Abstract
The objective of this work was to identify the main allergenic proteins and to assess the influence of high hydrostatic pressure on the potential allergenicity of high hydrostatic pressure pineapple juice. Fresh pineapple juice was treated with different pressures and the allergenic proteins were extracted and analyzed by sodium dodecyl sulfate polyacrylamide gel electropheresis, Western bloting and liquid chromatography-tandem mass spectrometry. The main allergenic proteins were detected as 24-kDa and 14-kDa bands by serum of pineapple-allergic patients. liquid chromatography-tandem mass spectrometry analysis of these bands showed that they actually consisted of a mixture of several isotypes of cysteine proteinases. Among these, bromelain (Ananas comosus) is a known allergen named Ana c 2 and it is highly homologous to other cysteine proteinases. Enzyme-linked immunosorbent assay assay of high hydrostatic pressure pineapple juice carried out at ambient temperature (18–22°C) showed that the higher the pressure, the higher the reduction in allergenicity, yielding a maximum reduction of about 20% under 500 MPa. However, in the case of 400 MPa and 50°C, the reduction rate of allergenicity was higher, up to 50% compared to that of the sample at 20°C without high hydrostatic pressure treatment. Taken together, the results suggested that high-pressure treatment could alter the potential allergenicity of pineapple juice allergens and identified Ana c 2 and a few other related cysteine proteinases as the main allergens.
INTRODUCTION
Pineapple is a widely grown fruit in the tropical and subtropical regions of the globe.[Citation1,Citation2,] Pineapple juice is one of the most important non-citrus juices and is highly consumed in many countries.[Citation3,Citation4] It has been on the market shelves for some years, mainly because of its pleasant unique aroma and flavor. Pineapple juice is normally sterilized by thermal treatment. Nevertheless, conventional thermal sterilization can cause detrimental change in the quality of the juice because of the high temperature used in the process.[Citation5,Citation6]
High hydrostatic pressure (HHP) has been known as a potential food preservation method for over a century, interests have increased since recent progress in the engineering of large-scale equipment has allowed this technology to be adapted to the needs of the food industry.[Citation7,Citation8] The use of HHP in the processing of pineapple juice is of great interest because of its ability to inactivate microorganisms at low temperature and without preservatives or other additives.[Citation9,Citation10] Pressure-treated pineapple juice has similar sensory properties as fresh pineapple juice, which is a major advantage in juice processing as it fulfills consumers’ demand for high quality, nutritious, and ‘‘natural tasting’’ products.[Citation11,Citation12] It has been known that some allergenic proteins contained in pineapple easily cause allergies.[Citation13] The allergenic properties of proteins could be altered by hiding, destroying, or disclosing allergenic epitopes through the conformational changes[Citation14] and high-pressure treatment and cause changes in the structure of the protein molecules due to cleavage of weak hydrogen bonds and van der waals forces and then affect the allergenicity of proteins.[Citation15−Citation18]
Food allergens have become a significant worldwide public health issue. Food allergen labeling rules, which were introduced by European Directives 2003/89/EC and 2007/68/EC, require that food manufacturers indicate 14 groups of potential allergens by reference to their sources if they are to be used as ingredients at any level in pre-packed foods.[Citation19−Citation21] Approximately 4% of the total population in China are susceptible to food allergens and fruit is one of the ten allergenic foods.[Citation22,Citation23]
At present, it is generally recognized that pineapple allergy is caused by bromelain. Bromelain is a collective name for proteolytic enzymes or proteases found in tissues, including the stem, fruit, and leaves of the pineapple plant family Bromeliaceae.[Citation24] The highest concentration is found in the stock of ripe pineapple. Hale LP et al.[Citation25] reported that bromelain can induce a strong antibromelain antibody in the serum of mice that were given a daily dose of bromelain via oral administering for 18 weeks, and the antibody response is dose-dependent. Bromelain appears to be highly immunogenic when given orally. The main proteolytic constitutents contained in pharmaceutical preparations of bromelain are also present in the pineapple fruit.[Citation26] Bromelain is known to cause IgE-mediated respiratory and gastrointestinal allergic reactions. Furthermore, there is evidence for immunological cross-reaction between the two plant proteases, bromelain and papain, in human subjects.[Citation27,Citation28] Development of antibody responses to bromelain could limit the consumption of pineapple juice by these individuals, especially pineapple juice without thermal sterilization.
Pineapple allergy is more common in Guangdong, Guangxi and other coastal areas of China where pineapples are widely grown compared to the more inland regions. The aim of this study was to distinguish the allergy related proteins in pineapple juice and the influence of HHP treatment in potential allergenicity of the pineapple juice.
MATERIALS AND METHODS
Materials
Human serum were provide by The First Affiliated Hospital of Anhui Medical University. They were collected from five patients with a clinical history of allergic reactions toward pineapples, each of these patients tested positive to pineapple. Serum IgE specific for pineapple was determined using ImmunoCap™ (Phadia, Uppsala, Sweden). Sera from healthy donors without allergy were included as controls. Written informed consent was obtained from all study participants. Allergic-sera were pooled together, in order to screen IgE binding pattern of major pineapple allergens. Pineapples (Ananas comosus) were purchased at a local market. Protein markers for SDS-PAGE were obtained from Thermo Fisher Scientific (USA). Chemicals and solvents of analytical and molecular biology grades were obtained from Sigma-Aldrich (Bornem, Belgium), Tedia (Fairfield, USA), and Bio-Rad (Nazareth, Belgium). Enzyme-linked immunosorbent assay (ELISA) Plates were brought from Costar (CA, USA), ultrafiltration centrifugal tube from Millipore (Billerica, USA), BioTraceTM polyvinylidene difluoride (PVDF) membrane was purchased from Pall Corporation (Rensacola, USA). Others were brought from Sangon BioTech (Shanghai, China).
HHP Processing
Pineapples were peeled and squeezed with a small manual juicer (SPL G008, Jiangmen, China) and the juice was filtered through six layers of cheese cloth. For HHP treatment, the obtained pineapple juice was dispensed in sterile polyethelene (PE) bottles and the bottles were sealed into plastic bags which were filled with water. Air bubbles were avoided. The plastic bags were subsequently treated in a 1-L high pressure vessel developed by the research center. The vessel is capable of producing a pressure of up to 600 MPa and at a temperature range from 0˜60°C. The sample was treated with 0, 200, 300, 400, or 500 MPa for 20 min at ambient temperature (18–22°C). A separate sample was treated at 400 MPa for 20 min at 20, 30, 40, or 50°C.
Protein Extraction and Purification
HHP treated pineapple juices were centrifuged at 4000 ×g (Sigma Laborzentrifugen, 3K15, Osterode am Harz Germany) and 4°C for 30 min. The supernatant was retained and solid ammonium sulfate was slowly added and with continuous stirring. The ammonium sulfate was added until 40% saturation was reached, and the mixture was continuously stirred for more than 2 h. It was then aliquoted into 10-mL PE centrifuge tubes and centrifuged at 6000 × g for 20 min. The supernatant in each tube was discarded and the pellet was dissolved in 1 mL ddH2O, and then desalted with the use of Ultrafiltration Centrifugal Filters. After desalting, the final volume of each sample was made to 5 mL with ddH2O, and the obtain samples were all stored at –20°C until needed. All steps were carried out at 0˜4°C.
SDS-PAGE and Western Blot Analysis
Sodium dodecyl sulfate polyacrylamide gel electropheresis (SDS-PAGE) was carried out using a Mini-PROTEAN® Tetra gel system (Bio-Rad Laboratories, Hercules, USA). The gel consisted of 15% separating gel and gel 5% w/v stacking gel. Samples were boiled for 1 min in the presence of reducing SDS-PAGE sample buffer before loading. Duplicate gels were run at the same time. After electrophoresis, one gel was stained with Coomassie Brilliant R250 to visualize the protein bands, whereas the other gel was subjected to Western blot analysis. Protein bands were transferred onto PVDF membrane at a constant current of 250 mA for 90 min using the Mini Trans-Blot® (Bio-Rad Laboratories, Hercules, USA). The membrane was blocked with blocking buffer [Tris-HCl buffer solution-Tween (TBST; TBS + 0.5% Tween 20) containing 5%w/v skim milk powder] at 37°C for 2 h or at 4°C for overnight. After that, it was incubated at 37°C for 90 min in blocking buffer containing 1:100 dilution of allergic patients’ serum. Next, the membrane was washed three times in TBST, with 5 min per wash, and then incubated at 37°C for 1 h in blocking buffer containing 1:5,000 dilution of Goat anti-Human IgE-linked horse radish peroxidase (HRP) (Abcam, Massachusetts, USA). Following incubation the membrane was again washed three times in TBST, and then detected by exposure to detection reagent (3.0 mM 4-chloro-1-naphthol, 17% methanol, 83% 0.01 M TBS, and add 5.0 mM H2O2) for 15 min in the dark.
LC-MS/MS
Protein bands of interest detected by Western blot were excised from the corresponding Coomassie-stained gel. They were sliced into 1-mm3 pieces and digested with trypsin according to the method described by Christiane Kruse Fæste et al.[Citation29] and then subjected to LC-MS analysis using a splitless nanoACQuity (Waters, Milford, USA) system coupled to TripleTOF 5600 mass spectrometer (AB SCIEX, Concord, ON). The system uses microfluidic traps and nanofluidic columns packed with Symmetry C18 (5 μm, 180 um × 20 mm) for online trapping, desalting, and nanofluidic columns packed with BEH130 C18 (1.7 μm, 100 μm × 100 mm) for analytical separations. The solvents used consisted of water: acetonitrile: formic acid at a ratio of 98:2:0.1 for A, and 2:98:0.1 for B. Separation was established by maintaining the column at 5% B for 1 min, followed by a linear gradient to 35% in 40 min, and then maintained at 35% for 5 min. The concentration of B was increased linearly to 80% in another 5 min and maintained at this concentration for 5 min.
For mass spectrometry analysis, a Nanospray III source (AB SCIEX, Concord, ON) and a pulled quartz tip as the emitter (New Objectives, Woburn, MA) were used. Data was acquired using an ion spray voltage of 2.5 kV, curtain gas of 30 PSI, nebulizer gas of 15 pounds per square inch (PSI), and an interface heater temperature of 150°C. For IDA, survey scans were acquired in 250 ms and as many as 30 product ion scans were collected if exceeding a threshold of 120 counts per second (counts/s) and with a 2+ to 5+ charge-state. Total cycle time was fixed to 3.3 s. A sweeping collision energy setting of 35 ± 5 eV was applied to all precursor ions for collision-induced dissociation. Dynamic exclusion was set for 1/2 of peak width (18 s), and then the precursor was refreshed off the exclusion list.
The Peakview® software (AB SCIEX, Concord, ON) was used to process MS/MS data. MS/MS spectra were automatically searched for sequence matches using the National Center for Biotechnology Information (NCBI) non-redundant protein database. Unassigned MS/MS spectra were automatically processed using the AutoMod algorithm. The peptide sequence tag was searched against the NCBI database for plant proteins to identify the protein from which it came from.
ELISA
The allergenicity of HHP-treated pineapple juice was investigated using indirect enzyme-linked immunesorbent assay (iELISA). A 96-well ELISA plate was coated with 0.1 mL samples from the section of “protein extraction and purication”(which had been dissolved in carbonate buffer solution (CBS) by appropriate dilution) and the plate was then incubated at 4°C for overnight. After incubation the plate was washed three times with phosphate buffer solution (PBS) containing 0.05% Tween 20 (phosphate buffer solution-Tween [PBST]), with 5 min per wash. The plate was blocked with PBST containing 5% skim milk (0.2 mL/well) at 37°C for 2 h. After that, the blocking buffer was removed and serum (0.1 mL of 1:100 dilution) was added to each coated well of the plate and the plate was incubated at 37°C for 1 h. Next, the plate was washed three times with PBST (5 min per wash) followed by addition of 1:5,000 dilution of Goat anti-Human IgE in blocking buffer (0.1 mL/well) and further incubation at 37°C for 1 h. Finally, detection was carried out by adding 100 μl of substrate (trimethylbenzene [TMB]) to each well, and the plate was incubated at room temperature for 10 to 30 min. The reaction was terminated by adding 100 μl of 2 M H2SO4 to each well, and the plate was read at 450 nm using a microplate reader (Epoch, Bio-Tek, highland park, USA). Non-specific binding was studied using the serum from non-allergic individuals and appeared to be negligible.
RESULTS AND DISCUSSION
Identification Pineapple Juice Allergens with High-Pressure Treatment
Freshly made pineapple juice was subjected to a number of treatment steps before the effect of pressure treatment on the juice allergens was investigated. The process of juice treatment and subsequent analysis by ELISA is shown in . The effect of high-pressure treatment on the protein profile of pineapple juice was investigated by treating the material from the final step of the juice preparation (F1 in ) with different pressures at ambient temperature. The result of which is shown in . Overall, it appeared no difference between the non-treated sample and samples treated with different pressures. One dark-intensity band of around 24 kDa and two light-intensity bands above it and several other bands with molecular weight below 15 kDa were clearly visible in all the samples. This indicated that high-pressure treatment did not seem to alter the varieties protein species in the pineapple juice or influence their primary structures.
FIGURE 1 Outline of the procedure for the preparation of pineapple juice and identification of major allergic proteins.
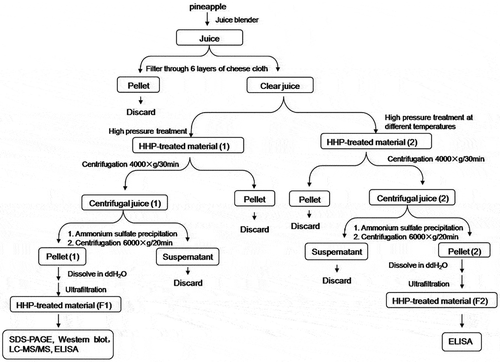
FIGURE 2 A: SDS-PAGE analysis of pineapple juice proteins after high-pressure treatment. Fraction F1 from the pineapple juice preparation (see Fig. 1) was treated with different pressures and then resolved in 15% gel; B: Western blots analysis of pineapple juice proteins after subjecting to high pressure treatment. Fraction F1 from the pineapple juice preparation (see Fig. 1) subjected to western blot analysis with allergic patients’ serum.
Among the various bands detected by SDS-PAGE, only two bands were detected by serum of pineapple-allergic patients. The sizes of these two bands were about 14 kDa and 24 kDa (). The presence of the 24-kDa band was consistent with that reported by H.R.Maurer,[Citation30] who showed that bromelain extracted from pineapple was 23.8 kDa. Other bands that were not detected using the serum of pineapple-allergic patients could be proteins such as peroxidase, acid phosphatase, and various protease inhibitors, all of which are known to be present in pineapple extracts.[Citation31,Citation32]
Confirmation of the Identified Allergens in Pineapple Juice by LC-MS/MS
To identify the two protein bands (14-kDa and 24-kDa) observed in SDS-PAGE and detected using allergic patients’ serum in western blot, the two bands were digested with trypsin and subjected to LC-MS/MS analysis. Through the use of Motorola automatic sequential computer operated tester (MASCOT) search engine, positive match results for the 24-kDa and 14-kDa bands were obtained and these are listed in and , respectively. shows that the 24-kDa band consisted of eight different proteins, all of which belong to cysteine proteinase of pineapple fruit. Four of these proteins were also present in the 14-kDa band (), and these were cysteine peptidase FB1035 precursor, bromelain and FBSB precursor from Ananas comosus, and Macrodontain I, which is also a cysteine peptidase from unripe fruits of Pseudananas macrodontes (Morr.) harms (Bromeliaceae), a species closely related to pineapple (Ananas comosus L.).[Citation33] As the amino acid sequence of these cysteine proteinases are highly homologous, it is possible that some of these proteins identified by LC-MS/MS may be false positive.
TABLE 1 Positive match result of 24kDa band of HHP pineapple juice
TABLE 2 Positive match result of 14-kDa band of HHP pineapple juice
TABLE 3 Aligned sequence of protein of Ana c 2 (bromelain [Ananas comosus]) that were identified by LC-MS/MS. Identified peptides of the 24-kDa and 14-kDa band in SDS-PAGE are, respectively, shown in bold and underlined
MS/MS analysis has shown that fruit bromelain is actually a mixture of several isotypes.[Citation34] The sizes of the two bands identified by western blot (24-kDa and 14-kDa) were different from the result of LC-MS/MS (Mr 36414, 39486, 39991), all of which indicates the sizes of the proteins are much larger than that of 24-kDa. Furthermore, some of the proteins identified in the 24-kDa band were also the same proteins identified in the 14-kDa band, for instances gi|2463588, gi|2342496, gi|2463584, and gi|24638018. This suggests that the proteins represented by the 14-kDa band could be proteolytic products of the proteins represented by the 24-kDa band.[Citation35] It also suggests that fruit bromelain represented by the 24-kDa band could indeed be the mature enzyme, a product derived from proteolyic cleavage of a proenzyme of larger molecular mass.[Citation35] Many proteases are synthesized as proenzymes, which need to undergo proteolytic cleavage, usually with a loss of segment of the N-terminus, becoming a mature enzyme.
Protein of bromelain (Ananas comosus) with a Mr of 39486 Da and pI 5.00 has already been identified as pineapple allergen Ana c 2 and its amino acid sequence is shown in . Ana c 2 which contains almost all the amino acid sequences shared by the other cysteine proteinases identified in the 24-kDa and 14-kDa bands of pineapple juice as a result of their high homology with Ana c 2. Thus, it is possible that these other proteinases may also be allergenic proteins, with different degrees of allergenicity. Marrugo et al.[Citation10] identified the 39-kDa protein as the main allergen of pineapples, which is in accordance with the present result. Though there are few reports about the epitopes of Ana c 2, studies in the relevant respects of antigenically active regions of bromelain have been carried out by researchers. Husain and Lowe[Citation36] and Goto. Murachi and Takahashi[Citation37] observed evidence of structural homology among bromelain, papain and ficin in terms of their amino acid sequences. Immunological cross-reactions between these enzymes as well as between stem and fruit bromelain have been reported by Sasaki, Kato, and Iida[Citation38] and Kato and Sasaki.[Citation39] Using radioallergosorbent test (RAST) inhibition, Bauer and Fruhmann[Citation40] demonstrated that papain, bromelain and some other allergens can mutually inhibit IgE specific to each antigen, and suggested that these allergens probably possess similar or even identical antigenically active regions, leading to immunological cross-reactivity. Louise Chambers[Citation41] and Laura P. Hale[Citation42] suggested that the proteolytic activity of bromelain and other cysteine proteases is critical for inducing immunogenicity and stated that the use of inactive allergens may be useful for desensitizing allergic reaction. Since the enzymatic activity of bromelain is dependent on the thiol group of cysteine residue within the active site,[Citation36,Citation43] it was speculated that the allergenicity of bromelain may also be associated with thiol group and disulfide bond.
Influence of Pressure on Allergenicity of HHP Pineapple Juice
Samples of HHP-treated material (F1) used for ELISA were diluted with CBS by 200 fold to reduce the protein concentration to that below saturation concentration 20 ug/mL required in the preliminary experiments.[Citation44] The effect of pressure on the potential allergenicity of pineapple allergens was investigated by subjecting the F1 fraction of the pineapple juice preparation to ELISA assay. The result of the assay is shown in . HHP treatment appeared to decrease the potential allergenicity of the allergens, as seen from the lower activity of high pressure-treated sample compared to the untreated sample. The potential allergenicity was reduced by about 16 and 20%, respectively when the sample was treated with 400 and 500 MPa. Increases in proteolytic activity of bromelain and other cysteine proteases have been considered as important for inducing the immunogenicity of allergens in fruit juice.[Citation45,Citation46] Therefore, decreases in the level of allergenicity of pineapple juice proteins under increasing pressures observed in the experiment were consistent with decreases in proteolytic activity for high pressure-treated pineapple juice reported by Tao Min et al.[Citation47] The use of inactivated allergens would be expected to eliminate the occurrence of allergic reaction.
FIGURE 3 (a) Immunoreactivity changes of proteins from pineapple juice after treated with different pressures; (b) immunoreactivity changes of pineapple juice proteins after treatment with 400 MPa under different temperatures. Control sample were kept at 20°C without high-pressure treatment.
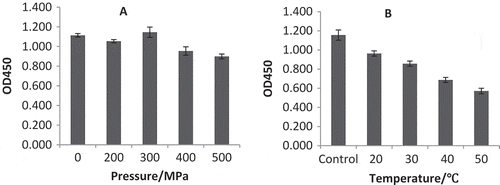
The influence of high pressure only on the level of allergenicity in pineapple juice proteins was not obvious. Thus, to further reduce the allergenicity of the allergens, high pressure (400 MPa) treatment was carried out under increasing temperatures, from 20 to 50°C. Marisa Rattanathanalerk[Citation48] found the quality loss of the pineapple juice which was treated at least 55°C and 10 min, and Jutamongkon and Charoenrein[Citation49] demonstrated that fruit bromelain activity only retained 51% after 8 min at 60°C. So 50°C was selected as the highest temperature to reduce the negative influence of temperature. The pressure selected was 400 MPa mainly due to the influence of pressure at 400 and 500 MPa almost had no difference in allergenicity and 400MPa is more energy-saving in pineapple juice processing. Greater reduction in allergenicity was observed when the sample was treated with high pressure and moderate temperature at the same time (), with the greatest reduction obtained at 50°C, which is about 50% reduction compared to the control (non-treated sample at 20°C).
Gomes et al.[Citation50] reported that high pressure influences cysteine protease by altering the precise spatial arrangement of residues in the active site and/or oxidation of the active site of the enzyme. Others investigators found that high pressure could also cause protein denaturation, leading to changes in protein folding and conformation.[Citation51] All these changes can affect the structure of the allergen and thereby influence its potential allergenicity. The combined action of high pressure and moderate temperature would force the protein into a more tightly packed structure, obscuring the allergen epitopes,[Citation52−Citation54] and this may explain the greater reduction in allergenicity of the pineapple juice proteins when treated with 400 MPa at 5°C compared to the same pressure treatment carried out at 20°C.
CONCLUSION
In conclusion, the novelty of this work lies in the development of an integrated approach using complementary techniques to identify the main allergens in pineapple juice and investigate the influence of pressure treatment on their potential allergenicity. The combination of SDS-PAGE, Western bloting, and LC-MS/MS allowed the specific detection and identification of the major allergens. The major allergenic protein species in pineapple juice, the 24-kDa and 14-kDa bands resolved by SDS-PAGE were identified as bromelain, and these were in fact a mixture of several isotypes of cysteine proteinases. Among these, bromelain (Ananas comosus), a cycteine proteinase with Mr 39486 and pI 5.00 is a known allergen named Ana c 2. Ana c 2 is highly homologous to the various cysteine proteinases identified in 24-kDa and 14-kDa bands by LC-MS/MS. High-pressure treatment could indeed reduce the level of potential allergenicity of pineapple juice allergens, but a combined treatment with high pressure (400 MPa) and moderate temperature (50°C) show the best result, which is probably due to the change in protein structure that either destroyed or masked the epitope of the allergens responsible for the allergic reaction. The mechanism that HHP altered the potential allergenicity of pineapple juice allergens will be a subject for further study.
ACKNOWLEDGMENTS
The authors wish to express their gratitude to Fengji Tan, Feilong Meng, and Yi Yan for their excellent technical assistance in this study and for proof-reading the manuscript.
FUNDING
This research was funded by the National High-Tech Research and Development Program of China (863 Program; No. 2011AA100801-05).
Additional information
Funding
REFERENCES
- Corzo, C.A.; Waliszewski, K.N.; Welti-Chanes, J. Pineapple fruit bromelain affinity to different protein substrates. Food Chemistry 1999, 133, 631–635.
- Gujral, H.S.; Oberoi, D.P.S.; Singh, R.; Gera, M. Moisture diffusivity during drying of pineapple and mango leather as affected by sucrose, pection, and maltodextrin. International Journal of Food Properties 2013, 16, 359–368.
- Hodgson, A.S.; Hodgson, L.R. Pineapple Juice. In Fruit Juices Processing Technology; Nagy, S.; Chen, C.C.; Shaw, P.E.; Eds.; Agscience, Inc.: Auburndale, FL, 1993; 378–435.
- Sriwatanapongse, A.; Balaban, M.; Teixeira, A. Thermal inactivation kinetics of bromelain in pineapple juice. Transactions of the ASAE 2000, 43, 1703–1708.
- Laorko, A.; Tongchitpakdee, S.; Youravong, W. Storage quality of pineapple juice non-thermally pasteurized and clarified by microfiltration. Journal of Food Engineering 2013, 116, 554–561.
- de Barros, S.T.D.; Andrade, C.M.G.; Mendes, E.S.; Peres, L. Study of fouling mechanism in pineapple juice clarification by ultrafiltration. Journal of Membrane Science 2003, 215, 213–224.
- Al-Nabulsi, A.; Shaker, R.; Osaili, T.; et al. Impact of high hydrostatic pressure and heat treatment on milk gel properties: A comparative rheological study. International Journal of Food Properties 2012, 15, 613–627.
- Zhu, S.; Marcotte, M.; Ramaswamy, H.S.; Shao, Y.; Le-Bail, A. Determination of in-situ thermal conductivity, thermal diffusivity, volumetric specific heat and isobaric specific heat of selected foods under pressure. International Journal of Food Properties 2012, 15, 169–187.
- Buzrul, S.; Alpas, H.; Largeteau, A.; Demazeau, G. Inactivation of Escherichia coli and Listeria innocua in kiwifruit and pineapple juices by high hydrostatic pressure. International Journal of Food Microbiology 2008, 124, 275–278.
- Welti-Chanes, J.; López-Malo, A.; Palou, E.; Bermúdez, D; Guerrero-Beltrán, J.A. Fundamentals and Applications of High Pressure Processing of Foods. In Novel Food Processing Technologies; Barbosa-Cánovas, G.V.; Tapia, M.S.; Cano, M.P.; Eds.; Taylor & Francis Ltd.: New York, 2005; 157−182.
- Paull, R.E.; Chen, W.J. Minimal processing of papaya (Carica papaya L.) and the physiology of halved fruit. Postharvest Biology and Technology 1997, 12, 93−99.
- Jang, M.S.; Sanada, A.; Ushio, H.; Tanaka, M.; Ohshima, T. Inhibitory effects of “Enokitake” mushroom extracts on polyphenol oxidase prevention of apple browning. Lebensmittel-Wissefnschaft and Technologie 2002, 35, 697−702.
- Marrugo, J.A.; Mercado, D.; Hernandez, L.D.; Perez, N. Immunochemical study of a pineapple (Ananas comosus) extract. Journal of Allergy and Clinical Immunology 2004, 113, 152.
- Sathe, S.K.; Teuber, S.S.; Roux, K.H. Effects of food processing on the stability of food allergens. Biotechnology Advances 2005, 23, 423–429.
- Hayakawa, I.; Linko, Y.Y.; Linko, P. Mechanism of high pressure denaturation of proteins. Lebens-Wiss. u.-Technol 1996, 29, 756–762.
- Iametti, S.; Donnizzelli, E.; Vecchio, G.; Rovere, P.P.; Gola, S.; Bonomi, I. Macroscopic and structural consequences of high-pressure treatment of ovalbumin solutions. Journal Agriculture and Food Chemistry 1998, 46, 3521−3527.
- Iametti, S.; Transidico, P.; Bonomi, F.; Vecchio, G.; Pittia, P.; Rovere, P. Molecular modifications of β-lg upon exposure to high pressure. Journal Agriculture and Food Chemistry 1997, 45, 23−29.
- Tedford, L.A.; Smith, D.; Schaschke, C.J. High pressure processing effects on the molecular structure of ovalbumin, lysozyme, and β-lg. Food Research International 1999, 32, 101−106.
- European Parliament and Council. Directive 2000/13/EC of March 20, 2000 on the approximation of the laws of the Member States relating to the labelling, presentation, and advertising of foodstuffs. Official Journal of the European Communities 2000, L109, 29–42.
- European Parliament and Council. Directive 2003/89/EC of November 10, 2003 amending Directive 2000/13/EC as regards indication of the ingredients present in foodstuffs. Official Journal of the European Union 2003, L308, 15–18.
- European Commission Directive 2007/68/EC of November 27, 2007 amending Annex IIIa to Directive 2000/13/EC of the European Parliament and of the Council as regards certain food ingredients. Official Journal of the European Union 2007, L310, 11–14.
- Bai, Q. The alleigic problems of certain food. Food and Nutrition in China 2005, 7, 54–55.
- Lv, X.; Liu, X.; Yang, X. Preliminary survey on status of food allergy in young Chinese students. Chinese Journal of Food Hygiene 2005, 17, 119–121.
- Devakate, R.V.; Patil, V.V.; Waje, S.S.; Thorat, B.B. Purification and drying of bromelain. Separation and Purification Technology 2009, 64, 259–264.
- Hale, L.P. Proteolytic activity and immunogenicity of oral bromelain within the gastrointestinal tract of mice. International Immunopharmacology 2004, 4, 255–264.
- Hale, L.P.; Greer, P.K.; Trinh, C.T.; James, C.L. Proteinase activity and stability of natural bromelain preparations. International Immunopharmacology 2005, 5, 783–793.
- Gailhofer, G.; Wilders-Rruschnig, M.; Smolle, J.; Ludvan, M. Asthma caused by bromelain: An occupational allergy. Clinical & Experimental Allergy 1988, 18, 445–450.
- Baur, X. Studies on the specificity of human IgE-antibodies to the plant proteases papain and bromelain. Clinical & Experimental Allergy 1999, 9, 451–457.
- Kruse Fæste, C.; Christians, U.; Egaas, E.; Jonscher, K.R. Characterization of potential allergens in fenugreek (Trigonella foenum-graecum) using patient sera and MS-based proteomic. Journal of Proteomic 2010, 73, 1321–1333.
- Maurer, H.R. Bromelain: Biochemistry, pharmacology, and medical use. Cellular and Molecular Life Sciences 2001, 58, 1234–1245.
- Kelly, G.S. Bromelain: A literature review and discussion of its therapeutic applications. Alternative Medicine Review 1996, 1, 243–257.
- Tochi, B.N.; Wang, Z.; Xu, S.-Y.; Zhang, W. Therapeutic application of pineapple protease (bromelain): A review. Pakistan Journal of Nutrition 2008, 7, 513–520.
- López;, L.M. Sequeiros, C.; Natalucci C.L.; Brullo, A.; Maras, B.; Barra, D.; Caffini, N.O. Purification and characterization of macrodontain I, a cysteine peptidase from unripe fruits of pseudananas macrodontes(morr.) harms (bromeliaceae). Protein Expression and Purification 2000, 18, 133–140.
- Secor, E.R. Jr.; Szczepanek, S.M.; Singh, A.; Guernsey, L. LC-MS/MS identification of a bromelain peptide biomarker from ananas comosus merr. Evidence-Based Complementary and Alternative Medicine 2012, 1–10.
- Larocca, M.; Rossano, R.; Santamaria, M.; Riccio, P. Analysis of pineapple [Ananas comosus (L.) Merr.] fruit proteinases by 2-D zymography and direct identification of the major zymographic spots by mass spectrometry. Food Chemistry 2010, 123, 1334–1342.
- Husain, S.S.; Lowe, G. The amino acid sequence around the active-site cysteine and histidine residues of stem bromelain. Biochemical Journal 1970, 117, 341.
- Goto, K.; Murachi, T.; Takahashi, W. Structural studies on stem bromelain isolation, characterization, and alignment of the cyanogen bromide fragments. FEBS Letters 1976, 62, 93.
- Sasaki, M.; Kato, T.; Iida, S. Antigenic determinant common to four kinds of thiol proteases of plant origin. Journal of Biochemistry 1973, 74, 635.
- Kato, T.; Sasaki, M. Biological significance and localization of antigenic determinant common to thiol proteases of plant origin. Journal of Biocemistry 1974, 76, 1021.
- Baur, X.; Fruhmann, G. Allergic reactions, including asthma, to the pineapple protease bromelain following occupational exposure. Clinical Allergy 1979, 9, 443–450.
- Chambers, L.; Brown, A.; Pritchard, D.I.; Sreedharan, S. Enzymatically active papain preferentially induces an allergic response in mice. Biochemical and Biophysical Research Communications 1998, 253, 837–840.
- Hale, L.P.; Fitzhugh, D.J.; Staats, H.F. Oral immunogenicity of the plant proteinase bromelain. International Immunopharmacology 2006, 6, 2038–2046.
- Kelly, G.S. Bromelain: A literature review and discussion of its therapeutic applications. Alternative Medicine 1996, Rev 1, 243–257.
- Xiao, N.; Chen, Y.; Yu, R. The Principle and Method of Biochemistry Experiment; Peking University: Beijing, China, 2006; 240–242.
- Chambers, L.; Brown, A.; Pritchard, D.I.; Sreedharan, S. Enzymatically active papain preferentially induces an allergic response in mice. Biochemical and Biophysical Research Communication 1998, 253, 837–840.
- Hale, L.P.; David, J.F.; Herman, F.S. Oral immunogenicity of the plant proteinase bromelain. International Immunopharmacology 2006, 6, 2038–2046.
- Min, T.; Jian, P.; Wen-Cheng, Z.; Hui-Ming, X.; Lu, W. Effect of ultra-high pressure treatment on bromelain activity in pineapple juice. Food Science 2013, 34 (15), 162–165.
- Rattanathanalerk, M.; Chiewchan, N.; Srichumpoung, W. Effect of thermal processing on the quality loss of pineapple juice. Journal of Food Engineering 2005, 66, 259–265.
- Jutamongkon, R.; Charoenrein, S. Effect of temperature on the stability of fruit bromelain from smooth cayenne pineapple. Kasetsart Journal (Natural Science) 2010, 44, 943–948.
- Gomes, M.R.A.; Sumner, I.G.; Ledward, D.A. Effects of high pressure on papain activity and structure. Journal of the Science of Food and Agriculture 1997, 75, 67–72.
- Iametti, S.; Donnizzelli, E.; Pittia, P.; Rovere, P.; Squarcina, N.; Bonomi, F. Characterization of high-pressure treated egg albumin. Journal of Agriculture and Food Chemistry 1999, 47, 3611−3616.
- Tedford, L.-A.; Kelly, S.M.; Price, N.C.; Schaschke, C.J. Combined effects of thermal and pressure processing on food protein structure. Institution of Chemical Engineers 1998, 76, 8–86.
- Katsaros, G.I.; Katapodis, P.; Taoukis, P.S. Modeling the effect of temperature and high hydrostatic pressure on the proteolytic activity of kiwi fruit juice. Journal of Food Engineering 2009, 94, 40–45.
- Zhu, S.; Marcotte, M.; Ramaswamy, H.; Shao, Y. Evaluation and comparison of thermal conductivity of food materials at high pressure. Food and Bioproducts Processing 2008, 86, 147–153.
