Abstract
The objectives of the present study were to produce snack-type extrudates and to investigate their ability to encapsulate and protect β-carotene (0.05% w/w in sunflower oil) using single layer and layer-by-layer emulsions as an ingredient. The dry feed composed of wheat flour (60% w/w dry solids), maltodextrin (DE 23-27, 20% w/w dry solids), and lactose (20% w/w dry solids). The extrudates (0.6 aw) were ground and sealed in vials under vacuum, placed in vacuum-sealed plastic pouches, and stored at 20, 40, and 60°C. Analysis of the beta-carotene content during storage was carried out using HPLC with a C30 column and diode array detector. The results showed rapid loss of β-carotene during the first six days at all temperatures. Further losses of β-carotene at 20 and 40°C occurred gradually leveling off at 27 days. It was noted that the percentage of retention of β-carotene was generally higher in layer-by-layer extrudates with layer-by-layer upon storage for 27 days. It can be concluded that the layer-by-layer emulsion may enhance protection of bio-sensitive compounds in glassy membranes.
INTRODUCTION
Extrusion is a significant food processing technology used to produce breakfast cereals, ready-to-eat snack foods, as well as other textured foods since the mid-1930s. By using extrusion, raw materials can be converted to various intermediate and finished food products.[Citation1] Increased production and consumption of snack foods has led to expanding choices of products being made available to consumers.[Citation2] “Snack foods” are light meal products that are easy to handle, ready-to-eat, and small in size.[Citation3] Extrusion processes enable continuous operation in cooking and shaping. Extrusion is often a high-temperature, short-time process (HTST) that involves molecular transformations and chemical reactions.[Citation4] The high mechanical shear in the twin screw extrusion process causes breaking of covalent bonds in biopolymers. The structural disruption and mixing promotes changes in functional properties of food ingredients as well as providing texture.[Citation5]
Starch as an ingredient plays a very important role in extrusion since changes in starch structure such as gelatinization, dextrinization, fragmentation, and fusion will affect the texture and expansion of the final product.[Citation3] Extrusion may denature proteins and produce complexes between lipids and starch as well as between lipids and proteins.[Citation6] The advantages of an extrusion process includes the continuous production of high-quality products, the capability to produce products with textural advantages such as crispiness and mouthfeel, low operating cost, high productivity, and reduced cooking time.[Citation7,Citation8] The gelatinization of starch increases the digestibility of the products.[Citation9]
There has been increasing knowledge on the composition of food products and its influence on foods nutritional quality. The presence of bioactive compounds in food products plays a significant role in preventing chronic and degenerative diseases in humans.[Citation10] Carotenoids provide 70% of the vitamin A in the human diet. Due to its high antioxidant capacity as well as provitamin A activity, β-carotene has received more attention compared to other carotenoids.[Citation11] β-carotene occurs naturally in plants either in crystalline form (carrots) or non-crystalline form (mangoes).[Citation12] Nonetheless, β-carotene is only partially soluble at room temperature in oil and insoluble in water, β-carotene in crystalline form has poor bioavailability.[Citation13] The beneficial biological activity of carotenoids can be lost when they are exposed to low pH, high temperature, light, and oxygen. Beta-carotene degradation is usually caused by isomerization[Citation14] and oxidation.[Citation15]
The bioavailability and solubility of β-carotene can be improved by incorporating β-carotene in the lipid phase of oil-in-water (O/W) emulsion. The lipophilic β-carotene can be dissolved into the oil before homogenization to form an O/W emulsion.[Citation16] The stability of an emulsion may be increased with the application of layer-by-layer (LBL) technology on protein coated oil particles. LBL emulsion has better stability toward changes in pH, ionic strength, and heat in thermal processing and drying, lipid oxidation, freeze thaw cycles, and high-salt concentrations.[Citation17,Citation18]
The thicker interfacial layer provides the particles with higher resistance toward disruptions.[Citation19] There are interests in the use of O/W emulsions as delivery system for lipophilic bioactive compounds as they can be used in a wide range of food applications. Microencapsulation, protection, and delivery of bioactive compounds in food materials can be enhanced with the application of layered interfaces of emulsion systems.[Citation20] Oil droplets in such emulsions form particles covered by alternating protein and polyelectrolyte layers and the particles become entrapped within a continuous glass-forming wall matrix during extrusion. The objectives of the present study were to produce snack-type extrudates and to investigate their solids ability to encapsulate and protect beta carotene prepared to an emulsion using single-layer (SL) and LBL interface structures.
MATERIAL AND METHOD
Material
Wheat flour (Musgrave Retail Partners, Cork, Ireland; 14.31% H2O), α-lactose monohydrate (Sigma-Aldrich, St. Louis, Mo., U.S.A.; 2.39% H2O), and maltodextrin (MD250, GPC, U.S.A., 6.26% H2O) were used as solid feed. Whey protein isolate (WPI, Isolac, Carbery Food Ingredients, Balineen, Ireland) was used as emulsifier (the primary layer). Gum arabic (GA; Sigma Aldrich G9752 Stenheim, Germany) was used as a polyelectrolyte (the secondary layer). Sunflower oil (Musgrave Excellence™, Spain) was used as the lipid phase and the solvent for β-carotene (crystalline Type I, synthetic, >93% (UV), powders, Sigma-Aldrich, USA). All other chemicals were purchased from Sigma-Aldrich.
Emulsions Preparation
Preparation of primary emulsions
WPI was dispersed in deionized water (12%, w/w in oil) at room temperature and stirred for 1 h to enhance the hydration of the proteins. pH was adjusted to 3.5 by citric acid (10% w/w). The oil phase was prepared by dispersing β-carotene (0.05%, w/w) in sunflower oil at 50°C by mixing with magnetic stirrer in a beaker until a homogeneous dispersion was obtained. Light exposure of the oil was avoided during the process by covering the beaker with aluminium foil. The oil phase (400 g) and water phase (400 g) with oil:protein ratio of 60:1 were mixed, pre-homogenized using an Ultra-Turrax (T25 Digital, IKA-Werke GmbH & Co. KG, Staufen, Germany) at 10,000 rpm for 60 s. The pre-emulsions were subsequently homogenized at room temperature using a two-stage valve homogenizer (APV-1000, APV Homogenizer Group, Wilmington, MA, USA) with three cycles at 250 bar (approximately 20% of the total pressure was applied for the second stage). The protein-stabilized primary emulsion was used as a wet feed in the extruder.
Preparation of secondary emulsions by layer-by-layer electrodeposition technique
LBL emulsions were prepared by first dispersing GA (0.15% w/w,) in deionized water at room temperature and stirred for 1 h. The GA solution was then adjusted to pH 3.5 with citric acid solution (10% (w/w)). The primary emulsion obtained earlier was mixed with GA solution at room temperature and stirred for 30 min to form LBL. The protein-GA stabilized LBL emulsion was used as a wet feed in the extruder.
Extrusion
The dry ingredients feed contained wheat flour (60%) 20% (w/w) for maltodextrin (DE 23-27) and lactose, respectively. A homogenous mixture of these dry ingredients was prepared by using a mixer (Kenwood KM330, Kenwood Limited, Hampshire, UK). The mixing process was performed at 60 rpm for 5 min. The dry fed into a twin-screw pilot extruder (MPF model, APV Baker, Peterborough, UK) was 73.4 g/min. The barrel had four heating zones and hosted twin screws with screw diameter of 19 mm and length to diameter ratio (L/D) of 25:1. The screw speed was adjusted as 300 rpm. The emulsions were diluted with water at a ratio of 4:1 (4 parts emulsion w/w: 1 part water w/w) enabling them to be fed into the peristaltic pump. The diluted emulsion (wet feed) was supplied using a peristaltic pump (504 U MK, Watson Marlow Ltd) at a rate of 12.153 g/min. The temperatures in the four zones were adjusted to 105, 120, 145, and 155°C, respectively, and these temperatures were kept constant during processing. To obtain a standard curve, the solid mixture was fed into the solid mixture feeder at the rates of 0–3 to give values of 0–101.5 g/min (y = 33.919x R2 = 0.9961) On the other hand, the diluted emulsion were fed into peristaltic pump at rate of 0–25 giving values of 0–20.9 g/min (y = 0.8629x R2 = 0.9967). The extrudates were cooled to room temperature and ground with a mixer (KM330, Kenwood Limited, Hampshire, UK, 30 s at minimum speed). Aliquots (2 g) of the powdered extrudates were transferred to 10 mL clear glass vials (Schott, Müllheim, Germany).The vials were sealed and closed with septa under vacuum in a freeze dryer (Lyovac, GT 2, Steris, Hurth Germany). Closed vials were subsequently sealed in plastic packages (PA/PE 90, Fispak Ltd., Dublin, Ireland) under vacuum (99%) using a vacuum packaging machine (Polar 80 KL, Henkelman B. V., Den Bosch, The Netherlands). Samples were stored in temperature controlled incubators at 20 (cooling incubator, KBP 6151 series 6000, Termarks, Bergen, Norway), 40 (TS 8136, Termarks), and 60°C (TS 8136, Termarks) and protected from light, water loss, and uptake from the environment. The packages with vials retained vacuum during the storage indicating a closed system. Samples were analyzed at intervals during storage for up to 27 days.
Physical and Chemical Analysis
Determination of moisture content and water activity
The moisture content of the extrudates was determined by difference in weight before and after drying in a vacuum oven at 65°C for 24 h. The mean water content ± standard deviation (SD) of triplicate samples for each material was measured. The water activity of the extrudates was measured by water activity meter (Aqua Lab 4TE, Decagon Devices, Inc., Pullman, WA). The mean water activity ± SD of duplicate samples for each material was measured after extrusion and at every interval point before HPLC analysis.
Color measurements
The color (L*, a*, and b* values) of the extrudates were measured by using a Colorimeter (Model CR-300, Konica Minolta, Japan) and the results were expressed in accordance with the CIE Lab System. The standard white tile was used as the reference.
Differential scanning calorimetry (DSC)
Glass transition temperatures, Tg, of the extrudates were measured using DSC (Mettler Toledo 821e with liquid N2 cooling). The extrudates were milled and transferred into pre-weighted DSC aluminium pans (40 μL, Mettler Toledo Schwerzenbach, Switzerland). The pans were hermetically sealed and reweighted. An empty pan was used as a reference. The samples were scanned at 5°C/min from –60 to 50°C, cooled at 10°C/min to –60°C, and a second heating scan at 5°C/min was run from –60 to 100°C. The Tg values were recorded using STARe software, version 8.10 (Mettler Toledo Schwerzenbach, Switzerland) as onset temperatures of the glass transition.
Dynamic mechanical analyses (DMA)
A dynamic mechanical analyser (Tritec 2000 DMA, Triton Technology Ltd., UK) was used to determine the dynamic mechanical properties of the extrudates. Samples of the milled extrudates were prepared in metal pocket-forming sheets (Triton Technology Ltd., UK). A thin sandwich pocket was formed by crimpling the sheet along a pre-scored line which was then attached directly between the clamps of the DMA sample assembly. (The length, width, and thickness of the sample pocket between the clamps were measured.) The samples were scanned between 0.5 to 20 Hz using the single cantilever bending mode from –50 to 140°C with a cooling rate of 5°C/min and a heating rate of 3°C/min. Liquid nitrogen was used for cooling. The α-relaxation temperature was determined from the peak of loss modulus (E’’) above glass transition.
Extraction and HPLC analysis
Two gram samples of the extrudates at various intervals of storage were hydrated and suspended in 15 mL of deionized water by vortexing (Scientific Industries Inc., G-560E, NY, USA) at room temperature for 5 min to release suspended oil particles. In order to destabilize emulsified droplets and extract beta-carotene, 4 mL of methanol:ethlyacetate (1:1 v/v) solution containing 0.25% butylated hydroxyl toluene (BHT) was added and vortexed for 30 s. Oil was saponified by adding 1 mL of saturated (20%) potassium hydroxide in methanol (2M) and vortexed for 30 s to separate the lipid carrier (saponized fraction) from the β-carotene (unsaponized). Finally remaining β-carotene was extracted using 1 mL of dichlorometane and the sample was vortexed for 30 s. The organic phase was separated by adding 4 mL of n-hexane and the sample was further vortexed for 30 s. The extracts were left to stand for 30 min. The top layer was separated using a pipette and centrifuged (Sigma 1-15, Model 78307, D-37520, Ostenode am Harz, Germany) at 10,000 rpm for 5 min. The supernatant was filtered through a filter (Minisart RC 15, Sartorius Stedim Biotech GmbH, Goettingen, Germany), upon transfer to 1 mL HPLC vials. Injections of 200 μL were used into the HPLC system. The β-carotene contents of the extrudates were quantitated using an HPLC (Dionex ICS3000, Sunnyvale, CA, USA), autosampler (AS-1, Dionex, Sunnyvale, CA, USA), and photodiode-array detector (PDA ICS Series, Dionex, Sunnyvale, CA, USA). The HPLC column was a 250 mm × 4.6 mm i.d., 5 μm, reversed-phase Acclaim C30 analytical column with a 4 mm × 4 mm i.d. guard column of the same material (Dionex, Sunnyvale, CA, USA). An eluent gradient composed of acetonitrile at 85 to 65%, methanol:ethyl acetate (1:1) at 15 to 35% and 0.5% acetic acid in water was used for separation of carotenoids that were analysed at 450 nm. The amounts of β-carotene were calculated from the standard curve of all-trans β-carotene. Standard curve was prepared using freshly prepared SL emulsion containing all-trans β-carotene. The amount of emulsion present in the final extrudates was known and was calculated to represent the emulsion present in the extrudates. Based on calculation, 0.07 g of emulsion was present in 1 g sample. Standard curve was prepared using 0.025, 0.05, 0.1, 0.2, 0.3, and 0.4 g of emulsions giving an equation of y = 13.7246x (R2 = 0.98). β-carotene degradation data were fitted to first-order kinetics: −kt = ln A/A0, and the rate constants (k) were derived from the slopes of linear regression lines. Activation energy was obtained using the Arrhenius relationship
Statistical Analysis
The data were analyzed using statistical software SPSS 16.0 (SPSS Inc., USA). The data were also subjected to an analysis of variance (ANOVA) and Duncan’s multiple range test (α = 0.05) was used to determine the differences between means.
RESULTS AND DISCUSSION
Physical Properties of Extrudates and Color
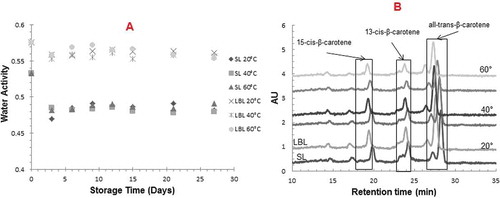
The moisture content of extrudates with SL and LBL emulsion was found to be as 7.47 ± 0.05 g H2O/100 g of solids and 8.71 ± 0.02 g H2O/100 g of solids, respectively (p < 0.05). The constant weights of samples during storage showed no loss of water. Changes in the aw of extrudates during storage is shown in . The initial water activity values were 0.5330 ± 0.004 aw, and 0.5760 ± 0.004 aw for SL and LBL extrudates, respectively, (p < 0.05). The aw remained constant throughout storage of 27 days at the 3 different storage temperatures. A slight gradual decrease during the first three days of storage for both extrudates was noted. SL and LBL extrudates showed a similar Tg (onset) as they have the same wall materials with almost identical water content and water activity. The extrudates has Tg of approximately 3 ± 0.44°C (onset). Structural relaxation of multicomponent food systems may provide more information on the changes in material characteristics around and above the glass transition. DMA measurements were used for the determination of the α-relaxation temperatures (Tα). The Tα of the extrudates were found to be as 55°C at 0.5 Hz.
FIGURE 1 A: Changes in aw values of SL and LBL samples stored at 20, 40, and 60°C for 27 days; B: Peaks identified in chromatogram from SL and LBL extrudates stored at 20, 40, and 60°C.
Color is an important quality factor which reflects the quality and sensory attractiveness of the food materials.[Citation21] The influence of storage temperature and emulsion type on the color values (L*, a*, and b*) of the extrudates are shown in , and , respectively. The results showed that the color values (L*, a*, and b*) of the extruded snack were greatly influenced by emulsion type (SL and LBL), and storage temperature (p < 0.05). The brightness (L*), and yellowness (b*) values of extrudate with SL emulsion (71.83 ± 0.23 and 35.49 ± 0.38) were lower than extrudate with LBL (73.06 ± 0.57 and 41.34 ± 0.34). However, redness value (a*) of extrudates that contained SL emulsion (2.46 ± 0.14) was found to be higher than for LBL (1.05 ± 0.20). As beta carotene was encapsulated in the oil phase, the thicker interfacial layer of LBL emulsion compared to SL emulsion due to the presence of a layer of WPI and GA may have reduced the a* value of the LBL sample. Harnsilawat et al.,[Citation21] and Gu et al.,[Citation22] reported that the thicker interfacial layer of LBL emulsion particles compared to a single emulsifier increased the steric repulsion between particles. In addition, the particles have resistance toward disruptions due to the thick interfacial layer.[Citation19] The differences between the color values of extrudate snacks stored at different temperatures were found to be statistically significant (p < 0.05).
FIGURE 2 Changes in A: L* values; B: a* values; C: b* values of SL and LBL samples stored at 20, 40, and 60°C for 27 days.
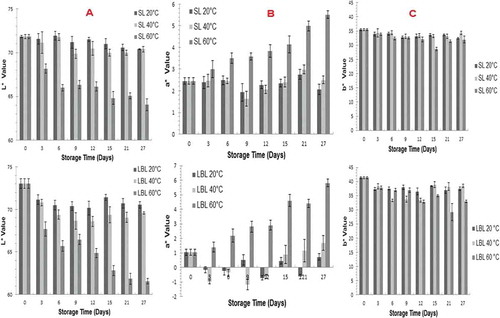
Generally, the L* values decreased and a* values increased with storage time especially at 60°C. The same trends for carrot extrudates and carrot pomace extrudates during storage were observed by Kumar et al.[Citation23] and Dar et al.[Citation24] The color changes were rapid at the beginning of storage but reduced with time. During storage, sugar crystallization of the wall material of extrudates may occur resulting in the release of the oil containing β-carotene which has orange-red color causing an increase in redness during storage. The brightness loss of the extrudates may also be caused by non-enzymatic browning during the storage. and showed that when the storage temperature was increased from 20 to 60°C, rapid brightness loss and increasing redness were observed (p < 0.05).
Desorby et al.[Citation25] reported that while brightness and redness values are good indicators for β-carotene degradation, yellowness value is not a sufficient indicator as they were not dominant. The rapid color changes in the samples at 60°C could be related with β-carotene loss in the sample. The study by Zielinska and Markowski[Citation26] found that high temperature leads to color degradation as a result of loss of carotenoids and β-carotene in carrots. Similar trend was observed between β-carotene loss and the changes of L* and a* values where losses were higher at 40°C compared to 20°C. Only small changes were observed in b* values and no trend could be observed between β-carotene losses, and changes in b* values. However, Gaspar et al.[Citation27] stated that L*, a*, b* values cannot be correlated with β-carotene loss.
HPLC Analysis
The retention of non-crystalline β-carotene in extrudates containing SL and LBL emulsions was monitored during storage at 20, 40, and 60°C for 27 days using HPLC. The mobile phase and stationary phase of the C30 column used in the present study were reported to effectively separate the isomers of β-carotene. Three significant peaks of β-carotene were found in the HPLC chromatograms (). The three β-carotene isomers were identified as 15-cis-β–carotene (19.19 ± 0.81 min), 13-cis-β–carotene (23.51 ± 0.65 min), and all-trans-β-carotene (27.31 ± 0.87 min). Total carotene content and the quantity of individual isomers were calculated from peak area measurements and calibration data. The mechanism of β-carotene degradation has been extensively reviewed by previous researchers.[Citation16,Citation25,Citation28,Citation29] Generally, various factors during food processing and storage, e.g., heat, acid, light, oxygen, metal ions, accelerate oxidation, and isomerization of carotenoids, lead to the degradation and loss of bioavailability.[Citation29,Citation30] Autoxidation is known as the major cause of carotenoid loss in dehydrated foods.
The β-carotene losses after the extrusion process were 50.25 and 37.70% from the theoretical amount for extrudates with SL and LBL emulsions, respectively. These losses were found to be lower than the results of Guzman-Tello and Cheftel[Citation28] and Emin et al.,[Citation9] who reported 70–73% reduction in β-carotene during the extrusion process, respectively. The results may show that the encapsulation of beta carotene in the oil droplets increased the retention. It was also noted the use of LBL emulsion reduced the loss of β-carotene suggesting higher ability of LBL emulsions in protecting bioactive compounds. A study by Rao et al.,[Citation31] on the stability of astaxanthin in oils at different temperatures found significant losses of carotenoids during heating at 120 and 150°C without changes in the fatty acid profiles of edible oils. The barrel temperature of the 4th zone in the extruder in the study was 155°C. This could lead to high losses of β-carotene in the material. In addition to the process conditions, food formulation, and moisture content of feed which defines the food matrix are the major factors influencing carotenoids retention.[Citation32] High moisture content of feed can offer limited protection toward losses in protein water solubility, quality, and molecular structure extrudates containing WPI at constant extrusion temperature.[Citation33] The wet feed of extrudates in this study were fed with diluted emulsion containing high water content and this may provide the encapsulated β-carotene within the extrudates some protection against losses. On the other hand, Pérez-Navarrete et al.,[Citation3] reported that due to lipid degradation from the high processing temperatures, the screw speed used for extrusion, the fatty acids in the raw material form complexes with amylose making extraction more difficult.
β-carotene retention values were plotted versus storage times and the data were fitted using a first order kinetics equation (). However, the reaction kinetic did not properly represent the results obtained. It was observed that the β-carotene retention showed two different trends giving two first order degradation of β-carotene slopes. During the first six days of storage, the degradation rate was faster and the slope was found to be steeper. However, the loss of β-carotene was slower upon storage from day 6 to 27. The higher initial loss in the first six days could be due to the residual oxygen present within the matrix of the extrudates. The initial plot of the degradation kinetics showed that the degradation kinetics was best fitted using two different kinetic. The degradation kinetics of β-carotene followed a first order kinetics followed by a second first order kinetics according to the first order kinetic plots obtained by using equation –kt = ln A/A0. A similar observation was also observed by Desorby et al.,[Citation25] Carotenoids degradation is a typical first order reaction.[Citation24,Citation25,Citation29,Citation33–Citation38] This same trend of carotenoids loss was also observed in the other studies.[Citation15,Citation24,Citation35] Even though oxidation is the major cause of carotenoid degradation, isomerization may play a major role in carotenoid degradation during processing.[Citation39] Chandler and Schwartz[Citation40] found that heating causes increased in isomerization and at the same time decreased all-trans-β-carotene. Significant amount of isomerisation was observed for both extrudates which includes SL and LBL emulsions. The isomerization amount of β-carotene was found to be as 45.06% for extrudates with SL emulsion and 46.46% for extrudates with LBL emulsion. At high temperatures (>120°C) where significant disruption of the food matrix occurred, extensive β-carotene isomerization was observed.[Citation41,Citation42]
FIGURE 3 A: Beta-carotene retention (%) during storage at various temperatures for 27 days; B: Final retention percentages of beta-carotene for extrudates formulated with SL and LBL emulsions after storage at various temperatures for 27 days.
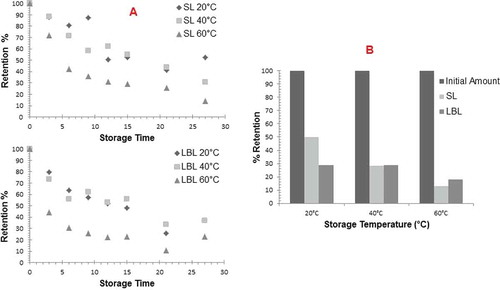
The loss of β-carotene in the extrudates increased with increasing storage temperature. For storage between 0–6 days, reaction rates were generally higher for samples with LBL emulsion. Reaction rates of β-carotene losses were higher in LBL samples between 6 to 27 days as well. The effect of storage temperatures on the β-carotene degradation followed the Arrhenius type of equation both in 0–6 days; with activation energy of 25.40 kJ/mol in SL and 39.39 kJ/mol in LBL (). Nevertheless, the activation energy of extrudate with LBL emulsion for 6–27 days (11.01 kJ/mol) was found to be lower than extrudate with SL emulsion (17.27 kJ/mol). The fast degradation in the LBL system at the beginning may result in it reaching constant value and stay almost stable during storage at 6–27 days. The differences between activation energies of extrudates showed that the different behavior of β-carotene degradation in the two emulsions. The degradation kinetic of β-carotene was found to be significant. The activation energy reflects the temperature dependent of any compound. The higher activation energy reflects that the β-carotene degradation was more temperature dependent. The loss of β-carotene in LBL system was more temperature dependent at day 0–6. However, at day 6–27, loss of β-carotene was more temperature dependent in SL system. It was noted that the beta-carotene amount was slightly higher in extrudates with LBL at all temperatures. The percentage of retention of β-carotene in LBL system was higher upon storage for 27 days at 40 and 60°C (). The higher stability of LBL emulsion.[Citation43,Citation44] may enhance protection of bioactive compounds in glassy membranes. The retention percentage of beta-carotene was calculated as the ratio of the beta-carotene amount of extrudates after extrusion to the beta-carotene amount of extrudates after storage period. The percentage of retention of β-carotene was 28.59% in LBL compared to 28.34% in SL upon storage at 40°C, 17.75% in LBL, and 13.12% in SL upon storage at 60°C. However, lower percentage of retention of β-carotene was observed in LBL at 20°C. Despite the higher β-carotene retention in the LBL during extrusion process, Serfert et al.[Citation45] reported that the oxidative stability of SL microcapsules with lecithin was higher than in the bilayer microcapsules which included lecithin and chitosan.
TABLE 1 Kinetic loss parameters of beta-carotene for extrudates prepared with single layer (SL) and layer-by-layer (LBL) emulsions after storage at 20, 40, and 60°C
Degradation of dispersed lipophilic compounds in hydrophilic solids depends upon matrix stability and lipid physicochemical properties.[Citation12] During storage, sugar crystallization of the wall material of extrudates may occur resulting in the release of dispersed compounds with subsequent exposure to oxygen and heat, and degradation of bioactive components. Further losses of beta-carotene at 20 and 40°C occurred gradually leveling off at 27 days. However, there was a rapid loss of β-carotene content at 60°C during the storage period. In the glassy state, most structural changes occur very slowly.[Citation46,Citation47] However, storing the samples at temperature above the Tg caused rapid structural changes and sugar crystallization of the wall material of extrudates and rapid release of the dispersed β-carotene. Overall, it can be seen that LBL system gave a better stability compared to SL system samples based on the percentage of retention and rate constant from day 6–27. The higher stability of LBL emulsion could play a role in protecting bioactive compounds during extrusion giving a higher initial amount.
CONCLUSION
Extrudates were successfully produced using SL and LBL emulsions as wet feed. The results showed that loss of β-carotene was higher in extrudates with LBL emulsion than in extrudates with SL emulsion. Loss of β-carotene in LBL was more temperature dependence from day 0–6 and in SL from day 6–27. The percentage of retention of β-carotene over storage was generally higher in LBL. Modification to the LBL emulsion can be done to increase its stability toward heat as well as mechanical stress in extrusion. The L* and b* values decreased while a* value of extrudates increased with storage time. The formation of more resistant interfacial film by LBL however does not provide protection against the loss of β-carotene in extrudates after storage at room temperature; conversely, the protection was effective at relatively high temperatures.
REFERENCES
- Omueti, O.; Morton, I.D. Development by extrusion of soyabari snack sticks: A nutritionally improved soya-maize product based on the Nigerian snack (kokoro). International Journal of Food Science 1996, 47, 5–13.
- Kӧksel, H.; Ryu, G.H.; Basman, A.; Demiralp, H.; Ng, P.K.W. Effects of extrusion variables on the properties of waxy hulless barley extrudates. Nahrung/Food 2004, 48, 19–24.
- Pérez-Navarrete, C.; González, R.; Chel-Guerrero, L.; Betancur-Ancona, D. Effect of extrusion on nutritional quality of maize and Lima bean flour blends. Journal of the Science of Food and Agriculture 2006, 86, 2477–2484.
- Castells, M.; Marin, S.; Sanchis, V.; Ramos, A.J. Fate of mycotoxins in cereals during extrusion cooking: A review. Food Additives & Contaminants 2005, 22, 150–157.
- Singh, S.; Gamlath, S.; Wakeling, L. Nutritional aspects of food extrusion: A review. International Journal of Food Science & Technology 2007, 42, 916–929.
- Hagenimana, A.; Ding, X.; Fang, T. Evaluation of rice flour modified by extrusion cooking. Journal of Cereal Science 2006, 43, 38–46.
- Brennan, C.; Brennan, M.; Derbyshire, E.; Tiwari, B.K. Effects of extrusion on the polyphenols, vitamins, and antioxidant activity of foods. Trends in Food Science & Technology 2011, 22, 570–575.
- Sirawdink, F.F.; Ramaswamy, H.S. Protein rich extruded products from tef, corn, and soy protein isolate blends. Journal of Applied Sciences and Technology 2011, 2, 75–90.
- Emin, M.A.; Mayer-Miebach, E.; Schuchmann, H.P. Retention of β-carotene as a model substance for lipophilic phytochemicals during extrusion cooking. LWT–Food Science and Technology 2012, 48, 302–307.
- van Dokkum, W.; Frølich, W.; Saltmarsh, M.; Gee, J. The health effects of bioactive plant components in food: Results and opinions of the EU COST 926 action. Nutrition Bulletin 2008, 33, 133–139.
- Naves, M.M.V.; Moreno, F.S. Beta-carotene and cancer chemoprevention: From epidemiological association to cellular mechanisms of action. Nutrition Research 1998, 18 (10), 1807–1824.
- Harnkarnsujarit, N.; Charoenrein, S.; Roos, Y.H. Reversed phase HPLC analysis of stability and microstructural effects on degradation kinetics of β‑carotene encapsulated in freeze-dried maltodextrin-emulsion systems. Journal of Agricultural and Food Chemistry 2012, 60, 9711−9718.
- Ribeiro, H.S.; Cruz, R.C.D. Biliquid foams containing carotenoids. Engineering in Life Sciences 2005, 5 (1), 84–88.
- Sweeney, S.J.; Marsh, A.C. Effect of processing on provitamin A in vegetables. Journal of the American Dietetic Association 1971, 59, 238–243.
- Simpson, K.L. Chemical Changes in Natural Food Pigments. In Chemical Chances in Food During Processing; Richardson, T.; Finley, J.W.; Eds.; AVI: Westport, CT, 1985; 411.
- Qian, C.; Decker, E.A.; Xiao, H.; McClements, D.J. Physical and chemical stability of b-carotene-enriched nanoemulsions: Influence of pH, ionic strength, temperature, and emulsifier type. Food Chemistry 2012, 132, 1221–1229.
- Gharsallaoui, A.; Saurel, R.; Chambin, O.; Cases, E.; Voilley, A.; Cayot, P. Utilisation of pectin coating to enhance spray-dry stability of pea protein-stabilised oil-in-water emulsions. Food Chemistry 2010, 122, 447–454.
- Gu, Y.S.; Decker, E.A.; McClements, D.J. Influence of pH and ι-carrageenan concentration on physicochemical properties and stability of β-lactoglobulin-stabilized oil-in-water emulsions. Journal of Agricultural and Food Chemistry 2004, 52, 3626–3632.
- Guzey, D.; McClements, D.J. Influence of environmental stresses on O/W emulsions stabilizedby β-lactoglobulin-pectin and β-lactoglobulin-pectin-chitosan membranes produced by the electrostatic layer-by-layer deposition technique. Food Biophysics 2006, 1, 30–40.
- Klinkesorn, U.; Sophanodora, P.; Chinachoti, P.; Decker, E.A.; McClements, D.J. Encapsulation of emulsified tuna oil in two-layered interfacial membranes prepared using electrostatic layer-by-layer deposition. Food Hydrocolloids 2005, 19, 1044–1053.
- Quek, S.Y.; Chok, N.K.; Swedlund, P. The physicochemical properties of spray-dried watermelon powders. Chemical Engineering and Processing 2007, 46, 386–392.
- Gu, Y.S.; Decker, E.A.; McClements, D.J. Production and characterization of oil-in-water emulsions containing droplets stabilized by multilayer membranes consisting of β-lactoglobulin, ι-carrageenan, and gelatin. Langmuir 2005, 21, 5752–5760.
- Kumar, N.; Sarkar, B.C.; Sharma, H.K.; Jha, S.K. Colour kinetics and storage characteristics of carrot, pulse, and rice by-product based extrudates. British Food Journal 2012, 114, 1279–1296.
- Dar, A.H.; Sharma, H.K.; Kumar, N. Effect of extrusion temperature on the microstructure, textural, and functional attributes of carrot pomace-based extrudates. Journal of Food Processing and Preservation 2014, 38, 212–222.
- Desorby, S.A.; Netto, F.M.; Labuza, T.P. Comparision of spray-drying, drum-drying and freeze-drying for β-carotene encapsulation and preservation. Journal of Food Science 1997, 62, 1158–1162.
- Zielinska, M.; Markowski, M. Color characteristics of carrots: Effect of drying and rehydration. International Journal of Food Properties 2011, 15, 450–466.
- Gaspar, M.C.D.M.P.; Soares, R.A.M.; Cardenas, T.D.C.; Lima, S.C.D.T.C.; Areas, J.A.G. Effect of extrusion on β-carotene content and storage stability of corn and bovine lung snacks. Alimentos e Nutrição Araraquara 2012, 23, 529–535.
- Guzman-Tello, R.; Cheftel, J.C. Colour loss during extrusion cooking of β-carotene wheat flour mixes as an indicator of the intensity of thermal and oxidative processing. International Journal of Food Science & Technology 1990, 25, 420–343.
- Achir, N.; Randrianatoandro, V.A.; Bohuon, P.; Laffargue, A.; Avallone, S. Kinetic study of β-carotene and lutein degradation in oils during heat treatment. European Journal of Lipid Science and Technology 2010, 112, 349−361.
- Liang, R.; Shoemaker, C.F.; Yang, X.; Zhong, F.; Huang, Q. Stability and bioaccessibility of β-carotene in nanoemulsions stabilized by modified starches. Journal of Agricultural and Food Chemistry 2013, 61, 1249−1257.
- Rao, A.R.; Sarada, R.; Ravishankar, G.A. Stabilization of astaxanthin in edible oils and its use as an antioxidant. Journal of the Science of Food and Agriculture 2007, 87, 957–965.
- Waramboi, J.G.; Gidley, M.J.; Sopade, P.A. Carotenoid contents of extruded and non-extruded sweet potato flours from Popua New Guınea and Australia. Food Chemistry 2013, 141, 1740–1746.
- Qi, P.X.; Onwulata, C.I. Physical properties, molecular structures, and protein quality of texturized whey protein isolate: Effect of extrusion moisture content. Journal of Dairy Science 2011, 94, 2231–2244.
- Wagner, L.A.; Warthesen, J.J. Stability of spray-dried encapsulated carrot carotenes. Journal of Food Science 1995, 60, 1048–1053.
- Elizalde, B.E.; Herrera, M.L.; Buera, M.P. Retention of β-carotene encapsulated in a trehalose-based matrix as affected by water content and sugar crystallization. Journal of Food Science 2002, 67, 3039–3045.
- Rodrı´guez-Huezo, M.E.; Pedroza-Islas, R.; Prado-Barraga´n, L.A.; Beristain, C.I.; Vernon-Carter, E.J. Microencapsulation by spray-drying of multiple emulsions containing carotenoids. Journal of Food Science 2004, 69, 351–356.
- Zepka, L.Q.; Borsarelli, C.D.; da Silva, M.A.A.P.; Mercadante, A.D. Thermal degradation kinetics of carotenoids in a cashew apple juice model and its impact on the system color. Journal of Agricultural and Food Chemistry 2009, 57, 7841−7845.
- Lim, A.S.L.; Griffin, C.; Roos, Y.H. Stability and loss kinetics of lutein and β-carotene encapsulated in freeze-dried emulsions with layered interface and trehalose as glass former. Food Research International 2014, 62, 403–409.
- Qiu, D.; Chen, Z.H.; Li, H.R. Effect of heating on solid β-carotene. Food Chemistry 2009, 112, 344–349.
- Chandler, L.A.; Schwartz, S.J. Isomerization and losses of trans-beta-carotene in sweet-potatoes as affected by processing treatments. Journal of Agricultural and Food Chemistry 1988, 36, 129–133.
- Marx, M.; Stuparic, M.; Schieber, A.; Carle, R. Effects of thermal processing on trans-cis-isomerization of β-carotene in carrot juices and carotene-containing preparations. Food Chemistry 2003, 83, 609–617.
- Lemmens, L.; Vleeschouwer, K.D.; Moelants, K.R.N.; Colle, I.J.P.; Loey, A.M.V.; Hendrickx, M.E. β-carotene isomerization kinetics during thermal treatments of carrot puree. Journal of Agricultural and Food Chemistry 2010, 58, 6816–6824.
- Harnsilawat, T.; Pongsawatmanit, R.; McClements, D.J. Influence of pH and ionic strength on formation and stability of emulsions containing oil droplets coated by β-lactoglobulin-alginate interfaces. Biomacromolecule 2006, 7, 2052–2058.
- Ogawa, E.A.; Decker, D.; McClements, J. Production and characterization of O/W emulsions containing cationic droplets stabilized by lecithin-chitosan membranes. Journal of Agricultural and Food Chemistry 2003, 51, 2806–2812.
- Serfert, Y.; Schröder, J.; Mescher, A.; Laackmann, J.; Shaikh, M.Q.; Rätzke, K.; Gaukel, V.; Schuchmann, H.P.; Walzel, P.; Moritz, H.U.; Drusch, S.; Schwarz, K. Characterization of the spray drying behaviour of emulsions containing oil droplets with a structured interface. Journal of Microencapsulation 2013, 30, 325–334.
- Buera, P.; Schebor, C.; Elizalde, B. Effects of carbohydrate crystallization on stability of dehydrated foods and ingredient formulations. Journal of Food Engineering 2005, 67, 157–165.
- Ramoneda, X.A.; Ponce-Cevallos, P.A.; Buera, M.D.P.; Elizalde, B.E. Degradation of β-carotene in amorphous polymer matrices. Effect of water sorption properties and physical state. Journal of the Science of Food and Agriculture 2011, 91, 2587–2593.
