Abstract
In this study, hazelnut oil oleogels prepared with sunflower wax and carnauba wax were analyzed and compared with a commercial shortening. Oil binding capacities of sunflower wax oleogels were higher than 99%, while carnauba wax had a maximum value of 97.6% for 10% addition level. At 3% addition level of carnauba wax, no gel developed. The crystal formation time of sunflower wax was shorter. Although the highest (8.5%) solid fat content was observed in the 10% carnauba wax containing oleogel (HC10) sample, it was 30.4% in the commercial shortening sample at 20°C. The peak melting temperature of commercial shortening was 52.3°C, and among all organogels, sunflower wax oleogel at 3% addition level had the closest value (58.4°C). The melting enthalpies of the oleogels ranged from 4.3 to 20.3 J/g, while it was 10.9 J/g for the commercial shortening sample. The firmness and stickiness values in the oleogel samples were lower than that of commercial shortening sample. On the other hand, there was no significant change of firmness and stickiness during storage, indicating good stability (p ≤ 0.001). Especially the sunflower wax oleogels were very homogenous and smooth in structure. The polarized light microscopy pictures revealed needle-like crystals for sunflower wax and aggregate-like crystals for carnauba wax oleogels. The x-ray diffraction measurements of the crystals showed the β´ types of the polymorphic structures. Furthermore, the oleogels were very stable against oxidation during the storage period. Hazelnut oil organogels prepared with sunflower wax can be good source material for shortening or margarine-like products.
INTRODUCTION
Edible oils naturally exist in solid, semi-solid or liquid state, and this physical state can determine their application areas in food products.[Citation1,Citation2] The physical state of oil depends on the chain length and saturation level of its fatty acids, cis/trans isomers content and the position of fatty acids in the glycerol. As versatile raw materials, the physical properties of liquid oils have been modified by some technological applications such as hydrogenation, fractionation, interesterification, blending, and crystallization. Although these present technologies are useful, they may have some disadvantages, such as increase in saturated and trans fatty acid content, expensive investment and higher operation cost.[Citation1,Citation3,Citation4]
Oil structuring has been a technological challenge for many research efforts. Organogelation is based on a completely different approach than hydrogenation or fractionation.[Citation2] Briefly, some small molecular weight organogelator agents are added into liquid oil and mixed, and the oil is entrapped within a thermo-reversible, 3-dimentional network created by the organogelators. Apparently there is no change in the fatty acid composition of the stock oil. For edible oils, sometimes the term “oleogel” is used to distinguish it from other organic phase gels. This technique is novel, easy to apply, relatively cheaper, and safer compared to hydrogenation. The most important challenge in organogelation researches is to find the most suitable organogelators. Oleogels are suggested for production of plastic fat stocks, nutraceutical encapsulation and delivery, controlled release of bioactives, production of creams and cookies, substitution in meat products and confectionary, minimizing oil migration in different processed foods, and others.[Citation2,Citation3,Citation5,Citation6]
Among others, natural waxes have been investigated for their organogelation properties in edible oils. Carnauba wax (CW) is produced from the leaves of Brazilian palm, Copernicia prunifera. It is usually more expensive than most of the other plant waxes.[Citation2] In a study,[Citation7] the organogel properties of rice bran wax, candelilla wax, and CW were compared. The melting temperature and enthalpy change of CW were determined to be 84°C and 137.6 J/g, respectively. It was shown that the minimum gel concentration for CW is 4%, and gelation time was 13.5 min. The hardness value of CW oleogels was the lowest among others. In that study, it was concluded that rice bran wax was the best as organogelators when compared with candelilla and CWs. Sunflower wax (SW) is produced from the seeds of Helianthus annuus plant.[Citation2] The organogelation properties of SW with soybean oil were investigated. The minimum quantity required for gelation were determined for many plant waxes, animal waxes, and synthetic waxes in that study, but only SW organogels were studied extensively. It was determined that SW can produce gels at 0.5–10% concentration with 47–67°C melting point range, and as the wax content increased in the organogels, the melting temperatures, enthalpies, and firmnesses enhanced. The microphotographs of SW organogels were also provided.[Citation8]
In this study, the oleogels of hazelnut oil (HO) prepared with CW and SW and their textural, structural, thermal, visual, and storage properties were examined in detail. Three different addition levels (3, 7, and 10%, w/w) of the waxes were also compared to find out the most suitable one as spreadable product. The oleogels produced in this study were also compared with a commercial shortening (CS) product to reveal the level of similarities. In addition, storage stability for texture and oxidation was monitored at both room and refrigerator temperatures for three months. Furthermore, the x-ray diffraction (XRD) data is provided for the oleogels developed. To our best knowledge, the literature is lacking for SW oleogels for XRD pattern data.
MATERIALS AND METHODS
Materials
Refined HO (Çotanak Oil Co., Ordu, Turkey) was purchased from marketplaces. The fatty acid composition of the oil (% weight) provided by the producer, as 0.03% myristic, 5.87% palmitic, 0.2% palmitoleic, 2.64% stearic, 82.7% oleic, 9.50% linoleic, 0.07% linolenic, 0.13% arachidic, and 0.02% behenic acids. SW (6607L) was purchased from KahlWax (Kahl GmbH & Co., Trittau, Germany). It is a yellowish solid pellet with soft/characteristic odor, with 0.85–1.05 g/cm3 density at 20°C, having a melting range of 74–80°C, acid value of 2–8 mg KOH/g, saponification value of 80–96 mg KOH/g, peroxide value (PV) of 0 mval/kg. It is classified as free from dangerous chemicals according to Annex I to Directive 67/548/EEC. CW (5023) was also purchased from KahlWax (Kahl GmbH & Co., Trittau, Germany). It is a yellow to brown solid powder/flakes with faint odor, having density of 0.99–1.00 g/cm3 at 20°C, with melting range of 78–88°C, acid value of 5–15 mg KOH/g, saponification value of 80–95 mg KOH/g. No dangerous materials were acknowledged and the wax was claimed as safe product.
Preparation of the Oleogels
The addition levels of waxes were 3, 7, and 10% as weight ratio. First HO and each of the SW and CW were placed into beakers and heated in a water bath at 90°C. When the waxes melted completely, and all are at the same temperature, the waxes were added into the oil and stirred vigorously for 5 min. Then this mixture was poured into sterile plastic cups (150 mL) and glass tubes with caps for subsequent analyses. The oleogels were formed at ambient temperature without any shear effect overnight. Finally, the planned analyses were performed for each sample. For the storage trial, the samples were stored at 20°C in a dark place, and at 4°C in a refrigerator for three months and analyzed periodically. The oleogel production was repeated twice, and all analyses within each production replicate were at least twice. In , the pictures of the HO oleogels are shown. Throughout this manuscript, the samples were abbreviated with codes: HO-hazelnut oil; SW-sunflower wax; CW-carnauba wax; CS-commercial shortening; HS3, HS7, and HS10-oleogels of HO at concentrations of 3, 7, and 10% SW; HC3, HC7, and HC10-oleogels of HO prepared with addition of CW in concentrations of 3, 7, and 10%.
Oil Binding Capacity (OBC)
The OBC method was adapted from Da Pieve et al.[Citation9] First, 1 mL of the melted oleogel sample was put into previously weighed eppendorf tube (weight a) and conditioned in refrigerator for 1 h. Then the tube was weighed again (weight b). The tubes were centrifuged at 9167 g for 15 min at room temperature, and turned over onto a filter paper for drainage of released oil. Finally, the tubes were weighed (weight c) again and OBC was calculated by Eq. (1).
Crystal Formation Time (CFT)
The previously formed oleogel samples were first completely melted in water bath (90°C) and kept for 2 h for isothermal setting. Then they were taken out from the water bath to room temperature, and meantime chronometer was started. The CFT was recorded when the tubes turned 90° and no flow observed.[Citation7]
Solid Fat Content (SFC)
The method of ISO 8292-2[Citation10] was followed for the percent of SFC measurement. The analysis were carried out at 20 and 30°C with a Minispec Bruker NMR Analyzer mq20 (Bruker Optics, Inc., Billerica, MA, USA) calibrated with 0, 31, and 73.5% solid fat containing standard solutions.
Color Measurement
The color of the oleogel samples was measured with Minolta CR-400 colorimeter (Konica Minolta Sensing, Osaka, Japan). First, the instrument was calibrated with white tile, and then blink reading on an empty glass tube was done to set off the effects of tube. Finally, the color reading were accomplished on several different points of the glass tubes filled with the organogel samples, and the values of L, a*, and b* were recorded.[Citation11]
Thermal Analysis
The thermal cycling technique of Dassanayake et al.[Citation7] was adapted for this analysis. The thermal analysis was performed with a Perkin-Elmer 4000 Series differential scanning calorimeter (DSC; Groningen, The Netherlands). The instrument was calibrated with Indium and Zinc standards. First, the DSC was purged with nitrogen at 50 mL/min flow rate. Then the oleogel samples were weighed around 5–7 mg into aluminum pans and sealed hermetically. The temperature program was heating the samples from room temperature to 140°C by 10°C/min; then cooling to –20°C by 10°C/min rate and keeping for 3 min at that temperature for full crystal formation and finally heating again to 100°C by 5°C/min rate. The thermal parameters were calculated by Pyris 1 Manager Software on the instrument.
Instrumental Texture Analysis
The firmness and stickiness of the oleogels were measured with a Texture Analyzer TA-XT2i (Stable Microsystems, Surrey, UK) equipped with a custom-built block and 45° conic acrylic probe. The samples stored at room temperature (20°C) or refrigerator (4°C) were taken out and measured at that temperature to observe the effect of storage temperature. Texture was also monitored during 90 days storage to observe the effects of storage time. The penetration test was accomplished by penetrating the probe into 23 mm depth by 3.0 mm/s speed, and then pulling the probe out at 10 mm/s speed. The texture parameters were calculated by Texture Exponent v.6.1.1.0 software (Stable Microsystems).[Citation12]
Crystal Morphology
The crystal morphology of the oleogels was observed at room temperature with an Olympus BX51 polarized light microscope (Olympus Optical Co., Ltd., Tokyo, Japan) equipped with a CCD color video camera (Canon Inc., Tokyo, Japan).[Citation13]
XRD Analysis
The wide and small-angle XRD patterns of the oleogels were taken with a Rigaku D-Max Rint 2200 model XRD (Rigaku Int. Corp, Tokyo, Japan). The angular scans from 2.0° to 50° (2θ) were performed by 2°/min scan rate. A Cu source x-ray tube (λ = 1.54056 Å, 40 kV and 40 mA) and MDI Jade 7 software (Materials Data Inc, Livermore, USA) was used for the data analysis.
Oxidative Stability
During the 90 days storage at the two different temperatures, the oxidative stabilities of the samples were monitored by measuring the PV (Cd 8-53 method).[Citation14]
Statistical Analysis
The duplicate measurements within each replicate of oleogel productions were performed for the samples and the results were represented as mean values with standard deviations. The data were analyzed by ANOVA and the multiple comparisons of the means accomplished by Tukey’s test. Statistic analysis was performed with Minitab v.16.1.1.[Citation15]
RESULTS AND DISCUSSION
Physico-Chemical Properties
The OBC, CFT, SFC and color values (L, a*, and b*) of the oleogel samples are shown in . Clearly, the oil binding capacities (OBC) of the CW oleogels at 3 and 7% addition levels were much lower than that of SW oleogels. No stable gels were observed for CW oleogels at 3% addition level, and the crystal formation periods were also longer. The results indicated that the 10% CW containing oleogel exhibited maximum SFC (8.5%) at 20°C, whereas at the same temperature CS had 30.4% SFC. The SFCs measured at 30°C were lower than that measured at 20°C. Co and Marangoni[Citation2] have indicated that there was no change in the SFC of organogels, as one of the main advantages listed for organogelation. This situation is confirmed in this study as well. There was a proportional increase of SFC in the oleogels depending on the addition and saturation levels of the organogelators used. The instrumental color values of the oleogel samples showed similarity for a* and b* values with CS, while the level of brightness (L value) was somewhat different. The colors and appearances of the oleogels can be visualized from .
TABLE 1 The oil binding capacity (OBC), crystal formation time (CFT), solid fat content (SFC), and color values (L, a*, and b*) of the oleogel samples
Thermal Properties
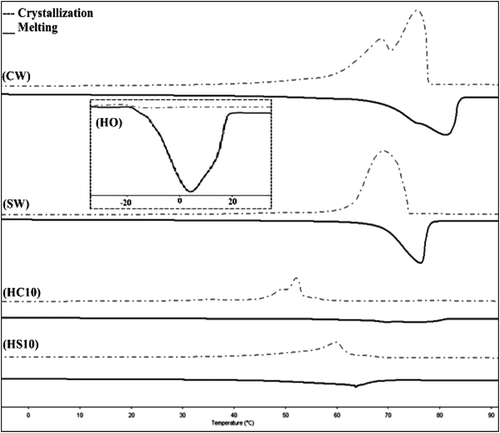
In any plastic fat product, crystallization and melting behaviors are essential components of its technological properties.[Citation1] Hence, the thermal properties of the oleogel samples together with HO, SW, and CW are measured with DSC and from the thermograms () the thermal parameters were calculated by the software and the results are presented in . The melting temperatures and enthalpies of the SW and CW oleogels were quite similar. On the other hand, the melting temperatures of SW containing oleogels were a little lower than that of CW containing oleogels. The melting peak temperatures of the CS was 52.3°C, and among the oleogel samples, 3% SW containing oleogel had the closest value (58.4°C) to that of the CS sample. As the level of added wax increased, the melting temperatures and enthalpies also increased, as expected.[Citation2] The melting enthalpies of the oleogels were different from that of CS sample. The melting enthalpies increased as the level of added organogelators enhanced. The melting temperatures of SW and soybean oil organogels were reported to be between 47 and 65°C within 0.5–10% addition levels. At the same addition range, the enthalpy change of melting was reported between 0.2 and 15 J/g.[Citation8] Hence, 3–7% SW containing HO can be suggested as an alternative source for shortening fat stock. CW oleogels had similar performance as long as only the thermal properties concerned. Dassanayake et al.[Citation7] reported 84°C melting point and 137.6 J/g melting enthalpy for CW, and we measured 81.04°C melting point and 182.15 J/g melting enthalpy for the same wax. The differences can be attributed to the materials source and purity differences. It was concluded that rice bran wax was better than CW for organogel properties.[Citation7] Similarly, we conclude that SW is better than CW regarding the oleogel properties.
TABLE 2 The thermal properties of the oleogel samples
Textural Properties and Storage Stability of the Oleogels
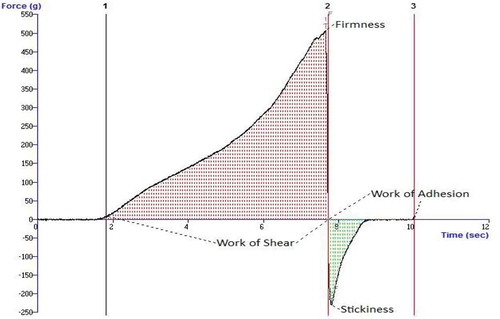
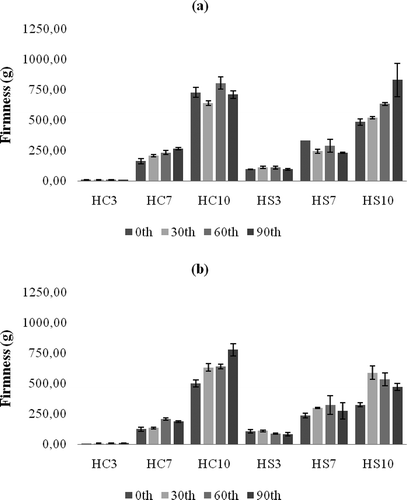
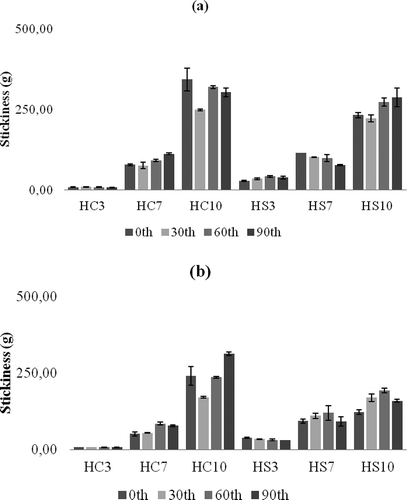
The textural properties of plastic fats are as important as thermal properties, and they define the spreadability, adhesion, and oil separation phenomena.[Citation1] An example graph of the texture measurement is shown in . The firmness values measured for the oleogel samples are shown in . For both waxes, as the addition level increased, the firmness of corresponding oleogels increased proportionally (p ≤ 0.001). At room temperature, the firmness values have mostly not changed during storage, but only the firmness value of 10% CW containing sample has increased. There was no significant difference between the samples (p = 0.794) for the two organogelators in terms of firmness values. Similarly, there was no significant difference of the firmness values for the samples stored at refrigerator or room temperature (p = 0.469). This situation is quite important for the reduction of cooling requirements. Oleogels are self-stable, thermoreversible and plastic products which do not require cooling systems.[Citation2,Citation3] The lowest firmness value among the samples was 6.9 g with 3% CW oleogel stored at 20°C at the beginning of storage, and the highest value was 831.8 g in 10% SW oleogel stored for 90 days at 4°C. The CS sample had the lowest firmness value (533.8 g) in sample stored at 20°C for 30 days, and the highest value (5824.8 g) in a sample stored at 4°C for 60 days. Clearly CS had higher firmness values, but the oleogel samples prepared with 10% organogelator had firmness values close to the values measured in the CS sample. In fact, all oleogel samples were very stable during storage and the perceptible firmness was almost the same as that of the CS sample. The stickiness or adhesion, the other very important textural parameters, was measured and the results are presented in . The stickiness values of CW oleogels were not significantly different (p = 0.731) from that of the corresponding SW oleogels at both storage temperatures. Similarly to firmness, storage at 4°C has yielded a little higher stickiness values for the two types of the oleogels (p = 0.416). As the added wax level increased, the stickiness was also enhanced (p ≤ 0.001) for both types. Among all oleogels prepared, the lowest stickiness value was found as 7.2 g for 3% CW oleogel stored at 4°C for 90 days. The highest stickiness value was 343.3 g for 10% CW oleogel stored at 4°C for 0 days. The range of stickiness for CS samples was from 274.06 g measured in a sample stored at 20°C for 0 day to 1917.4 g measured in another sample stored at 4°C for 90 days. The measured stickiness values of the CS were much higher than that of the oleogel samples. It was indicated that the correlation between hardness (firmness) and spreadability, and of cohesiveness and spreadability were higher, but correlation between adhesiveness and spreadability was lower among the analyzed table fats.[Citation16] Hence, the evaluation of both firmness and stickiness together might be more meaningful for the estimation of spreadability of plastic fats. As stickiness of a plastic fat sample increases, more force is required to pull back the probe, indicating the possible difficulty of spreading.[Citation12] In another study,[Citation17] pistachio oil spreads were characterized. It was shown that firmness, adhesiveness, and compressibility of spreads were declined by added cocoa butter, whereas the same attributes were improved by xanthan gum additions. In a recent study,[Citation18] margarines from organogels were prepared and firmness values were compared with themselves and with commercial spread and margarine products. It was indicated that SW organogels are more suitable than rice bran and candelilla wax organogels for margarine formulations as long as firmness considered, although they did not perform a sensory analysis.
Polarized Light Microscopy and XRD Patterns
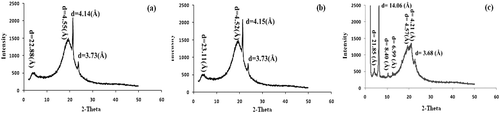
The polarized light microscopy pictures of the oleogels are shown in . Since no true crystals were present in 3% CW sample, its picture is not presented. Although SW oleogels had needle-like crystal structures, CW oleogels seem rather more aggregate-like. A very similar optical micrograph of CW in olive oil is presented by Dassanayake et al.[Citation7] They also suggested that CW organogels yield spherulitic structures and at 1% addition level, it is in a viscous sol state rather than an organogel structure. On the other hand, needle-like crystals in SW-soybean oil organogels[Citation8] seem very similar to those viewed in this study. We have also taken the wide and small angle XRD patterns of the oleogel samples ( and ). The XRD patterns of 10% CW and SW oleogels were very similar to each other. In fact, similar peaks in the CS sample are observed with much higher intensities. In addition to the common peaks, there was a distinct peak at around 14.06 Å in the CS sample which creates the main difference from the oleogels (). There are three common peaks at wide-angle region for both wax oleogels at around 2.99–3.73, 4.14–4.15, and 4.50–5.55 Å. Similar peaks at 0.372 and 0.413 nm were shown in olive oil and CW organogels.[Citation7] Usually peaks at that region indicate orthorhombic perpendicular subcell packing type crystals, and this type is found very similar to the β’ crystals of margarines and spreads with smooth texture and better mouthfeel properties defined.[Citation1,Citation7] Although the crystal microphotographs were shown in the study of Hwang et al.,[Citation8] the XRD patterns of SW organogels have not been investigated. This study provides the XRD patterns data of SW oleogels for the literature. SW oleogels had the very similar wide-angle region peaks and yielded very smooth and uniform crystalline network. Especially, SW containing oleogels were very stable during storage in terms of textural properties. As suggested previously for rice bran wax organogels[Citation7] and SW organogels,[Citation8] we also suggest that especially SW oleogels can be an alternative hard fat stock in some products formulations.
TABLE 3 The XRD measurement results of the oleogels samples
Oxidative Stability
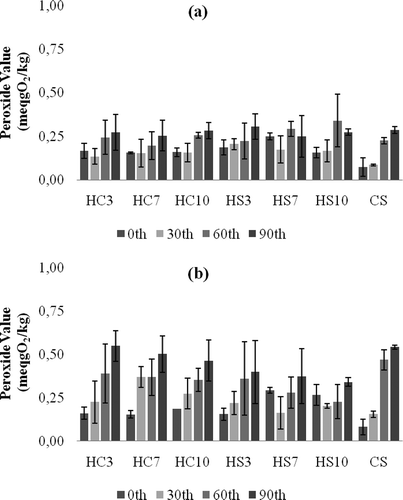
The oxidative stability of the oleogel samples and CS sample were monitored during three months storage at room and refrigerator temperatures by the PV measurements, and the results are shown in . Generally at both storage temperatures for all samples, the increase in the PVs through the storage time was minimal. Also, the increase was much less in refrigerator stored samples (p = 0.001). Among all stored oleogel samples, the highest PV (0.6 meqgO2/kg) was measured in 3% CW containing oleogel stored at 20°C for 90 days. At the same temperature (20°C), the PV for CS sample has reached its max value of 0.5 meqgO2/kg after 90 days. These results indicate that HO oleogels are very stable in terms of oxidation. This might be due to the fatty acid composition (see materials section) and higher amounts of tocols and sterols found in HO.[Citation19] The effect of mineral fortification on oxidative stability of reduced-fat spreads has shown that iron and copper added samples were very susceptible to oxidation, whereas low level of zinc addition led to acceptable oxidative stability in the spreads developed.[Citation20] In our study, there was no antioxidant or any other addition into the oleogels.
CONCLUSIONS
In this study, some important properties of the CW and SW containing oleogels of HO in comparison with a CS were determined. The SW oleogels were binding more liquid oil and forming the gel more quickly than that of the CW oleogels. The SFC of the oleogels were much lower than that of the CS sample. In general, the melting temperatures of the CW oleogels were higher than that of the SW oleogels. The melting temperature of 3% SW containing oleogel was the most similar one to the melting temperature of the CS sample. The firmness and stickiness values of the oleogels were fairly stable during the 90 days storage period. Both firmness and stickiness were a little higher in refrigerator stored oleogels than that of the room temperature stored samples. Compared to the CS, the measured texture values of the oleogels were lower. During the three months of storage, the textural and oxidative stabilities of SW oleogel samples were better than those of CW oleogels. Overall, from the determined properties of the HO oleogels, it would be concluded that, these HO-SW oleogels can be used in the productions of shortenings or similar products with acceptable suitability.
NOMENCLATURE
| HO- | = | hazelnut oil |
| CW- | = | carnauba wax |
| SW- | = | sunflower wax |
| CS- | = | commercial shortening |
| OBC- | = | oil binding capacity |
| CFT- | = | crystal formation time |
| SFC- | = | solid fat content |
| DSC- | = | differential scanning calorimetry |
| PV- | = | peroxide value |
| PLM- | = | polarized light microscopy |
| XRD- | = | x-ray diffraction |
FUNDING
This research is funded by the Scientific and Technical Council (TÜBİTAK) of Turkey as the COST 112O038 project within COST FA 1001 Action. The authors are grateful for the support.
Additional information
Funding
REFERENCES
- O’Brien, R.D. Fats and Oils: Formulating and Processing for Applications. CRC Press: New York, NY, 2004.
- Co, E.D.; Marangoni, A.G. Organogels: An alternative edible oil-structuring method. Journal of the American Oil Chemists’ Society 2012, 89, 749–780.
- Pernetti, M.; van Malssen, K.F.; Flöter, E.; Bot, A. Structuring of edible oils by alternatives to crystalline fat. Current Opinion in Colloid and Interface Science 2007, 12, 221–231.
- Stortz, T.A.; Zetzl, A.K.; Barbut, S.; Cattaruzza, A.; Marangoni, A.G. Edible oleogels in food products to help maximize health benefits and improve nutritional profiles. Lipid Technology 2012, 24 (7), 151–154.
- Bot, A.; Veldhuizen, Y.S.J.; den Adel, R.; Roijers, E.C. Non-TAG structuring of edible oils and emulsions. Food Hydrocolloids 2009, 23, 1184–1189.
- Marangoni, A.; Garti, N. Food oil gels: New strategies for structuring edible oils. INFORM 2011, 22 (5), 317–320.
- Dassanayake, L.S.K.; Kodali, D.R.; Ueno, S.; Sato, K. Physical properties of rice bran wax in bulk and organogels. Journal of the American Oil Chemists’ Society 2009, 86, 1163–1173.
- Hwang, H.S.; Kim, S.; Singh, M.; Winkler-Moser, J.K.; Liu, S.X. Organogel formation of soybean oil with waxes. Journal of the American Oil Chemists’ Society 2012, 89 (4), 639–647.
- Da Pieve, S.; Calligaris, S.; Co, E.; Nicoli, M.C.; Marangoni, A.G. Shear nanostructuring of monoglyceride organogels. Food Biophysics 2010, 5, 211–217.
- ISO. Animal and vegetable fats and oils—Determination of solid fat content by pulsed NMR-Part 2: Indirect method. ISO/TC 34/SC 11, On-line version, 2008.
- Pomeranz, Y.; Meloan, C.N. Food Analysis: Theory and Practice. Chapman & Hall Pub. Inc.: New York, NY, 1994.
- Moskowitz, H.R. Food Texture—Instrumental and Sensory Measurements. Marcel Dekker: New York, NY, 1987.
- Toro-Vazquez, J.F.; Morales-Rueda, J.A.; Dibildox-Alvarado, E.; Charo´-Alonso, M.; Alonzo-Macias, M.; Gonza´lez-Cha´vez, M.M. Thermal and textural properties of organogels developed by candelilla wax in safflower oil. Journal of the American Oil Chemists’ Society 2007, 84, 989–1000.
- AOCS. Peroxide Value, Acetic Acid-Chloroform Method, Cd 8-53. Official Methods and Recommended Practices of the America Oil Chemists Society, 5th Ed; AOCS Press: Champaign, IL, 1997.
- Minitab. Minitab 16.1.1 Statistical Software. Minitab, Inc., State College, PA, USA, 2010.
- Glibowski, P.; Zarzycki, P.; Krzepkowska, M. The rheological and instrumental textural properties of selected table fats. International Journal of Food Properties 2008, 11, 678–686.
- Mousazadeh, M.; Mousavi, M.; Emam-Djomeh, Z.; Hadinezhad, M.; Gharibzahedi, S.M.T. Formulation optimization of pistachio oil spreads by characterization of the instrumental textural attributes. International Journal of Food Properties 2014, 17, 1355–1368.
- Hwang, H.S.; Singh, M.; Bakota, E.L.; Winkler-Moser, J.K.; Kim, S. Margarine from organogels of plant wax and soybean oil. Journal of the American Oil Chemists’ Society 2013, 90, 1705–1712.
- Alasalvar, C.; Amaral, J.S.; Shahidi, F. Functional lipid characteristics of Turkish tombul hazelnut (Corylus avellana L.). Journal of Agricultural and Food Chemistry 2006, 54, 10177–10183.
- Stathopoulos, C.E.; Chockchaisawasdee, S.; Doyle, J.; O’Kennedy, B.T.; Mounsey, J.S. Effect of mineral fortification on textural and oxidative stability of reduced-fat spreads. International Journal of Food Properties 2009, 12, 368–378.