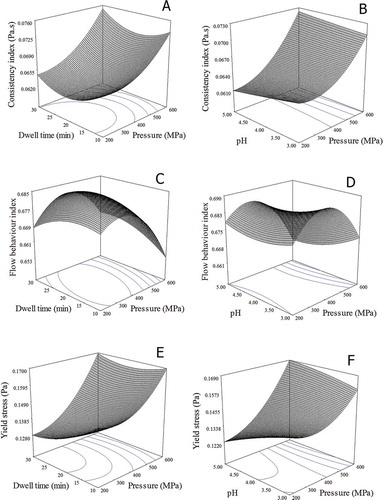
From the regression coefficients obtained from ANOVA, it was observed that pressure (X1) had the significant effect on the consistency index (p < 0.05) which increased with an increase in pressure and ranged between 0.0590 and 0.0789 Pa.s (). However, no significant effect of dwell time (X2) and pH (X3) was observed. Linear decrease in consistency with an increase in pH, as evident by a negative coefficient of X3 was observed. This might be due to glucomannans in aloe vera juice, which are sensitive to pH and degrade with pH alteration.[Citation4] Interaction terms X1X3 and X1X2, and quadratic terms (X12 and X22) had positive effect on Y1 (p > 0.05), whereas an interaction term X2X3 showed negative interaction term (p > 0.05).
High pressure causes changes in hydration capacity of pectic substances, so aloe vera juice might have undergone similar changes leading to formation of compact structures, which might have increased the consistency.[Citation37,Citation38] Similar results were obtained by Opazo-Navarrete et al. for high pressure treated aloe vera suspensions[Citation38] and Panteloglou et al.[Citation23] for gum arabic dispersions.
Effect of Pressure, Dwell Time, and pH on Flow Behavior Index (n)
The flow behavior index values of all the treatments are shown in . The flow behavior index decreased significantly with an increase in pressure (p < 0.05) and ranged between 0.64 and 0.70 indicating shear thinning behavior. The flow behavior index is mainly dependent on the distribution of small and large particles; in this case the high pressure might have affected the particles and their functional groups which were responsible for the decrease in flow behavior index with increasing pressure level. However, similar to consistency, no significant effect of pH and dwell time was observed (p > 0.05). The obtained results were in agreement with those reported by Liu et al.,[Citation21] they reported decrease in the flow behavior index with an increase in pressure for mango pulp due to presence of large pectic substances. The regression model equation obtained with a correlation coefficient of 0.907 as given in Eq. (9):
It was inferred that the fit obtained can be used to predict and explain flow behavior over the selected range of process conditions. Linear terms X1 and X3 had negative coefficients indicating a decrease in Y2 with an increase in applied pressure and pH. Quadratic terms X12 and X22 also showed negative coefficients, whereas the quadratic term X32 had positive coefficient (Eq. 9). The response surface plots as shown in and , describe interaction effect of X1X2 and X1X3. A close examination of revealed that, there was a minor change in the flow behavior index up to 400 MPa and large decrease at pressures higher than 400 MPa. Interacting terms did not show any significant effect on the response variable Y2. Opazo-Navarrete et al. reported flow behavior index values ranging between 0.16–0.25 for HPP aloe gel suspensions.[Citation38] The observed variation was possibly due to variations in sample composition and preparation methods used.
Effect of Pressure, Dwell Time, and pH on Yield Stress (σo)
Yield stress is an important parameter which shows stress required for initiating flow; it has practical significance in food formulations.[Citation24] and show the response surface indicated that beyond 400 MPa there was less effect of pH on the yield stress in aloe vera juice. The obtained regression equation (R2 = 0.879) for yield stress is given in Eq. (10):
Pressure (X1) had a significant effect on Y3 (p < 0.05), which showed increasing trend and varied between 0.12 and 0.18 Pa (). Whereas, effect of linear terms X2 and X3 on Y3 were not significant (p > 0.05). Similar trend of increase in yield stress with increase in pressure were reported for mango pulp.[Citation21] Response surface plots ( and ) for the effect of interacting terms X1X2 and X1X3 confirmed the increase in yield stress of aloe vera juice with applied pressure, whereas the effect of dwell time and pH was insignificant (p > 0.05). Quadratic term X12 also showed a significant effect on response (Y3) as evident from ANOVA. The pressure application might have induced the formation of weak gel networks thus, increasing the yield stress of aloe vera juice.[Citation40] HPP affects the sol-gel transition of the polysaccharide forming gels, responsible for the increase in the yield stress.[Citation41]
Effect of Pressure, Dwell Time, and pH on PME Activity
Inactivation of PME is considered desirable in many fruit based products since this enzyme is responsible for destabilization of juice, gelation, and loss of consistency.[Citation22] shows cloud loss in aloe vera juice caused by PME activity. PME catalyzes the demethylation of carboxyl groups on pectic substances, which further take part in cross-linking reactions with ions like calcium and precipitates.[Citation42] The estimated percent of inactivation of PME activity of fresh and HP treated aloe juice is shown in . Fresh aloe juice sample (without treatment) had PME activity of 0.053 PE units/mL, which was taken as reference and maximum inactivation up to 30.19% was achieved after pressure application of 736 MPa for 20 min at pH 4. Activation of PME was observed at low pressure of 200 MPa as shown in . Samples treated at 200 MPa for 30 min at pH 3 showed the maximum enhanced activity of 11.53%. This increase might be due to effective extraction of enzymes caused by the damage to cell wall membranes by pressure application and their contact with the substrate.[Citation10] Similar instance of PME activation at 300 MPa for tomato juice has been reported by Hsu.[Citation43] They observed 1.7 times increase in PME activity compared to control. The partial activation can be used to stabilize PME while simultaneously inactivating undesirable enzymes during processing.[Citation13] The percent of relative activity data obtained for aloe vera juice was statistically analyzed and it was found that pressure level had the most significant effect on the PME inactivation (p < 0.05). showed the interactive effect of pH and pressure at fixed dwell time of 20 min. It was observed that pH and pressure had antagonistic relation and at 275 MPa pressure level, no PME inactivation was observed. In case of , which shows the interactive effect of pressure and dwell time in the form of contour plot for percent of inactivation of PME at fixed pH of 4, it was observed that dwell time and pressure had additive effect on the PME inactivation. Many studies have reported the effect of HPP on PME activity, but no such information was found out for aloe vera juice. Pressure level decreased the activity of PME significantly (p < 0.05), whereas dwell time and changes in pH had no significant effect on its activity (p > 0.05; ) within the studied domain of process parameters. Increase of 5 to 12% in PME activity was observed for samples treated at 200 MPa (). The regression model (R2 = 0.849) for PME activity is given in Eq. (11):
FIGURE 4 Effect of pressure level, dwell time, and pH on % residual activity of in situ PME enzyme in aloe vera juice.
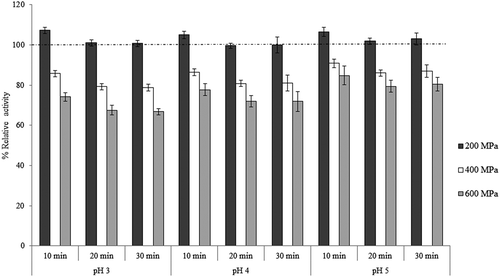
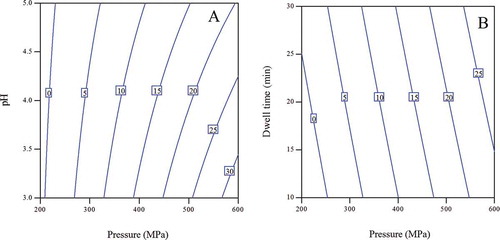
FIGURE 5 Contour plots of pressure-pH (a) and pressure-dwell time (b) showing iso --- (%) inactivation lines for in situ PME enzyme of aloe vera juice within the studied domain of pressure, dwell time, and pH.
Linear terms X1 and X2 had negative coefficients indicating a decrease in response, whereas positive effect on PME inactivation was observed with decrease in X3. Quadratic term X12 showed significant effect (p < 0.05), whereas terms X22 and X32 did not affect the PME activity (p > 0.05). Interacting terms X1X2 and X2X3 had a significant effect on PME activity (p < 0.05). Effect of HPP on PME activity is previously described by Basak and Ramaswamy for orange juice.[Citation26] They reported that the PME activity decreased with an increase in pressure applied and decrease in pH, where orange juice samples at pH of 3.2 gave higher inactivation of PME compared to the samples of pH 3.7.
Effect of Pressure, Dwell Time, and pH on Microorganisms
In fresh aloe juice the AMC, Y&M, LAB, and TC counts were enumerated and found to be 6.63, 6.66, 6.48, and 6.51 log cfu mL–1, respectively. These obtained initial counts were relatively higher for a fresh plant product. This may be due to the added contamination from soil during cutting, washing, cleaning, and blending operations which might have increased the microbial load in the fresh aloe vera juice. The microbiological counts obtained for after HPP are presented in . The obtained microbial data fitted best to the quadratic regression model having good correlation coefficient (R2 > 0.90). ANOVA indicated that the overall model had significant effect on destruction of microbial populations (p < 0.05) in aloe vera, however, significance of individual independent parameters varied for different microbes studied in the present work. Reduction in AMC was significantly enhanced (p < 0.05) by pressure (X1) and quadratic term of pressure (X12), whereas, pH and dwell time did not affect its reduction (p > 0.05). Similar effect was seen for Y&M, LAB, and TC counts. In addition to this, TC were also significantly affected (p < 0.05) by pH (X3) and interaction of pressure and pH (X1X3).
TABLE 3 Experimental data for microbiological characteristics of aloe vera juice
HPP has been reported to cause rupture of the microbial cell membrane and the cells were unable to recover this physical damage, which results in a loss to maintain the homeostasis and eventually leads to cell death. Several studies have reported the beneficial effects of HPP in improving the microbiological quality of many fruit juices.[Citation44–Citation47] The effectiveness of HPP in decreasing the mesophilic aerobic and yeast counts in aloe vera gel within 300 to 500 MPa pressure range was studied and reduction of the microbial counts to less than 10 cfu mL–1 for samples processed at pressure more than 400 MPa (up to 2 log reduction) was reported.[Citation45] This observation is contradictory to the results of TPC and Y&M of pressurized samples obtained in the present study (), which can be explained by higher initial microbial loads in fresh aloe vera juice samples. Based on the obtained total viable counts in the present study (), it was observed that AMC required application of maximum pressure level (600 MPa) and severest acidic conditions (pH 3) to reduce their counts below detection (1 log CFU mL--1), whereas, coliforms were undetectable at >200 MPa pressure levels and < pH 4 itself. AMC includes both gram positive as well as gram negative bacteria and some fast growing Y&M may also be found; whereas, coliforms are essentially a gram negative group of bacteria. In general, gram positive bacteria have been reported to be more baroresistant than gram negative due to their complex cell wall composition.[Citation48] Hence, this may explain comparatively higher resistance of the aerobic mesophiles (depicted by AMC) in the present study.
Contour plots are a 2D representation of interaction effects of two parameters on a response shown in the form of iso-lines and are applied as a useful tool to represent the interaction effect. P-t and P-pH contours showing iso – (%) Reduction lines of indigenous microbes of aloe vera juice are presented in , where “P” is pressure (MPa) and “t” is dwell time (min). AMC decreased with increase in pressure, dwell time, and decrease in pH ( and ). Additive effect of pH and pressure was seen up to 400 MPa (). Effectiveness of pressure for AMC destruction reduced beyond 320 MPa, as larger increase in pressure level was required for the same extent of reduction. This might be due to the fact that, up to 320 MPa, almost 80% of the aerobic mesophiles were killed by pressure application and remaining population might have strains with higher pressure resistance, requiring higher pressure application for their destruction. At pH greater than 4 and pressures more than 410 MPa, antagonistic effect of these two parameters was observed on AMC (). Up to 400 MPa, no effect of dwell time was seen on reduction of AMC (), wherein, more than 80% decrease in AMC was noted. This implies that maximum reduction of AMC was caused during pressure come-up stage due to rapid compression. Compression stage has been shown to be highly effective for destroying microorganisms.[Citation48,Citation49] Similarly, Y&M count also reduced with increase in pressure, dwell time, and decrease in pH ( and ). Though, effectiveness of pressure reduced beyond 310 MPa (up to which 85% reduction was achieved), as for the same level of Y&M reduction, larger increase in applied pressure was required. With increase in pressure, synergistic effect of pH 3 to 4 and antagonistic effect from pH 4 to 5 was observed, whereas, synergistic effect of dwell time and pressure was seen from 10 to 20 min duration, and it was more visible for pressures less than 340 MPa. In case of LAB, additive effect of pressure and pH was noted up to pH 4, beyond which effectiveness of pH in decreasing LAB counts reduced (). Similarly additive effect of dwell time and pressure was seen up to 80% inactivation of LAB, beyond which effectiveness of dwell time reduced (). Additive effect of pH and pressure is seen up to 300 MPa for TC counts, beyond which this effect is visible for pH less than 4 (). Also, no effect of dwell time was seen on TC, as can be deduced from the horizontal iso lines in the contour ().
FIGURE 6 P-t and P-pH contour plots showing iso --- (%) reduction lines for AMC (a and b), Y&M (c and d), LAB (e and f) and TC (g and h) counts in aloe vera juice within the studied domain of pressure, dwell time, and pH.
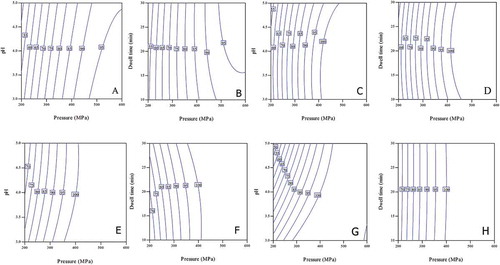
Complete inactivation of all microorganisms was observed at 600 MPa for 10 min at pH 3 (<1 cfu mL–1) achieving up to 6.66 log reduction (). Similarly, more than 5 log reduction in natural microflora was reported in mango pulp after application for 600 MPa pressures for 5 min dwell time.[Citation47] According to WHO acceptable limit for microbial quality of aloe vera juice is below 10 cfu mL–1,[Citation50] hence HP treatment at 400 MPa for 20 min and pH lower than 4 was regarded to be sufficient for aloe vera juice to reduce the microbial load within acceptable limits. In addition, contour plots helped to understand the interaction effects of pressure, pH, and dwell time on microbial counts in aloe vera juice throughout the studied domain of process parameters. Also, contour plots suggested that aerobic mesophiles (as represented by AMC) were most resistant to HPP, followed by Y&M, LAB, and TC.
CONCLUSIONS
In the present study, effect of HPP on rheological properties, PME activity, and microbiological characteristics of aloe vera juice were evaluated using RSM. Predictive model equations were developed for consistency index, flow behavior index, yield stress, and PME activity having good fit. Power law and Casson model were found to be most suitable rheological models to describe the flow characteristics of aloe vera juice. Pressure level significantly affected all the response variables followed by pH and dwell time. Complete inactivation of microorganisms was achieved at a pressure level of 600 MPa for dwell time of 10 min at pH 3 with aerobic mesophiles being most baroresistant.
FUNDING
We sincerely thank National Agricultural Innovation Project (NAIP) and Indian Council for Agricultural Research (ICAR) for funding the research work. The first author would like to thank CSIR, New Delhi for providing research fellowship (ACK. No. 142175/2K12/1).
Additional information
Funding
REFERENCES
- Ramachandra, C.T.; Rao, P.S. Shelf-life and colour change kinetics of Aloe vera gel powder under accelerated storage in three different packaging materials. Journal of Food Science and Technology 2011, 50, 1–8.
- Gruenwald, J. Novel botanical ingredients for beverages. Clinics in Dermatology 2009, 27, 210–216.
- Eshun, K.; He, Q. Aloe vera: A valuable ingredient for the food, pharmaceutical, and cosmetic industries—A review. Critical Reviews in Food Science and Nutrition 2004, 44, 91–96.
- Eberendu, A.R.; Luta, G.; Edwards, J.A.; McAnalley, B.H.; Davis, B.; Rodriguez, S.; Henry, C.R. Quantitative colorimetric analysis of aloe polysaccharides as a measure of Aloe vera quality in commercial products. Journal of AOAC International 2005, 88, 684–691.
- Yaron, A.; Cohen, E.; Arad, S.M. Stabilization of aloe vera gel by interaction with sulfated polysaccharides from red microalgae and with xanthan gum. Journal of Agricultural and Food Chemistry 1992, 40, 1316–1320.
- Channe Gowda, D.; Neelisiddaiah, B.; Anjaneyalu, Y.V. Structural studies of polysaccharides from aloe vera. Carbohydrate Research 1979, 72, 201–205.
- Rodriguez, E.R.; Martin, J.D.; Romero, C.D. Aloe vera as a functional ingredient in foods. Critical Reviews in Food Science and Nutrition 2010, 50, 305–326.
- Bermudez-Aguirre, D.; Barbosa-Canovas, G.V. An update on high hydrostatic pressure, from the laboratory to industrial applications. Food Engineering Reviews 2011, 3, 44–61.
- Al-Nabulsi, A.; Shaker, R.; Osaili, T.; Clark, S.; Harte, F.; Barbosa-Cánovas, G. Impact of high hydrostatic pressure and heat treatments on milk gel properties: A comparative rheological study. International Journal of Food Properties 2012, 15, 613–627.
- Rastogi, N.K.; Raghavarao, K.S.M.S.; Balasubramaniam, V.M.; Niranjan, K.; Knorr, D. Opportunities and challenges in high pressure processing of foods. Critical Reviews in Food Science and Nutrition 2007, 47, 69–112.
- Matser, A.M.; Krebbers, B.; van den Berg, R.W.; Bartels, P.V. Advantages of high pressure sterilization on quality of food products. Trends in Food Science & Technology 2004, 15, 79–85.
- Oey, I.; Lille, M.; Van Loey, A.; Hendrickx, M. Effect of high-pressure processing on colour, texture, and flavour of fruit- and vegetable-based food products: A review. Trends in Food Science & Technology 2008, 19, 320–328.
- Eisenmenger, M.J.; Reyes-De-Corcuera, J.I. High pressure enhancement of enzymes: A review. Enzyme and Microbial Technology 2009, 45, 331–347.
- Chakraborty, S.; Kaushik, N.; Rao, P.S.; Mishra H.N. High-pressure inactivation of enzymes: A review on its recent applications on fruit purees and juices. Comprehensive Reviews in Food Science and Food Safety 2014. DOI:10.1111/1541-4337.12071 (Accepted manuscript)
- Reyes, E.R.; Guanoquiza, M.I.; Tabilo-Munizaga, G.; Vega-Galvez, A.; Miranda, M.; Pérez-Won, M. Microbiological stabilization of Aloe vera (Aloe barbadensis Miller) gel by high hydrostatic pressure treatment. International Journal of Food Microbiology 2012 3, 218–224.
- Vega-Galvez, A.; Miranda, M.; Aranda, M.; Henriquez, K.; Vergara, J.; Tabilo-Munizaga, G.; Perez-Won, M. Effect of high hydrostatic pressure on functional properties and quality characteristics of Aloe vera gel (Aloe barbadensis Miller). Food Chemistry 2011, 129, 1060–1065.
- Gundurao, A.; Ramaswamy, H.S.; Ahmed, J. Effect of soluble solids concentration and temperature on thermo-physical and rheological properties of mango puree. International Journal of Food Properties 2011, 14, 1018–1036.
- Karaman, S.; Kesler, T.; Goksel, M.; Dogan, M.; Kayacier, A. Rheological and some physicochemical properties of selected hydrocolloids and their interactions with guar gum: Characterization using principle component analysis and viscous synergism index. International Journal of Food Properties 2014, 17, 1655–1667.
- Razavi, S.M.; Karazhiyan, H. Rheological and textural characteristics of date paste. International Journal of Food Properties 2012, 15, 281–291.
- Rao, M.A. Rheology of Fluid and Semisolid Foods: Principles and Applications, Gaitherburg, MD: Aspean Publishers, Inc., 2007; 25–28.
- Liu, F.; Wang, Y.; Bi, X.; Guo, X.; Fu, S.; Liao, X. Comparison of microbial inactivation and rheological characteristics of mango pulp after high hydrostatic pressure treatment and high temperature short time treatment. Food and Bioprocess Technology 2013, 6, 2675–2684.
- Verlent, I.; Hendrickx, M.; Rovere, P.; Moldenaers, P.; Van Loey, A. Rheological properties of tomato-based products after thermal and high-pressure treatment. Journal of Food Science 2006, 71, S243–S248.
- Panteloglou, A.G.; Bell, A.E.; Ma, F. Effect of high-hydrostatic pressure and pH on the rheological properties of gum arabic. Food Chemistry 2010, 122, 972–979.
- Balogh, T.; Smout, C.; Nguyen, B.L.; Van Loey, A.M.; Hendrickx, M.E. Thermal and high-pressure inactivation kinetics of carrot pectinmethylesterase: From model system to real foods. Innovative Food Science & Emerging Technologies 2004, 5, 429–436.
- Guiavarc’h, Y.; Segovia, O.; Hendrickx, M.; Van Loey, A. Purification, characterization, thermal, and high-pressure inactivation of a pectin methylesterase from white grapefruit (Citrus paradisi). Innovative Food Science & Emerging Technologies 2005, 6, 363–371.
- Basak, S.; Ramaswamy, H.S. Ultra high pressure treatment of orange juice: A kinetic study on inactivation of pectin methyl esterase. Food Research International 1996, 29, 601–607.
- Zhang, C.; Trierweiler, B.; Li, W.; Butz, P.; Xu, Y.; Rufer, C.E.; Zhao, X.Y. Comparison of thermal, ultraviolet-c, and high pressure treatments on quality parameters of watermelon juice. Food Chemistry 2011, 126, 254–260.
- Ahmed, J.; Ramaswamy, H.S.; Hiremath, N. The effect of high pressure treatment on rheological characteristics and colour of mango pulp. International Journal of Food Science & Technology 2005, 40, 885–895.
- Kok, M.S. Rheological study of galactomannan depolymerisation at elevated temperatures: Effect of varying pH and addition of antioxidants. Carbohydrate Polymers 2010, 81, 567–571.
- Ahmed, J.; Ramaswamy, H.S. Effect of high-hydrostatic pressure and concentration on rheological characteristics of xanthan gum. Food Hydrocolloids 2004, 18, 367–373.
- Liu, Y.; Zhao, X.Y.; Zou, L.; Hu, X.S. Effect of high hydrostatic pressure on overall quality parameters of watermelon juice. Food Science and Technology International 2013, 19, 197–207.
- Downes, F.; Ito, K. Compendium of Methods for the Microbiological Examination of Foods, Washington, DC: APHA, 2001.
- Obeng, D.P.; Morrell, S.; Napier-Munn, T.J. Application of central composite rotatable design to modelling the effect of some operating variables on the performance of the three-product cyclone. International Journal of Mineral Processing 2005, 76, 181–192.
- Box, G.E.P.; Hunter, J.S. Multi-factor experimental designs for exploring response surfaces. The Annals of Mathematical Statistics 1957, 28, 195–241.
- Dak, M.; Verma, R.C.; Jaaffrey, S.N.A. Effect of temperature and concentration on Rheological properties of “Kesar” mango juice. Journal of Food Engineering 2007, 80, 1011–1015.
- Tiziani, S.; Vodovotz, Y. Rheological effects of soy protein addition to tomato juice. Food Hydrocolloids 2005, 19, 45–52.
- Torres, B.; Tiwari, B.K.; Patras, A.; Wijngaard, H.H.; Brunton, N.; Cullen, P.J.; O’Donnell, C.P. Effect of ozone processing on the colour, rheological properties, and phenolic content of apple juice. Food Chemistry 2011, 124, 721–726.
- Opazo-Navarrete, M.; Tabilo-Munizaga, G.; Vega-Gálvez, A.; Miranda, M.; Pérez-Won M. Effects of high hydrostatic pressure (HHP) on the rheological properties of Aloe vera suspensions (Aloe barbadensis Miller). Innovative Food Science & Emerging Technologies 2012, 16, 243–250.
- Swami Hulle, N.R.; Patruni, K.; Rao, P.S. Rheological properties of aloe vera (Aloe barbadensis Miller) juice concentrates. Journal of Food Process Engineering 2014. DOI:10.1111/jfpe.12093
- Abbasi, S.; Dickinson, E. High-pressure-induced rheological changes of low-methoxyl pectin plus micellar casein mixtures. Journal of Agricultural and Food Chemistry 2002, 50, 3559–3565.
- Mozhaev, V.V.; Heremans, K.; Frank, J.; Masson, P.; Balny, C. Exploiting the effects of high hydrostatic pressure in biotechnological applications. Trends in Biotechnology 1994, 12, 493–501.
- Xu, Z.H.; Zhang, L.Y.; Wang, Y.T.; Bi, X.F.; Buckow, R.; Liao, X.J. Effects of high pressure CO2 treatments on microflora, enzymes, and some quality attributes of apple juice. Journal of Food Engineering 2011, 104, 577–584.
- Hsu, K.C. Evaluation of processing qualities of tomato juice induced by thermal and pressure processing. LWT-Food Science and Technology 2008, 41, 450–459.
- Kaushik, N.; Kaur, B.P.; Rao, P.S. Application of high pressure processing for shelf life extension of litchi fruits (Litchi chinensis cv. Bombai) during refrigerated storage. Food Science and Technology International 2013. DOI:10.1177/1082013213496093
- Vega-Gálvez, A.; Giovagnoli, C.; Pérez-Won, M.; Reyes, J.E.; Vergara, J.; Miranda, M.; Uribe, E.; Di Scala, K. Application of high hydrostatic pressure to aloe vera (Aloe barbadensis Miller) gel: Microbial inactivation and evaluation of quality parameters. Innovative Food Science & Emerging Technologies 2012, 13, 57–63.
- Landl, A.; Abadias, M.; Sárraga, C.; Viñas, I.; Picouet, P.A. Effect of high pressure on the quality of acidified Granny Smith apple puree product. Innovative Food Science & Emerging Technologies 2010, 11, 557–564.
- Kaushik, N.; Kaur, B.P.; Rao, P.S.; Mishra, H.N. Effect of high pressure processing on color, biochemical, and microbiological characteristics of mango pulp (Mangifera indica cv. Amrapali) 2014, 22, 40–50.
- Smelt, J.P.; Hellemons, J.C.; Patterson, M. Effects of high pressure on vegetative microorganisms. In: Ultra High Pressure Treatments of Foods; Hendrickx, M.E.G.; Knorr, D.; Eds.; New York, NY: Kluwer Academic/Plenum Publishers, 2002; 55–76.
- Farkas, D.F.; Hoover, D.G. High pressure processing. Journal of Food Science 2000, 65, 47–64.
- World Health Organization. (1999). WHO Monographs on Selected Medicinal Plants, Vol. 1; Geneva: World Health Organization.