Abstract
The thermophysical properties (thermal conductivity, thermal diffusivity, and specific heat) of big-eyed tuna and pacific cod were measured at various temperatures (5–50°C) by the modified version of current probe method. The optimal prediction models for these thermophysical properties were determined. The random model was applied to predict the thermal conductivity of seafood in a wide range of temperature and it provided the accurate predictions for the samples. The thermal diffusivities of the samples could be predicted by Martens’s equation. An additive relationship exists between the specific heat of the sample, the composition, and the specific heat of each component.
INTRODUCTION
Thermal treatments such as pasteurization, concentration, drying, and cooling are frequently used in food processing, transportation, storage, and cooking. Knowledge of the thermophysical properties of foods is important not only for process design but also for the prediction and control of various changes that occur in food during thermal processing. The thermophysical properties of foods, such as thermal conductivity, thermal diffusivity, and specific heat, have been reviewed by Singh,[Citation1] Sweat,[Citation2] Saravacos and Maroulis[Citation3] and others. However, because of the wide variations in foods, it is practically impossible to obtain reliable thermophysical property data for all kinds of food and at various conditions. Therefore, the predictive methods for the thermophysical properties of foods were needed.
Thermal conductivity, specific heat, and density of mango puree[Citation4] and papaya puree[Citation5] were measured using a line heat source probe, differential scanning calorimetry, and pycnometer, respectively. In these literatures,[Citation4,Citation5] the thermal diffusivity was determined from the definition equation of thermal diffusivity. A dual-needle probe was used to measure in-situ pressure dependent thermal conductivity, thermal diffusivity, and specific heat values of some foods, such as tomato puree, soy protein isolate, and so on.[Citation6]
Thermal conductivities of some seafood, such as squid,[Citation7] shrimp,[Citation8,Citation9] scallop,[Citation8] skipjack tuna,[Citation10] oyster,[Citation11] and loco,[Citation12] have been measured by a transient line source technique or probe method. In those reports, only the thermal conductivity of the material was determined from the temperature change in the line heat source or probe. Kumber et al.[Citation13] measured the thermal conductivity and thermal diffusivity of 12 kinds of fresh and frozen fish by Nix’s method. The differential scanning calorimetry has been used to determine the specific heat of shrimp,[Citation9] skipjack tuna,[Citation10] oyster,[Citation11] and loco.[Citation12] However, there is a lack of information about the thermophysical properties of seafood compared with other food materials. Thermal conductivity, thermal diffusivity, and specific heat of seafood have never been simultaneously measured under identical conditions.
The thermal conductivity, thermal diffusivity, and specific heat of foods were affected by temperature, moisture content, and composition of the solid part, i.e., the protein, fat, carbohydrate, dietary fiber, and minerals. In addition, thermal conductivity and thermal diffusivity depend on the structure (the spatial distribution of the components) of the food. Therefore, the prediction models of the thermophysical properties of food including the effects of those parameters would be valuable for practical use. Pereira et al.[Citation14] measured the thermal conductivity of guava and passion fruit pulps by the hot wire probe method, and these values were compared with the values calculated from the three kinds of structural models for thermal conductivity (the parallel, perpendicular, and Maxwell-Eucken models). However, there have been few reports of structural models to predict the thermal conductivity of seafood.
In this study, the thermophysical properties (thermal conductivity, thermal diffusivity, and specific heat) of big-eyed tuna and pacific cod were measured under identical conditions. There have been no reports about these thermophysical properties of big-eyed tuna and pacific cod. A modified version of current probe method[Citation15] was used for the determination of the thermal conductivity and thermal diffusivity. The specific heat of the sample was estimated from the definition of thermal diffusivity. The objectives of this study were: (1) under identical conditions, to simultaneously obtain the three kinds of thermophysical properties of the samples by the modified version of current probe method; (2) to examine the effect of temperature on the thermophysical properties of the samples; and (3) to determine the most suitable prediction model for each thermophysical property of the samples.
MATERIALS AND METHODS
Samples
The fillets of big-eyed tuna (Thunnus obesus) and pacific cod (Gadus macrocephalus) were purchased from a local market. The moisture content of the samples was determined using the moisture analyzer (A&D Co., Ltd., MX-50, Tokyo, Japan) in which 5 g of the sample was dried at 160°C until the decrease of the sample’s mass was less than 0.05 g per one minute. The moisture contents for big-eyed tuna and pacific cod were 74.0 and 80.4% (w.b.), respectively; these values were almost equal to the values in standard table of food compositions in Japan.[Citation16] Therefore, the compositions of each sample were calculated from measured data of moisture content and data from the standard table of food compositions in Japan,[Citation16] and given in .
TABLE 1 Compositions (mass fraction and volume fraction) of each component for each sample
Measurement of Thermophysical Properties
Thermophysical properties of the samples were measured with the modified transient heat flow probe method[Citation15] at seven different temperatures (5, 10, 15, 20, 30, 40, and 50°C). The measurement condition was selected in consideration of the treatment for cooling and preparation of cooking of seafood. In this method, the thermal conductivity and thermal diffusivity are determined using the data of the change in temperature from the probe inserted in the sample. The probe consisted of a constantan heater wire and a T-type thermocouple in a stainless steel tube (1 mm in outer diameter and 100 mm in length). The details of measurement apparatus and estimation method of thermophysical properties used in this study were given in literature.[Citation15]
The samples were cut into rectangular parallelepiped 20 mm long, 30 mm wide, and 170 mm high for measurement of thermophysical properties. After the probe was inserted into the center of sample cross section 20 mm long and 30 mm wide, the sample was submerged in the water bath; thus, the temperature of the sample was controlled. To avoid the changes in moisture content of the sample, the sample wrapped with clear plastic food wrap (0.01 mm-thick polyvinylidene chloride) was put in the plastic bag (0.01 mm-thick polyethylene). The sample must be readied in the initial state of thermal equilibrium. When the temperature difference between the preset temperature and the sample temperature was less than 0.01°C, it was considered that they had reached the thermal equilibrium. Approximately 90 min were necessary to achieve this state of equilibrium. After the thermal equilibrium state had been established, the probe heater wire was energized, and the temperatures of the probe increased with time. The probe temperature changes were amplified and recorded with the data logger. The input and output range of the amplifier were adjusted to 100 μV and 10 mV, respectively, and the current applied to the heater wire in the probe was adjusted to 130 mA to raise the temperature of the probe to about 2°C. Current was supplied to the heater wire of the probe for 180 s. In this study, the measurements of thermophysical properties were replicated five times for each experimental condition. As the deviation of the five measurements from the mean value was less than ± 5%, the mean was taken as the measured value.
Estimation Method of Thermophysical Properties
To estimate the thermophysical properties of the samples, the measured probe temperature changes between 30–180 s were fitted by a least squares method to Eq. (1), and the values of the parameters F and G were determined.
Equation (1) shows that when the elapsed heating time is long enough, the relationship between the probe temperature difference and the logarithm of elapsed time is approximately linear, and the slope and intercept of this straight line are F and G, respectively. The parameters F and G were defined as follows:[Citation15]
Using the values of F, G, Pc, and Ic, the thermal conductivity and thermal diffusivity of the sample were calculated from Eqs. (2) and (3), respectively. In Eqs. (2) and (3), the values of correction coefficients (Pc and Ic) and heat quantity of the probe per unit time (q’) are Pc = 8.152 (1/m), Ic = 0.2912 (°C), and q’ = E × I = I2× R = 0.1302 × 14.23 = 0.2405 (W), respectively.
The specific heat cp of the sample was estimated by substituting the thermal conductivity λ and thermal diffusivity κ calculated from Eqs. (2) and (3), and the density ρ of the sample into the following equation for thermal diffusivity:
In this study, the values of density in Eq. (4) were calculated from Eq. (5).
The values of mass fraction of each component in Eq. (5) are given in . The values of density of each component in Eq. (5) are taken from a reference.[Citation17]
Regression and Statistical Analysis
Regression and statistical analysis of the measured data were carried out by Microsoft Excel 2010®. The three statistical parameters, i.e., the root mean square error (RMSE), the coefficient of determination (R2), and the relative error (RE) were used to evaluate the accuracy of the fitting process using Microsoft Excel 2010®.
RESULTS AND DISCUSSION
Temperature Change of the Probe
The measured data for the probe temperature changes over 30–180 s at each measurement condition were fitted by a least squares method to Eq. (1). A comparison between the measured results and the results calculated from Eq. (1) at a temperature of 15°C for pacific cod is shown in . The solid line and symbols in show the calculated values and the measured results, respectively. In this case, the values of parameters F, G, RMSE, R2, RE in Eq. (1) were F = 0.3093 (°C), G = –0.2469 (°C), RMSE = 3.936×10–3 (°C), R2 = 0.9993 (–), and RE = 0.1–1.5 (%; Average of RE = 0.3 [%]). As shown in , the measured data for the temperature changes of the probe over 30–180 s matched well with the calculated values. For all measurement conditions, the RMSE, R2, and RE had nearly equal values as the measurement condition in . The temperature changes of the probe between 30–180 s were expressed by Eq. (1) with good accuracy. Therefore, the values of parameters F and G determined from the probe temperature changes over 30–180 s were used to estimate the thermophysical properties of the samples.
FIGURE 1 (A) Comparison of the observed temperature changes of the probe with the results calculated from Eq. (1) for pacific cod at a temperature of 15°C. The solid line shows the calculated values from Eq. (1). (B) Empirical relationships between the thermal conductivity and the temperature for each sample. The solid line and broken line show calculated results from Eq. (6) for each sample. (C) Comparison of measured results of thermal conductivity with results calculated using Eqs. (7), (8), and (9) for big-eyed tuna. The solid line, broken line, and dot-dash-line show the results calculated from Eqs. (7), (8), and (9), respectively. (D) Empirical relationships between the thermal diffusivity and the temperature for each sample. The solid line and broken line show calculated results from Eq. (11) for each sample.
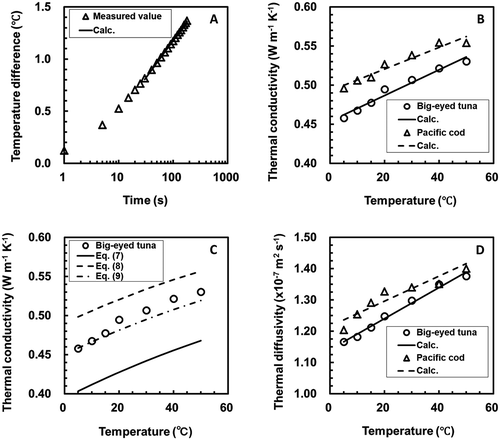
Thermal Conductivities of the Samples
The values of the thermal conductivity calculated from Eq. (2) were 0.45–0.54 W m–1 °C–1 for big-eyed tuna and 0.49–0.56 W m–1 °C–1 for pacific cod. The thermal conductivities of bluefin tuna[Citation18] as measured by the parallel plate method were 0.38–0.44 W m–1 °C–1 at temperatures from 13 to 20°C and moisture content levels from 68 to 78% (w.b.). The thermal conductivity of cod[Citation1] was 0.544 W m–1 °C–1 at a temperature of 2.8°C and moisture content of 83% (w.b.). The measured thermal conductivities of the samples were compared with the literature values.[Citation1,Citation18] As a result, there were no significant differences between the measured thermal conductivities of the samples and the literature values. This result demonstrates that the method adopted in this study for estimating thermophysical properties was appropriate.
Kumber et al.[Citation13] reported that the thermal conductivities of 12 kinds of fresh fish increased with temperature and were expressed as a linear function of temperature above freezing point. The thermal conductivity of oyster[Citation11] was presented as a quadratic function of temperature. shows the relationships between thermal conductivity and temperature for each sample, showing that the thermal conductivity of each sample increased linearly with increasing temperature. Therefore, the measured data for each sample were fitted by a least squares method to Eq. (6).
The solid line and broken line in show the calculated result for each sample. For all samples, the calculated values matched well with the measured data. The parameters, RMSE, R2, and RE of Eq. (6) for each sample are shown in . The relationship between thermal conductivity and temperature for all samples was represented by Eq. (6).
TABLE 2 The parameters, coefficients of determination (R2), root mean squared errors (RMSE), and relative errors (RE) of Eqs. (6) and (11) for each sample
Thermal conductivity of food is affected by the composition, temperature, and structure (the spatial distribution of the components) of the food. Therefore, a structural model of thermal conductivity would be valuable for practical use. However, the thermal conductivities of some seafood are reported only as a function of temperature and/or moisture content, and there have been few reports on structural models that predict the thermal conductivity of seafood. A parallel structural model and random structural model have been previously applied to estimate the thermal conductivity of calamari and king prawn.[Citation19] The experimental values of thermal conductivities for shrimp meat[Citation9] and shucked oysters[Citation11] were compared with the values calculated from the parallel structural model. In this study, theoretical values of thermal conductivity of each sample were calculated from series, parallel, and random heat transfer models, using the volume fraction and thermal conductivity of each component.
The series model:[Citation2]
The parallel model:[Citation2]
The random model:[Citation20]
In the series model (Eq. 7), heat conduction is assumed to occur perpendicular to alternating layers of each component. In the parallel model (Eq. 8), the layers of each component are arranged parallel to the direction of heat flow. The parallel model yields the highest values of thermal conductivity, while the lowest values are obtained by the series model. In the random model (Eq. 9), the phases of each component are assumed to be randomly mixed and the thermal conductivity is the geometric mean of the values for the individual phases. In this study, the volume fractions of each component and temperature (5, 10, 15, 20, 30, 40, and 50°C) in Eqs. (7)–(9) were obtained by substituting the values of mass fraction () and density[Citation17] for each component into Eq. (10).
The values of the volume fractions of each component had almost the same value regardless of temperature, remaining nearly constant from 5 to 50°C. The mean values of volume fraction of each component in the temperature range of 5–50°C were used in this study, and given in . The values of intrinsic thermal conductivity of each component and temperature in Eqs. (7)–(9) were taken from a reference.[Citation17]
shows a comparison of the measured results and those calculated by the three different models (Eqs. Citation7–Citation9) for big-eyed tuna. The solid line, broken line, and dot-dash-line show the results calculated from Eqs. (7), (8), and (9), respectively. The results calculated from Eq. (9) corresponded well with the measured results. The similar results shown in were obtained for pacific cod. The RMSE and RE of Eqs. (7), (8), and (9) for each sample are shown in . The random model provided the most accurate predictions for thermal conductivity of the samples. Therefore, it was confirmed that the thermal conductivities of the samples could be predicted by the random model (Eq. 9).
Thermal Diffusivities of the Samples
The values of thermal diffusivity of the samples obtained from Eq. (3) were 1.1 × 10–7 – 1.4 × 10–7 m2 s–1 for big-eyed tuna and 1.2 × 10−7 – 1.4 × 10–7 m2 s–1 for pacific cod. The thermal diffusivity of codfish in literature[Citation1] was 1.22 × 10–7 m2 s–1 at a temperature of 5°C and a moisture content of 83% (w.b.). Comparing the measured data to the literature value, the measured value were almost the same as the reported value. The thermal diffusivities of the samples increased with increasing temperature. This tendency was also seen in a report by Kumber et al.[Citation13] The values of thermal diffusivity plotted in for each sample were fitted by a least squares method to the following equation:
The values of the parameters are given in along with the RMSE, R2, and RE of Eq. (11) for each sample. The RMSE, R2, and RE values show that the measured data matched well with the calculated values. The temperature dependency of thermal diffusivity for each sample was represented by Eq. (11) as a linear function of temperature within the measurement conditions in this study. The following equation was obtained by Martens.[Citation21]
The thermal diffusivities calculated from Eq. (12) were compared with the measured data. The RMSE and RE were RMSE = 3.409 × 10–7 (m2 s–1), RE = 0.2–3.8 (%; Average of RE = 2.4 [%]) for big-eyed tuna and RMSE = 3.174 × 10–7 (m2 s–1), RE = 0.1–3.6 (%; Average of RE = 2.0 [%]) for pacific cod. Thus, it was confirmed that a comparison of the measured data with the calculated values from Eq. (12) showed good agreement, and the thermal diffusivities of the samples could be estimated using Martens’s equation (Eq. 12).
Specific Heats of the Samples
The specific heats determined from Eq. (4) were 3.6–3.8 kJ kg–1 °C–1 for big-eyed tuna and 3.7–4.0 kJ kg–1 °C–1 for pacific cod. The specific heat of each sample was almost constant over temperature. Choi and Okos[Citation17] proposed the following equation:
The specific heat of the sample was calculated from Eq. (13) and compared with the measured data. The specific heat of each component in Eq. (13) was taken from a reference.[Citation17] As a result, the values of RE were 1–5(%) for all samples, and the measured data were almost equal to the values calculated from Choi and Okos’s equation (Eq. 13). In this study, the thermal conductivities, thermal diffusivities, and specific heats of samples were simultaneously determined using a modified transient heat flow probe method. These values can be estimated from Eqs. (9), (12), and (13). The results obtained in this study will be useful in the design of equipment and in calculations for the thermal processing of seafood.
CONCLUSION
The thermal conductivity and thermal diffusivity of big-eyed tuna and pacific cod were measured at various temperatures (5–50°C). The specific heat of each sample was estimated from the definition of thermal diffusivity. The measured thermal conductivities of the samples matched well with the values calculated from the random model. The thermal diffusivity of each sample could be estimated by Martens’s equation. The observed specific heats of the samples were almost equal to the values calculated from Choi and Okos’s equation.
NOMENCLATURE
| = | Constant, | |
| = | Euler’s constant, 0.5772 (–) | |
| = | Volume fraction (–) | |
| = | Probe temperature difference (°C) | |
| = | Thermal diffusivity (m2 s–1) | |
| = | Thermal conductivity (W m–1 °C–1) | |
| = | Density (kg m–3) | |
| = | Specific heat (kJ kg–1 °C–1) | |
| = | Voltage (V) | |
| = | Current, 0.130 (A) | |
| = | Correction coefficient, 0.2912 (°C) | |
| = | Correction coefficient, 8.152 (m–1) | |
| = | Heat quantity of the probe per unit time (W), | |
| = | Resistance, 14.23 (Ω) | |
| = | Outer diameter of the probe, 0.0005 (m) | |
| = | Elapsed time (s) | |
| = | Temperature (°C) | |
| = | Constant (°C) | |
| = | Constant (°C) | |
| = | Constant (W m–1 °C–2) | |
| = | Constant (W m–1 °C–1) | |
| = | Constant (m2 s–1 °C–1) | |
| = | Constant (m2 s–1) | |
| = | Mass fraction (–) | |
| = | Components | |
| = | Components | |
| = | Water |
REFERENCES
- Singh, R.P. Heating and Cooling Processes for Foods. In Handbook of Food Engineering; Heldman, D.R., Lund, D.B., Eds; Marcel Dekker Inc.: New York, NY, 1992; 247–276.
- Sweat, V.E. Thermal Properties of Foods. In Engineering Properties of Foods. 2nd ed. ; Rao, M.A., Rizvi, S.S.H., Eds; Marcel Dekker Inc.: New York, NY, 1994; 99–138.
- Saravacos, G.D.; Maroulis, Z.B. Thermal Conductivity and Diffusivity of Foods. In Transport Properties of Foods; Marcel Dekker Inc.: New York, NY, 2001; 269–358.
- Gundurao, A.; Ramaswamy, H.S.; Ahmed, J. Effect of soluble solids concentration and temperature on thermo-physical and rheological properties of mango puree. International Journal of Food Properties 2011, 14 (5), 1018–1036.
- Tansakul, A.; Kantrong, H.; Saengrayup, R.; Sura, P. Thermophysical properties of papaya puree. International Journal of Food Properties 2012, 15 (5), 1086–1100.
- Nguyen, L.T.; Balasubramaniam, V.M.; Sastry, S.K. Determination of in-situ thermal conductivity, thermal diffusivity, volumetric specific heat and isobaric specific heat of selected foods under pressure. International Journal of Food Properties 2012, 15 (1), 169–187.
- Rahman, M.S.; Potluri, P.L. Thermal conductivity of fresh and dried squid meat by line source thermal conductivity probe. Journal of Food Science 1991, 56 (2), 582–583, 585.
- Murakami, E.G. Thermal processing affects properties of commercial shrimp and scallops. Journal of Food Science 1994, 59 (2), 237–241.
- Karunakar, B.; Mishra, S.K.; Bandyopadhyay, S. Specific heat and thermal conductivity of shrimp meat. Journal of Food Engineering 1998, 37 (3), 345–351.
- Zhang, J.; Farkas, B.E.; Hale, S.A. Thermal Properties of Skipjack Tuna (KATSUWONUS PELAMIS). International Journal of Food Properties 2001, 4 (1), 81–90.
- Hu, X.; Mallikarjunan, P. Thermal and dielectric properties of shucked oysters. LWT Food Science Technology 2005, 38 (5), 489–494.
- Reyes, A.; Pérez, N.; Mahn, A. Determination of specific heat and thermal conductivity of “Loco” (Concholepas concholepas). Food Bioprocess Technology 2013, 6 (7), 1873–1877.
- Kumbhar, B.K.; Agarwal, R.S.; Das, K. Thermal properties of fresh and frozen fish. International Journal of Refrigeration 1981, 4 (3), 143–146.
- Pereira, C.G.; Resende, J.V.; Pereira, G.G.; Giarola, T.M.O.; Prado, M.E.T. Thermal conductivity measurements and predictive models for frozen guava and passion fruit pulps. International Journal of Food Properties 2013, 16 (4), 778–789.
- Muramatsu, Y.; Sakaguchi, E.; Orikasa, T.; Tagawa, A. Simultaneous estimation of the thermophysical properties of selected liquid dairy products by a transient heat flow probe method. International Journal of Food Properties 2011, 14 (3), 557–569.
- Kagawa, Y. Standard Tables of Food Composition. In Standard Tables of Food Composition in Japan 2006 5th revised and enlarged ed. ; Kagawa, Y., Ed; Kagawa Nutrition University Publication: Tokyo, 2006; 147–187.
- Choi, Y.; Okos, M.R. Effects of Temperature and Composition on the Thermal Properties of Foods. In Food Engineering and Process Applications Vol. 1 Transport Phenomena; Maguer, M., Jelen, P., Eds; Elsevier Applied Science Publisher: New York, NY, 1986, 93–101.
- Maeda, A.; Takenaka, H. Measurement of thermal constants of fresh fish. The Eighth Japan Symposium on Thermophysical Properties 1987, 8, 61–64.
- Rahman, M.S.; Driscoll, R.H. Thermal conductivity of seafoods: Calamari, octopus and King prawn. Food Australia 1991, 43 (8), 356–360.
- Woodside, W.; Messmer, J.H. Thermal conductivity of porous media. Unconsolidated sands. Journal of Applied Physics 1961, 32, 1688–1699.
- Rahman, M.S.; Al-Saidi, G.S. Thermal Diffusivity of Foods: Measurement, Data, and Prediction. In Food Properties Handbook. 2nd ed. ; Rahman, M.S., Ed; CRC Press: Boca Raton, FL, 2009; 649–695.
