Abstract
Water removal during drying depends on the pathway of water migration from food materials. Moreover, the water removal rate also depends on the characteristics of the cell wall of plant tissue. In this study, the influence of cell wall properties on porosity and shrinkage of dried product was investigated. Cell wall stiffness depends on a complex combination of plant cell microstructure, composition of food materials and the water-holding capacity of the cell. In this work, a preliminary investigation of the cell wall properties of apple was conducted in order to predict changes of porosity and shrinkage during drying. Cell wall characteristics of two types of apple (Granny Smith and Red Delicious) were investigated under convective drying to correlate with porosity and shrinkage. A scanning electron microscope (SEM), 2kN Intron, pycnometer and ImageJ software were used in order to measure and analyse cell characteristics, water holding capacity of cell walls, porosity and shrinkage. The cell firmness of the Red Delicious apple was found to be higher than for Granny Smith apples. A remarkable relationship was observed between cell wall characteristics when compare with heat and mass transfer characteristics. It was also found that the evolution of porosity and shrinkage are noticeably influenced by the nature of the cell wall during convective drying. This study has revealed a better understanding of porosity and the shrinkage of dried food at microscopy (cell) level, and will provide better insights to attain energy-effective drying processes and improved quality of dried foods.
INTRODUCTION
Since the moisture content of fresh fruits and vegetables is more than 80%, they are classified as perishable commodities.[Citation1] Food is one of the most complex materials in natural form and the fundamental understanding of food drying has not been fully established.[Citation2] Lack of proper processing causes considerable damage and wastage of seasonal fruits in many countries, which is estimated to be 30–40% seasonal fruits in developing countries.[Citation3] Drying of foodstuffs is important, and is indeed the oldest method of food processing. Many physical and chemical changes occur in foods during the drying process. The quality of the dehydrated product is affected by a number of factors including quality of raw material, method of preparing, processing treatments, and drying conditions.[Citation4–Citation6] The first objective of drying is to remove water and hence to stabilize the food product. However, during food drying, many physico-chemical changes occur simultaneously, resulting in change in the overall quality of the dried food.[Citation7] Moreover, drying kinetics depends on the physical properties of food including food structure, components, maturity of the food sample, and drying conditions.[Citation8] In order to achieve better quality food and optimum drying conditions, deep insight into the drying phenomenon is essential.
Biological materials, especially plant tissues, are very complex in nature when one considers their anisotropy, porosity, and hygroscopic attributes. Fresh apple has a large amount of intercellular space, with 22–38% air spaces of its total tissue volume.[Citation9] In other words, fresh apple flesh is intensely porous, with an initial porosity 0.22–0.38.[Citation10] Among the different types of apples, the Granny Smith has one of the highest levels of initial porosity (0.33), due to its larger cell dimensions and thinner cell wall thickness. Larger cells in plant tissue generally are found loosely packed when compared with smaller cells. Despite having almost the same moisture content (0.86 and 0.87 g/g of sample for Granny Smith and Red Delicious, respectively) and density (789 and 766 kg/m3 for Granny Smith and Red Delicious, respectively), Granny Smith and Red Delicious apples show different drying rates. Moreover, the literature confirms the role of the mechanical properties of plant-based food on flavor, bio-availability of nutrients, and textural perception.[Citation11]
Plant cell membranes and walls are semi-permeable and are regarded as the components that affect the transfer of water to and from parenchyma cells.[Citation12,Citation13] The determination of the moisture content in fruits and vegetables is a complex task, as it involves both free and bound water. The determination of the separate types of water is even more critical than the estimation of the total water. The drying rate of the later falling rate period is too slower due to the internal mass transfer resistance increases and water is transported through a thicker layer of the dry solid matrix.[Citation14] The literature shows that in order to remove the last 10% of water from food material, it takes almost the same amount of time that is required to remove the first 90% of the water content. In other words, bound water migration from food materials during drying takes significant time and energy. In plant tissue, water distribution is intercellular, intracellular, and within the cell wall.[Citation15] Of the total water in plant tissue, more than 90% of water is intracellular and cell wall water.[Citation16]
Therefore, macroscopic structural behavior depends on turgor pressure, cell wall properties, and cell size.[Citation17–Citation19] Generally, the cell wall of plants comprises approximately 60% water, 2–8% pectin substances, 5–15% hemicellulose, 10–15% cellulose, 1–2% protein, and 0.5–3% lipids.[Citation20] These proportions vary due to a variety of factors, such as environmental conditions, stage of maturity, processing after harvest, and botanical origin of the plant.[Citation21] Determining the size and shape of the cell is one of the most important functions of the cell walls in plant tissue. In addition to this, the mechanical properties of the tissue depends on the cell wall properties.[Citation22] In addition to this, intact cell walls and cell membranes are the main hindrance for water migration from the cell.[Citation23] Dietary fiber is a non-homogeneous mixture of polysaccharides and lignin; and this mixture is mainly found in plant cell walls.[Citation24] This dietary fiber is mainly classified into soluble dietary fibre (SDF) such as pectin, gum, etc., and insoluble dietary fibre (IDF), such as lignin, cellulose, and hemicellulose.[Citation25,Citation26] These non-soluble compounds build the cell wall as a porous structure.[Citation27,Citation28] Of the dietary fibers, only the soluble can hold water.[Citation29] On the other hand, IDF causes less permeability of water due to its insoluble nature when compared with the SDF. Thus, the presence of more IDF leads to the development of a denser solid matrix cell wall. So, it can be seen that a ratio of soluble and IDF is an important factor when considering the permeability of the cell wall. More IDF means that there are more obstacles in the rigid skeleton for the path of water flow during drying.
Knowledge of the exact amount of bound water in the cell wall of fruit and vegetable tissue is crucial for realistic modeling of heat and mass transfer as well as in determining more efficient drying systems.[Citation30] However, there is currently insufficient study concerning the determination of cell wall bound water. In addition to bound water, recognition of the stiffness of the cell wall is essential to correlate the deformation (shrinkage/expansion) during drying. The compression test provides the mechanical response of the plant tissue.[Citation31] As stiffness is one of the structural properties of the tissue, it can be derived from the material’s properties and by considering the shape of the sample. In addition to stiffness, Poisson’s ratio and Young’s modulus are important apparent elastic properties of biomaterials for the anticipation of load-deformation behaviour of those materials. These elastic properties can be used in order to compare the relative strength of various plant tissues.[Citation32]
In general, the mechanical properties of plant tissues are assessed to predict post-harvesting process parameters.[Citation33] However, researchers have not dealt with the mechanical properties of cell walls (in general these are proportional to the mechanical properties of the tissue) in order to establish relationships between drying kinetics and physico-chemical characteristics and mechanical properties. In this study, two types of apples, namely Granny Smith and Red Delicious, were investigated to understand the characteristics of the cell wall. In addition, Influence of cell wall characteristics in terms of cell wall elastic properties (Young modulus, Poisson’s ratio and stiffness), cell wall thickness, cell diameter, and water distribution within the cell wall on the drying rate and dried food physical properties such as porosity and shrinkage were investigated.
MATERIALS AND METHODS
Sample Preparation
Granny Smith and Red Delicious apples were purchased from the local supermarket. First the apples were sliced into 11 mm diameter and 12 mm height cylinders for compression tests (), where each of the samples was subject to 3.5 mm deformation. Then 3.5 mm thick apple samples were dried in a convective dryer at 70°C for 180 min. Real-time mass measurements were provided with KERN ABT 220-5DM analytical balance with ±0.0001-g sensitivity. The compression speed was 2, 5, 10, and 20 mm/min, as proposed in ASAE standard 368.1.[Citation34] Experiments were carried out using the 2kN Instron universal testing machine. The increasing force caused a loosening of the rigidity of the sample, and led to a rupture at the rupture point. Force and deformation data from the compression test were recorded on a connected computer.
Young’s modulus (apparent elastic modulus, ASAE standard 368.1) was obtained from the slope of the force-deformation curve at the point of its highest gradient.[Citation35] The Poisson’s ratio was calculated by measuring the diminution before and after deformation under compression test. Young’s modulus, Poisson’s ratio and stiffness were calculated using the following formulas:[Citation36–Citation38]
where, A is the cross-sectional area of the sample.
A Quanta 200 scanning electron microscope (SEM) was used in order to analyze the microstructure of both fresh and dried apple slices. Meanwhile, a pycnometer (Pentapyc 52000e) was used to obtain the porosity of fresh and dried samples. Also, ImageJ 1.47v software was used to analyse the microstructure of the cell wall thickness and bulk shrinkage before and after drying, and to measure cell dimensions.
Cell Wall Water Estimation
An assumption is made that all cell wall water is treated as physically bound water due to its easy availability during the drying and moisture removal process. Therefore, measuring the cell wall thickness before and after drying can give the amount of water it holds. Some other assumptions of calculating cell wall water are as follows:
Shape of the cells is spherical. However, the ratio of intracellular and cell wall water minimizes the error of this assumption.
As most of the plant tissue shows the heterogeneous nature of cell orientation and content, average numbers of cell and intercellular spaces will be considered.
The density of cell wall water is the same as the density of intracellular water.
Cell dimension varies within the plant tissue, but the average cell dimension has been considered here.
Thus, it is assumed that the difference in the cell wall thicknesses of fresh and complete dried sample represents the amount of bound water. After calculating the volume difference of the cell walls, the following equation can be used to give the amount of bound water in the cell wall:
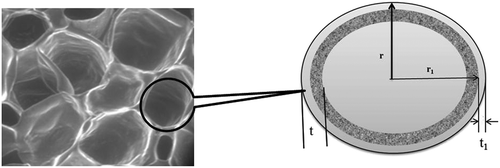
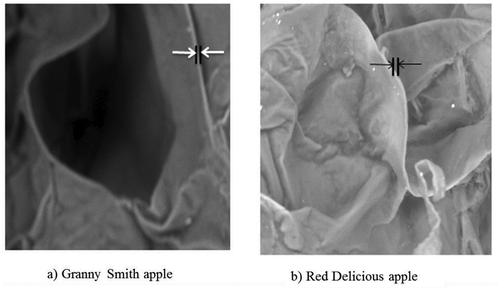
where, r and r1 are the radius of the fresh and dried plant tissue cell (), t is the initial thickness and t1 is the final thickness of the cell wall. Shrinkage coefficient of the cell wall thickness,
From Eq. (1), it is clearly apparent that the amount of cell water depends directly on the cell wall thickness, cell dimension, and cell wall shrinkage coefficient. Therefore, it is not enough to consider only cell wall water to represent the amount of water in food tissue rather than the ratio of intracellular and cell wall bound water, which can provide more insight regarding the overall bound water present in the plant tissue. So, the ratio of intracellular and cell wall water can be obtained from Eq. (2).
Therefore, it is possible to obtain quite accurate amounts of bound water from examining the microstructure of the fresh and dried products.
RESULTS AND DISCUSSION
Cell Stiffness and Drying Rate
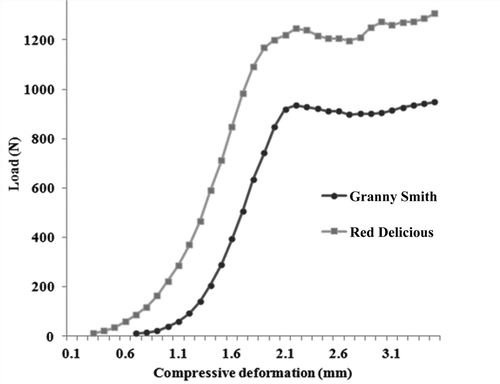
As shown in , in order to achieve the same amount of compressive deformation, Granny Smith apples require less energy than Red Delicious apples, as Red Delicious apple tissue is stiffer (23.03 kN/m) than Granny Smith apple (21.8 kN/m). The average Young’s modulus is also higher for Red Delicious (3.10 MPa), while Granny Smith shows Young’s modulus 2.81 MPa. It was found that the value of Poisson’s ratio is 0.169–0.344 for Granny Smith[Citation36] and 0.155–0.25 for Red Delicious apples.[Citation39] Overall, the cell wall stiffness is presumed higher in Red Delicious, as its tissue is stiffer than Granny Smith. Consequently, this higher stiffness affects the rate of moisture migration from the cells of the apple flesh.
There is a significant similarity between the compressive test and the drying process. The result, as shown in , indicates that the moisture migration rate is higher for Granny Smith when compared to Red Delicious. This result confirmed that in order to remove the same amount of water, the Red Delicious apple took more heat energy than the Granny Smith. Therefore, a positive relationship was found between water migration by mechanical and thermal energy as demonstrated by these similar trends. Interestingly, this correlation can be explained by the nature of the cell wall stiffness that facilitates or hinders the water migration in mechanical and thermal energy applications.
Cell Dimension and Porosity
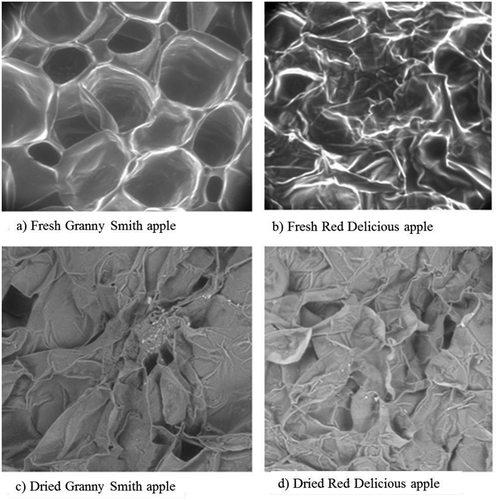
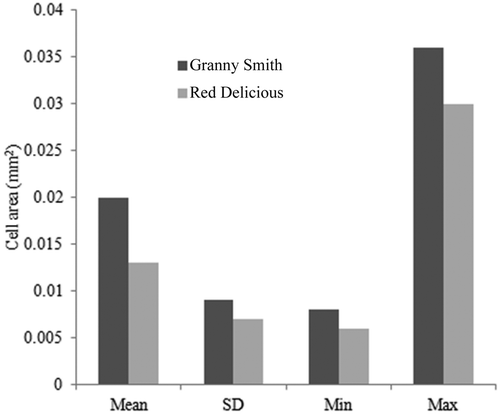
Cell dimension influenced the rigidity of the cell wall and tissue of plant materials. Greater cell dimensions caused the loose packing of cells, and consequently density, rigidity, intercellular spaces were affected significantly. compares the microstructures of fresh and dried Granny Smith and Red Delicious apples. The results obtained from microstructure analysis of the samples shows that the Granny Smith cells are larger than those of the Red Delicious, as shown in . This is consistent with previous literature, which maintained that the Granny Smith has a high initial porosity of is 0.33.[Citation9] It was also found from the pycnometer data that dried Granny Smith apple has a higher porosity due to its larger cell dimensions and the consequent loose packing of cells.
Cell Wall Bound Water
Cell wall thickness analysis, presented in , shows that the Red Delicious has thicker cell walls when compared with Granny Smith cell wall thickness in fresh state (9.31 ± 3.1 and 11.405 ± 3.2 µm for Granny Smith and Red Delicious, respectively). On the other hand, after drying the cell wall shrinks more (on average 78.68% shrinkage) in Red Delicious compared to Granny Smith (50% shrinkage) as shown in . This result indicates the presence of more bound water within the cell wall in Red Delicious.
TABLE 1 Cell wall thickness of fresh and dried apple
These findings concerning cell wall characteristics indicate that the amount of bound water in cell walls was higher in the Red Delicious apple. In addition, from Eq. (3), the ratio of intracellular and cell wall water shows Red Delicious cell water was quite a deal higher than for the Granny Smith apple. This result might be an explanation as to why Red Delicious apple takes more energy for compression and drying to give certain moisture content. It is relevant to compare the nature of these cell walls with the dietary fiber of apples. The ratio of IDF and SDF in Red Delicious is 4.37, whereas it is 3.21 for Granny Smith,[Citation40] which indicates that the cell wall of the Red Delicious is highly dense with insoluble fiber. In addition to this, it is a possible explanation as to why more energy is required to remove the same amount of water from Red Delicious apple when compared with the Granny Smith variety.
Bound Water and Bulk Shrinkage
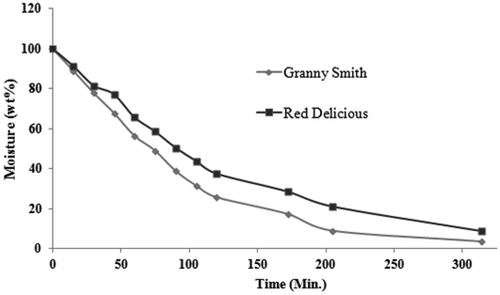
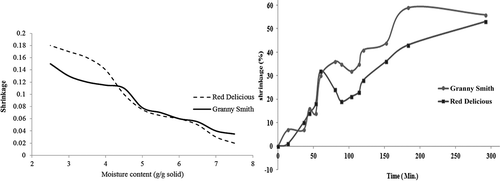
The amount of bound water influenced the trend of drying kinetics and physicochemical changes,[Citation41] in particular during the latter part of drying kinetics, as shown in . Shrinkage of the Granny Smith apple increased steadily with the decrease of moisture content when compared with the Red Delicious due to its higher intercellular and cell-to-cell moisture flux, up to intermediate moisture content. On the other hand, with respect to time and moisture content as shown in , shrinkage in the Red Delicious apple sharply increased at the end of the drying process, due to cell wall collapse, which began in that period.
This property of the cell walls also can explain why the cell wall collapses more in Red Delicious apple than in the Granny Smith apple. Taken together, the findings from the compression test, drying kinetics, shrinkage in the cell wall, and bulk shrinkage of tissue manifest that Red Delicious apple contains more trapped water in the cell wall than does the Granny Smith apple. Moreover, it emerged from the data that the porosity and shrinkage of dried food significantly depended on the nature of the cell wall. Further investigation is required to gain more insight into the cell wall properties in order to achieve optimum drying conditions and a better quality of dried food.
CONCLUSION
Drying kinetics and dried food quality are subject to drying conditions and fresh food properties. In this study, cell wall characteristics in terms of stiffness, wall thickness, bound water, and cell dimension were investigated in order to establish a relationship between with porosity and shrinkage. Drying kinetics and compressive deformation were found to trend similarly for the two varieties of apple slices. In other words, both drying kinetics and compressive drying confirm that Red Delicious apple took more energy than Granny Smith apple to release the same amount of water. This finding suggests that drying kinetics noticeably depends on the cell wall characteristics of plant tissues. Moreover, dried food quality, especially physical attributes such as porosity, shrinkage, and microstructure, are significantly affected by the cell wall characteristics. The findings of this study will bring new understanding of the relationship of the mechanical properties of plant tissue with drying kinetics and dried food quality.
ACKNOWLEDGMENT
This article was first presented in the First International Conference on Food Properties (iCFP 1) held in Kuala Lumpur, Malaysia on January 24–26, 2014 and it received recognition as one of the innovative papers.
REFERENCES
- Orsat, V.; Yang, W.; Changrue, V.; Raghavan, G.S.V. Microwave-assisted drying of biomaterials. Food and Bioproducts Processing 2007, 85(3),255–263.
- Barati, E.; Esfahani, J.A. Mathematical simulation of convective drying: Spatially distributed temperature and moisture in carrot slab. International Journal of Thermal Sciences 2012, 56(0),86–94.
- Karim, M.A.; Hawlader, M.N.A. Mathematical modelling and experimental investigation of tropical fruits drying. International Journal of Heat and Mass Transfer 2005, 48(23–24), 4914–4925.
- Puranik, V.; Srivastava, P.; Mishra, V.; Saxena, D.C. Effect of different drying techniques on the quality of garlic: A comparative study. American Journal of Food Technology 2012, 7(5),311–319.
- Joardder, M.U.H.; Kumar, C.; Karim, M.A. Effect of moisture and temperature distribution on dried food microstucture and porosity. Proceedings of From Model Foods to Food Models: The DREAM Project International Conference 2013.
- Kumar, C.; Karim, M.A.; Joardder, M.U.H. Intermittent drying of food products: A critical review. Journal of Food Engineering 2014, 121(0),48–57.
- Kompany, E.; Benchimol, J.; Allaf, K.; Ainseba, B.; Bouvier, J.M. Carrot dehydration for instant rehydration: Dehydration kinetics and modelling. Drying Technology 1993, 11, 451–470.
- Joardder, M.U.H.; Kumar, C.; Karim, M.A. Effect of temperature distribution on predicting quality of microwave dehydrated food. Journal of Mechanical Engineering and Sciences 2013, 5, 562–568.
- Vincent, J.F.V. Relationship between density and stiffness of apple flesh. Journal of the Science of Food and Agriculture 1989, 47, 443–462.
- Lewicki, P.P.; Pawlak, G. Effect of drying on microstructure of plant tissue. Drying Technology 2003, 21, 657–683.
- Ormerod, A.P.; Ralfs, J.D.; Jackson, R.; Milne, J.; Gidley, M.J. The influence of tissue porosity on the material properties of model plant tissues. Journal of Materials Science 2004, 39(2),529–538.
- Qi, H.; Sharma, S.K.; LeMaguer, M. Modeling multicomponent mass transfer in plant material in contact with aqueous solutions of sucrose and sodium chloride during osmotic dehydration. International Journal of Food Properties 1999, 2(1),39–54.
- Fanta, S.W.; Vanderlinden, W.; Abera, M.K.; Verboven, P.; Karki, R.; Ho, Q.T.; De Feyter, S.; Carmeliet, J.; Nicolaï, B.M. Water transport properties of artificial cell walls. Journal of Food Engineering 2012, 108(3),393–402.
- Aversa, M.; Curcio, S.; Calabrò, V.; Iorio, G. Measurement of the water-diffusion coefficient, apparent density changes, and shrinkage during the drying of Eggplant (Solanum Melongena). International Journal of Food Properties 2011, 14, 523–537.
- Joardder, M.U.H.; Kumar, C.; Karim, M.A. Better understanding of food material on the basis of water distribution using thermogravimetric analysis. In International Conference on Mechanical, Industrial, and Materials Engineering (ICMIME November, 1–3, 2013). 2013. Rajshahi, Bangladesh.
- Halder, A.; Datta, A.K.; Spanswick, R.M. Water transport in cellular tissues during thermal processing. AIChE Journal 2011, 57(9),2574–2588.
- Alamar, M.C.; Vanstreels, E.; Oey, M.L.; Moltó, E.; Nicolaï, B.M. Micromechanical behaviour of apple tissue in tensile and compression tests: Storage conditions and cultivar effect. Journal of Food Engineering 2008, 86(3),324–333.
- Rojas, A.M.; Delbon, M.; Marangoni, A.G.; Gerschenson, L.N. Contribution of cellular structure to the large and small deformation rheological behavior of kiwifruit. Journal of Food Science 2002, 67(6),2143–2148.
- Aguilera, J.M.; Lillford, P.J. Food Materials Science: Principles and Practice; Springer: Dordrecht, 2007.
- Bach Knudsen, K.E. The nutritional significance of “dietary fibre” analysis. Animal Feed Science and Technology 2001, 90(1–2), 3–20.
- Eastwood, M.A. The physiological effect of dietary fiber: An update. Annual Review of Nutrition 1992, 12, 19–35.
- Vincent, J.F.V. The Composite Structure of Biological Tissue Used for Food. In Food Materials Science; Aguilera, J.M. and Lillford, P.J.; Eds.; Springer: New York, 2008.
- Kusnadi, C.; Sastry, S.K. Effect of temperature on salt diffusion into vegetable tissue. International Journal of Food Properties 2011, 15(5),1148–1160.
- Flores, J.A.R. Effects of Soluble and Insoluble Dietary Fiber on Diet Digestibility And Sow Performance; University of Minnesota, Minneapolis, MN. 2003.
- Gorinstein, S.; Zachwieja, Z.; Folta, M.; Barton, H.; Piotrowicz, J.; Zember, M.; Weisz, M.; Trakhtenberg, S.; Martın-Belloso, O. Comparative content of dietary fiber, total phenolics, and minerals in persimmons and apples. Journal of Agricultural and Food Chemistry 2001, 49, 952–957.
- Gupta, P.; Premavalli, K.S. In-vitro studies on functional properties of selected natural dietary fibers. International Journal of Food Properties 2011, 14(2),397–410.
- Kethireddipalli, P.; Hung, Y.-C.; Phillips, R.O.; Mc Watters, K.H. Evaluating the role of cell material and soluble protein in the functionality of Cowpea (Vigna Unguiculata) pastes. Journal of Food Science 2002, 67(1),53–59.
- Lewicki, P.P. Effect of pre‐drying treatment, drying, and rehydration on plant tissue properties: A review. International Journal of Food Properties 1998, 1(1),1–22.
- Boulos, N.N.; Greenfield, H.; Wills, R.B.H. Water holding capacity of selected soluble and insoluble dietary fibre. International Journal of Food Properties 2000, 3(2),217–231.
- Joardder, M.U.H.; Kumar, C.; Karim, M.A.; Brown, R.J. Fractal Dimension of Dried Foods: A Correlation Between Microstructure and Porosity, In Food Structures, Digestion, and Health International Conference, Melbourne, Australia, October 21–24, 2013.
- Sirisomboon, P.; Pornchaloempong, P. Instrumental textural properties of Mango (cv Nam Doc Mai) at commercial harvesting time. International Journal of Food Properties 2011, 14(2),441–449.
- Kiani Deh Kiani, M.; Maghsoudi, H.; Minaei, S. Determination of Poisson’s ratio and Young’s modulus of red bean grains. Journal of Food Process Engineering 2011, 34(5),1573–1583.
- Gharibzahedi, S.M.T.; Emam-Djomeh, Z.; Razavi, S.H.; Jafari, S.M. Mechanical behavior of lentil seeds in relation to their physicochemical and microstructural characteristics. International Journal of Food Properties 2013, 17(3),545–558.
- Ekrami-Rad, N.; Khazaei, J.; Khoshtaghaza, M.-H. Selected mechanical properties of pomegranate peel and fruit. International Journal of Food Properties 2011, 14(3),570–582.
- Khan, A.A.; Vincent, J.V.F. Compressive stiffness and fracture properties of apple and potato parenchyma. Journal of Texture Studies 1993, 24(4),423–435.
- Grotte, M.; Duprat, F.; Piétri, E.; Loonis, D. Young’s modulus, Poisson’s ratio, and Lame’s coefficients of Golden Delicious apple. International Journal of Food Properties 2002, 5(2),333–349.
- Bentini, M.; Caprara, C.; Martelli, R. Physico-mechanical properties of potato tubers during cold storage. Biosystems Engineering 2009, 104(1),25–32.
- Emadi, B.; Abbaspour-Fard, M.H.; Kdv Yarlagadda, P. Mechanical properties of melon measured by compression, shear, and cutting modes. International Journal of Food Properties 2009, 12(4),780–790.
- Chappell, T.W.; Hamann, D.D. Poisson’s ratio and Young’s modulus for apple flesh. Transactions of the ASAE 1968, 11(5),608–612.
- Ferdous, G. Dietary fibre content of thirteen apple cultivars. Journal of the Science of Food and Agriculture 1997, 75(3), 333–340.
- Shanbhag, S.; Steinberg, M.P.; Nelson, A.I. Bound water defined and determined at constant temperature by wide-line nmr. Journal of Food Science 1970, 35(5),612–615.