Abstract
A line heat source probe method was used to determine thermal conductivity of the Croatian unleavened dough in a temperature range of 27–125°C. For estimation of the specific heat of the Croatian unleavened dough, a mixing method was used. The maximum value of 4.0 ± 0.8 kJ/(kg °C) was determined at 57.1°C for the potato dough, and the minimum value of 1.4 ± 0.6 kJ/(kg °C) was determined at 35.3°C for the Kroštula dough. Thermal conductivity first increased with temperature and then decreased after reaching its maximum value. Minimum value of 0.29 ± 0.04 W/(m °C) was determined at 123.8°C for the Kroštula dough. Further on, a set of empirical equations was established using regression analysis to correlate the results and to predict the specific heat and thermal conductivity of the Croatian unleavened dough within the studied temperature range and moisture levels.
Introduction
Thermophysical properties are unique and influence the design of any thermal process. Those properties include specific heat, thermal conductivity, and density. Individually, these properties may influence evaluation and design process. Specific heat and density are important components of analysis involving mass and/or energy balances. Thermal conductivity is the key property in the quantification of thermal energy transfer within a conductive material. Combination of these three properties is a thermal diffusivity, a key property in the analysis of unsteady-state heat transfer.[Citation1] All thermal processes involve changes in product temperature, and many also involve changes in product composition.
The specific heat of food is defined as the quantity of thermal energy associated with unit mass of food and unit change in temperature. This is often referred to as the heat capacity and it represents an essential component of the thermal energy analysis of a food product, a thermal process, or the processing equipment used for the heating or cooling of food. Thermal conductivity is a basic thermophysical property of any material, the magnitude of which expresses the rate of thermal energy transfer within the material.
Geometry of food material is generally irregular, and its composition heterogeneous. For the reason of these constraints, measurement of its thermophysical properties demands innovation, as well as the basic knowledge of heat transfer.[Citation2–Citation4] Specific heat is commonly determined using either the mixing method or the differential scanning calorimeter. Selection of the proper measurement technique for the specific heat of the bakery product should take into account the homogeneity and size of the sample, as well as the target temperature range.[Citation5–Citation14]
A number of methods are available for the measurement of thermal conductivity. These are classified as steady-state (guarded hot plate) or transient unsteady-state techniques (line heat source probe, temperature history). In the steady-state method, measurement time can be much longer (12 h or more, depending on the sample size) for poor thermally conductive samples than in the transient methods. During testing, moisture migration, and property changes can occur due to longer exposures to high temperature. Thus, this method may not be suitable for the measurement of the thermal conductivity of bakery products. Transient techniques are considered more suitable since the testing is very fast and yields (more) accurate results.[Citation5,Citation15–Citation22]
Owing to the complexity of food physical structure, prediction models for the thermal conductivity of food must account for the characteristics of the physical structure.[Citation23] Prediction models for thermophysical properties based on product composition have evolved. Many of these models are based on property magnitudes for the basic food components of foods: proteins, fat, carbohydrates, ash, and water. Knowledge of the thermal properties of these basic components as a function of temperature, provides the possibility to develop prediction models that will accommodate the needs of process design models. These models of thermophysical properties represent a significant opportunity to improve the efficiency of thermal processes for food and the ultimate design of equipment used for food processing. The models to be found in the literature provide less acceptable results compared to regression equations as they do not take into account the synergistic effect of all constituents of food.[Citation5] The objective of this study was to determine the specific heat and thermal conductivity as a function of temperature of the Croatian unleavened dough at over 100°C and additionally to determine the correlation relationship between temperature, specific heat, and thermal conductivity.
Material and methods
Sample Preparation
The “Mlinci” Croatian unleavened flat bread is a type of flat bread produced from wheat flour (68.2%), water (30.5%), vinegar (0.9%), and salt (0.4%). The dough is prepared by a method similar to that used for “Chapati” (Indian flat bread), and the bread is baked on a hot plate for a few minutes. Baked Mlinci can be stored for several months. Their final treatment includes cooking in water for approximately 1–2 minutes, and the bread is traditionally consumed as a side dish to oven-roasted turkey. Samples of Mlinci dough were prepared by mixing flour and salt with water and vinegar, followed by kneading in a low batch mixer for 15 min then allowing it to rest for 15–20 min.
Fresh “Kroštula” dough samples were prepared by mixing egg yolks (14.8%) with sugar (3.6%) and table salt (0.3%), after which soft wheat flour (59.2%), sour cream (16.9%), and rum (5.2%) were added. All ingredients were mixed well and allowed to rest in a covered container for 60 min. Kroštula dough is deep fried in fat in different shapes and is consumed as a dessert sprinkled with sugar. Samples of potato dough were prepared from whole potato flour and salt (0.2%). The whole potato flour was prepared before being added to half-white wheat flour; the whole potato flour was mixed with boiling water in a 1:5 ratio. Such mixtures of whole potato flour were added to half-white wheat flour in the ratio of 1:1. Potato dough is used to make dumplings or may be fried and served as soup noodles. The moisture contents of the samples were determined using the HR 73 halogen moisture analyser (Mettler Toledo, Mettler Toledo Croatia). The analyses were repeated three times.
Specific Heat Determination
Modified mixing method was developed by Kulacki and Kennedy[Citation11] to determine the specific heats of wheat, wheat flour, and cookie dough. The indirect method of mixtures based on literature reports by Hwang and Hayakawa,[Citation9] Gupta,[Citation7] and Maisuthisakul[Citation12] was used in the present work. Calorimeter used in the experimental work was constructed[Citation24] according to the mentioned literature references.
Since the used calorimeter (ordinary vacuum thermo flask of 350 mL capacity) was a composite of glass, plastic, and insulation material, it was easier to experimentally determine the heat capacity of the calorimeter rather than determining the mass and specific heat of each material separately and combining them. This method consisted of determining the temperature change of distilled water contained in the flask calorimeter at high temperature when the known quantity of distilled water at known lower temperature was added. The system was assumed to be adiabatic. Therefore, the heat capacity of the flask calorimeter is given by:[Citation14]
Samples of the Croatian unleavened dough for determination of heat capacity were prepared in the homogeneous form at 17 g each, filled, vacuumed, and sealed in polyethylene pouches (Vaxsy System, Zeptter, Zeptter Croatia) to prevent contact between the samples and the heat exchange medium. Heat capacity of the polyethylene pouches was disregarded during the calculations of the specific heat of the dough.
Pouches with dough samples at room temperature were dropped into the medium for heat transfer (oil, INA Delta 5, SAE 15W-40, Croatia) contained in the flask calorimeter at the higher uniform temperature (5°C higher than the desired experimental temperature).
Equilibrium was reached after 39 to 60 min, depending upon the characteristics of the sample material and the temperature of determination. Measured equilibrium temperature was the temperature at which the specific heat of unleavened dough was obtained. Considering the heat balance during the process, specific heat of the Croatian unleavened dough was calculated using the following heat balance equation:[Citation14]
Values of Hf used in the above equation were the average values of five replicates. The temperature was measured during experimental work using a type T thermocouple with a diameter of 0.0225 mm (Cole-Parmer, International, USA) in a temperature range of 34.3–95.8°C. The calorimeter was tested with distilled water. The obtained specific heat of distilled water at 46.03°C was 4.221 ± 0.025 kJ/(kg °C), which correlates well with the value found in the literature, 4.176 kJ/(kg °C);[Citation25] this measurement represents an error of 1.07%. Specific heat of the Croatian dough was determined for samples with different initial moisture contents in a temperature range of 34.3–95.8°C. The measurements were conducted in triplicate.
Thermal Conductivity Determination
Thermal conductivity of the Croatian unleavened dough was determined using the linear heat source probe method. This method is convenient and rapid, suitable for small samples, and could be developed into an accurate method for universal application. This method has frequently been used for the determination of thermal conductivity[Citation18,Citation22,Citation26–Citation30] for food and biological materials. Thermal conductivity measurements of the dough were performed using the probe method based on the line-heat source approach developed by Sweat.[Citation28]
The probe encloses a heater wire and thermocouple junction contained in a hypodermic needle (D = 4 mm, L = 233 mm). The construction of the line heat-source probe was described in detail by Sweat.[Citation29] The cylindrical sample holder was a film capsule (81 mm in length and of 41 mm in diameter) that was insulated at both ends. During the measurement of thermal conductivity, the probe was inserted longitudinally into a sample placed in the sample holder. The sample was equilibrated to the desired temperature using an oil (INA Delta 5, SAE 15W-40, Croatia) bath. Then, a current was applied and time-temperature data were recorded. Thermal conductivity was then calculated using the following equation:[Citation29]
A short experiment time was chosen to avoid the edge effect caused by heat transfer at the outer surface of the sample. To linearize the t-T plot, the initial time (t0) in the above equation was set equal to the time when the non-linear portion of the t-T plot ends.[Citation31] For each t-T plot, the slope was found using a simple linear regression. Prior to the measurement of the samples, the probe was calibrated with 99.5% glycerine. The measured average thermal conductivity (10 replicates) of glycerine at 30.15°C was 0.291 ±0.049 W/(m °C), an approximate 0.62% deviation from the expected value as the expected value of glycerine at 30°C was 0.289 W/(m °C).[Citation32] The resulting calibration factor for the probe was 1.006. Thermal conductivity of the Croatian dough was determined for samples with different initial moisture contents in a temperature range of 27.8–124.2°C. Measurements were repeated approximately ten times for each sample.
Results and discussion
The Kroštula, Mlinci, and Potato dough samples used in this experiment contained 29.27, 37.15, and 44.66% moisture, respectively. During the experimental determination of the specific heat and thermal conductivity, the moisture levels of the dough samples were constant. During the determination of the specific heat, the samples were sealed in polyethylene pouches; when determining the thermal conductivity, the samples were placed in a sample holder that was sealed at both ends with no possibility of water migration out of the samples.
Specific heat of dough samples was determined as a function of temperature up to 95.8°C (the temperature above the previously mentioned causes the leakage of polyethylene pouches due to high pressure occurring). The thermal conductivity of dough samples was determined as a function of temperature of up to 124.2°C (the temperature above the previously mentioned causes the overflow of the samples over the edge of the sample holder). The thermophysical properties of the dough being processed were changing as a function of temperature.
Variation of the average specific heat of the dough, estimated experimentally with temperature at various moisture levels is shown in . It can be observed that the experimental specific heat values of the dough increase with temperature, indicated by small peaks at 45–50 and 55–60°C. Although this was not further investigated, according to the literature, the first peak can be explained by the denaturation of the wheat and potato proteins.[Citation33]
FIGURE 1 Experimental specific heat as a function of temperature at different moisture levels; (○) Kroštula dough, (◊) Mlinci dough, and (Δ) Potato dough.
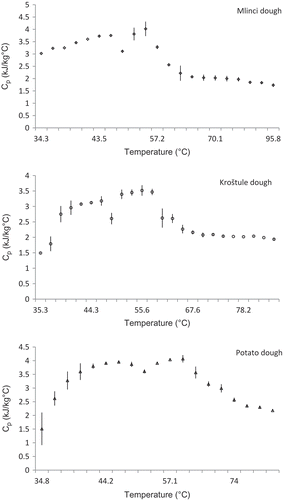
Temperature of denaturation decreased significantly in the case of the Croatian dough, where the moisture of the raw material ranged from 29.27 to 44.66%. The temperature at which the change took place ranged from 44.6 to 46.1°C (). Furthermore, the specific heat of the dough samples started decreasing at temperatures between 55.6 to 58.8°C and maintained a nearly constant value with the increasing temperature from 66.3 to 74°C (temperatures of gelatinization of wheat and potato starch, respectively). According to the literature,[Citation34,Citation35] it can be concluded that the decrease in cp values was actually the result of gelatinization.
The highest values of cp over the investigated temperature range occurred in the dough with the highest moisture content (potato dough; 4.04 ± 0.76 kJ/(kg °C) at 57.1°C), while the lowest values were recorded for the dough with lowest moisture content (Kroštula dough; 1.42 ± 0.61 kJ/(kg °C) at 35.3°C). It is interesting to note that the peaks corresponding to the denaturation of proteins and to the gelatinisation of starch were slightly shifted toward higher temperatures as the moisture content of the dough increased.
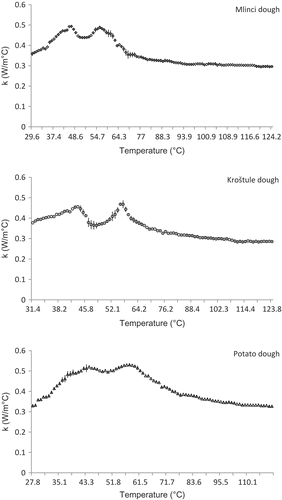
presents the variation of the average thermal conductivity estimated experimentally on the dough samples with temperature at various moisture levels. The highest values of k over the investigated temperature range occurred in the dough with the highest moisture content (potato dough; 0.5308 ± 0.0114 W/(m °C) at 56.7°C), while the lowest values were found in the dough with the lowest moisture content (Kroštula dough; 0.2853 ± 0.0416 W/(m °C) at 123.8°C). In , it can be observed that the experimental thermal conductivity of the dough increases with temperature, and small peaks (the first at 43–46°C and the second at 55–58°C) are evident as for the specific heat of the dough samples. The temperature at which the changes took place ranged from 44.6 to 46.2°C. The thermal conductivity of the dough started decreasing at temperatures from 57.7–57.8°C, then remained nearly constant with increasing temperatures of up to 90–95.5°C, where they were approximately equal to the values at the start of the investigation (27.8–31.4°C; ). All of these temperature-dependent changes in the experimentally determined thermal conductivity were also evident in the experimentally determined specific heat values. It can be concluded that the thermal properties of the Croatian dough, that is, the specific heat and thermal conductivity, were greatly affected by the physico-chemical changes that took place at ≈50°C and between 55 and 70°C, and in addition assumed according to the literature[Citation34,Citation35] that the changes are denaturation and gelatinization.
FIGURE 2 Experimental thermal conductivity as a function of temperature at different moisture levels; (○) Kroštula dough, (◊) Mlinci dough, and (Δ) Potato dough.
Thermal properties (specific heat and thermal conductivity) greatly depend on the temperature, state (frozen or unfrozen), composition parameters (moisture content, fat content, protein, and ash), and fiber orientation. However, many food and agricultural products contain individual constituents; hence, their thermal properties are also different. Choi and Okos[Citation36] developed a general model to predict the thermal properties of each specific food material based on its composition (fat, protein, water, carbohydrate, and ash) and temperature. It was assumed that each component has the same thermal properties regardless of its structure in different food materials. This, however, is not always true.[Citation1,Citation37] The mass fraction model, in general, satisfactorily predicts the value; however, several authors have reported significant disagreements between the calculated and measured values.[Citation5] It has been reported that the calculated values were lower than the measured values. This difference was explained with bound water. The specific heat of various undried foods is greater than that calculated from the specific heat of the dry solid and the moisture content. This is thought to be an effect of bound water, which may have a higher specific heat than the free water.
Since the porosity significantly affected thermal conductivity, the mass fraction model was not suitable for the estimation of the thermal conductivity of bakery products. As mentioned earlier, during processing, there are great structural changes due to chemical and physical reactions, where these changes often interact, strongly affecting the thermal properties. Thus, the regression model based on experimental data is applied more often than the structural model. Calculated specific heat values of dough obtained from models developed by Choi and Okos[Citation36] and Heldman and Singh[Citation37] were compared with the experimental data for dough investigated in this study. Composition of the Croatian dough was determined by standard AOAC method repeated in triplicate[Citation19] and presented in . Additionally, the experimental values of cp and k obtained in this study were compared with the calculated values from models proposed by Gupta[Citation7,Citation18] for the Indian unleavened bread Chapati. The values obtained from the mentioned models were found to have the highest mean percent error and were lower than the experimental data over the first part of temperature range, the part at which chemical and physical changes take place (up to 60°C). The experimental specific heat of the dough was higher than those predicted by theoretical models based on food composition. None of these models were found to be adequate for the whole investigated temperature range ( and ).
TABLE 1 Composition of Croatian dough expressed as mass basis percentages
TABLE 2 Specific heat and thermal conductivity of Croatian dough as functions of temperature in the range of 27.8–124.2°C
FIGURE 3 Specific heat data obtained in this work plotted with the data calculated from equations proposed by Choi and Okos [Citation36], Heldman and Singh [Citation37], and Gupta [Citation7] for Croatian unleavened dough.
![FIGURE 3 Specific heat data obtained in this work plotted with the data calculated from equations proposed by Choi and Okos [Citation36], Heldman and Singh [Citation37], and Gupta [Citation7] for Croatian unleavened dough.](/cms/asset/ccbe7e49-7a07-47d0-9ed0-eaccd4bda980/ljfp_a_971180_f0003_b.gif)
FIGURE 4 Thermal conductivity data obtained in this work plotted with the data calculated from equations proposed by Choi and Okos [Citation36] and Gupta [Citation18] for Croatian unleavened dough.
![FIGURE 4 Thermal conductivity data obtained in this work plotted with the data calculated from equations proposed by Choi and Okos [Citation36] and Gupta [Citation18] for Croatian unleavened dough.](/cms/asset/4069e842-0b88-4e52-b80f-c597d1679f47/ljfp_a_971180_f0004_b.gif)
The multiple peaks appearing in the specific heat and thermal conductivity curves of the Croatian dough make it difficult to reproduce cp and k over the entire studied temperature range using a single equation. Several functions were chosen to fit the data. For the specific heat, the temperature range of 34.3–94.3°C was divided into three sections to fully describe the behavior of the different dough formulations and moisture levels. The first temperature interval was chosen to cover the region leading to the first peak (linear increase). The second temperature interval extended from the onset of the first peak to the top of the following peak (polynomial regression). The third temperature interval extended from the top of the last peak to the finish stage (exponential regression). The same was performed for thermal conductivity. The temperature range of 27.8–124.2°C was divided into three sections: linear increase, polynomial regression, and exponential decline. lists the regression equations found for the dough as a function of temperature, for specific heat and thermal conductivity.
Conclusions
The line heat probe method and the mixing method were effective in measuring the thermal conductivity and specific heat of dough, respectively, at temperatures between 27 and 125°C. The results of the investigation clearly show the significant variation in the specific heat and thermal conductivity of different Croatian unleavened dough with temperature at different moisture levels. The developed models can be used to satisfactorily predict thermal conductivity and specific heat within the range of input variables studied, with a standard deviation of 0.2219 and an error of 5.83%.
Abbreviations
| cp | = | Specific heat (kJ/(kg °C)) |
| cw | = | Specific heat of water (kJ/(kg °C)) |
| Hf | = | Heat capacity of flask calorimeter (kJ/°C) |
| k | = | Thermal conductivity (W/(m °C)) |
| M | = | Mass (kg) |
| Q | = | Heat generation rate per unit length (W/m) |
| T | = | Temperature (°C) |
| Te | = | Final temperature (°C) |
| T0 | = | Initial temperture (°C) |
| t | = | Time (s) |
| te | = | Final time (s) |
| t0 | = | Initial time (°C) |
| Subscripts | = | |
| cw | = | Cold distilled water |
| d | = | Dough sample |
| e | = | Equilibrium condition of mixture |
| hw | = | Hot distilled water |
References
- Heldman, D.R. Prediction Models for Thermophysical Properties of Foods. In Food Processing Operations Modeling, Design, and Analysis; Irudayaraj, J., Ed.; Marcel Dekker, Inc.: New York, 2001; 1–24.
- Mahapatra, A.K.; Lan, Y.; Harris, D.L. Influence of moisture content and temperature on thermal conductivity and thermal diffusivity of rice flours. International Journal of Food Properties 2011, 14 (3), 675–683.
- Perussello, C.A.; Mariani, V.C.; Camargo do Amarante, A.C. Thermophysical properties of okara during drying. International Journal of Food Properties 2014, 17 (4), 891–907.
- Tansakul, A.; Kantrong, H.; Saengrayup, R.; Sura, P. Thermophysical properties of papaya puree. International Journal of Food Properties 2012, 15 (5), 1086–1100.
- Baik, O.D.; Marcotte, M.; Sablani, S.S.; Castaigne, F. Thermal and physical properties of bakery products. Critical Reviews in Food Science and Nutrition 2001, 41 (5), 321–352.
- El-Bushra, S.E. Construction of an isoperibol calorimeter to measure the specific heat capacity of foods between 20 and 90°C. Journal of Thermal Analysis Calorimetry 2001, 64, 261–272.
- Gupta, T.R. Specific heat of Indian unleavened flat bread (Chapati) at various stages of cooking. Journal of Food Process Engineering 1990, 13, 217–227.
- Hu, J.; Sari, O.; Eicher, S.; Rakotozanakajy, A.R. Determination of specific heat of milk at different fat content between 1°C and 59°C using micro DSC. Journal of Food Engineering 2009, 90, 395–399.
- Hwang, M.P.; Hayakawa, K.A. Specific heat calorimeter for foods. Journal of Food Science 1979, 44, 435–438, 448.
- Kemp, R.M.; North, M.F.; Leath, S.R. Component heat capacities for lamb, beef, and pork at elevated temperatures. Journal of Food Engineering 2009, 92, 280–284.
- Kulacki, F.A.; Kennedy, S.C. Measurements of the thermo-physical properties of common cookie dough. Journal of Food Science 1978, 43, 380–384.
- Maisuthisakul P.-O. 6th ASEAN Food Conference, Singapore, Singapore, November 24–27, 1997; Kong, L.K.; Yok Seng, L.Y., Eds.; Institute of Food Science & Technology: Singapore, 1997.
- Razavi, S.M.A.; Taghizadeh, M. The specific heat of pistachio nuts as affected by moisture content, temperature, and variety. Journal of Food Engineering 2007, 79, 158–167.
- Shrivastava, M.; Datta, A.K. Determination of specific heat and thermal conductivity of mushroom (Pleurotusflorida). Journal of Food Engineering 1999, 39, 225–260.
- Buhri, A.B.; Singh, R.P. Measurement of food thermal conductivity using differential scanning calorimetry. Journal of Food Science 1993, 8 (5), 1145–1147.
- Coimbra, J.S.R.; Gabas, A.L.; Minim, L.A.; Garcia Rojas, E.E.; Telis, V.R.N.; Telis-Romero, J. Density, heat capacity, and thermal conductivity of liquid egg products. Journal of Food Engineering 2006, 74, 186–190.
- Gratzek, J.P.; Toledo, R.T. Solid food thermal conductivity determination at high temperatures. Journal of Food Science 1993, 58 (4), 908–913.
- Gupta, T.R. Thermal conductivity of Indian unleavened flat bread (Chapati) at various stages of baking. Journal of Food Process Engineering 1993, 16, 227–235.
- AOAC Official Method of Analysis. Association of Official Analytical Chemists, 2002, 17th ed. AOAC, 10. Arlington, VA.
- Rahman, M.S.; Chen, X.D.; Perera, C.O. An improved thermal conductivity prediction model for fruits and vegetables as a function of temperature, water content, and porosity. Journal of Food Engineering 1997, 31, 163–170.
- Schmalko, M.E.; Morawicki, R.O.; Ramallo, L.A. Simultaneous determination of specific heat capacity and thermal conductivity using the finite-difference method. Journal of Food Engineering 1997, 31, 531–540.
- Tansakul, A.; Chaisawang, P. Thermophysical properties of coconut milk. Journal of Food Engineering 2006, 73 (3), 276–280.
- Carson, J.K. Measurement and modelling of thermal conductivity of sponge and yellow cakes as a function of porosity. International Journal of Food Properties 2014, 17 (6), 1254–1263.
- Šeruga, B.; Budžaki, S. International Congress “Flour-Bread ‘03” and 4th Croatian Congress of Cereal Technologists, Opatija, Croatia, 348–353, 2004; Ugarčić-Hardi, Ž., Ed.; Faculty of Food Technology Osijek: Osijek, 2004.
- Ražnjević, K. Toplinska svojstva vode (H2O) pri tlaku zasićenja [The thermal properties of water (H2O) at a pressure of saturation]. In: Termodinamičke tablice [Thermodynamic tables]; Ražnjević, K., Ed.; Školska knjiga: Zagreb, 1974; pp. 110.
- AbuDagga, Y.; Kolbe, E. Thermophysical properties of surimi paste at cooking temperature. Journal of Food Engineering 1997, 32, 325–337.
- Marcotte, M.; Taherian, A.R.; Karimi, Y. Thermophysical properties of processed meat and poultry products. Journal of Food Engineering 2008, 88, 315–332.
- Sweat, V.E. Experimental values of thermal conductivity of selected fruits and vegetables. Journal of Food Science 1974, 39, 1080–1083.
- Sweat, V.E. Thermal Properties of Foods. In Engineering Properties of Foods; Rao, M.; Rizvi, S.; Eds.; Marcel Dekker, Inc.: New York, 1986; p. 99–112.
- Tansakul, A.; Lumyong, R. Thermal properties of straw mushroom. Journal of Food Engineering 2008, 87, 91–98.
- Murakami, E.G.; Sweat, V.E.; Sastry, S.; Kolbe, E.; Hayakawa, K.; Datta, A. Recommended design parameters for thermal conductivity probes for nonfrozen food materials. Journal of Food Engineering 1996, 27, 109–123.
- Sweat, V.E.; Haugh, C.G. Thermal conductivity probe for small food samples. T ASAE 1974, 17 (1), 56–58.
- Šeruga, B.; Budžaki, S. Determination of thermal conductivity and convective heat transfer coefficient during deep fat frying of “Kroštula” dough. European Food Research and Technology 2005, 221, 351–356.
- De Graaf, L.A. Denaturation of proteins from a non-food perspective. Journal of Biotechnology 2000, 79, 299–306.
- Kovacs, M.I.P.; Fu, B.X.; Woods, S.M.; Khan, K. Thermal stability of wheat gluten protein: Its effect on dough properties noodle texture. Journal of Cereal Science 2004, 39, 9–19.
- Choi, Y.; Okos, M. Effects of Temperature and Composition on the Thermal Properties of Foods. In Food Engineering and Process Applications—Transport Phenomena; Le Maguer, M.; Jelen, P.; Eds.; Elsevier: New York, 1986; p. 93–101.
- Heldman, D.R.; Singh, R.P. Food Process Engineering; Van Nostrand Reinhold: New York, 1981.
